What are Solutions Homogeneous mixtures composition will not







- Slides: 24

What are Solutions? • • Homogeneous mixtures – composition will not vary from one sample of the mixture to another sample of the same mixture but will result in a uniform, homogeneous mixture: ethanol (CH 3 CH 2 OH mixed into H 2 O) – it appears to be one substance, though it really contains multiple materials – The definition of a solution is that it is a homogeneous mixture Most homogeneous materials we encounter are actually solutions – e. g. pure air The solute is the dissolved substance • The solvent is the substance the solute is dissolved in • When both the solute and solvent appear to have the same state, the solvent is the component present in the highest percentage. • Solutions in which the solvent is water are called aqueous solutions. •

Common Types of Solutions Solution Phase gaseous solutions Solute Phase Solvent Phase Example gas air (mostly N 2 & O 2) liquid solutions gas liquid solid liquid soda (CO 2 in H 2 O) vodka (C 2 H 5 OH in H 2 O) Table salt in water solid solutions solid brass (Zn in Cu) Solubility • • Solutions that contain Hg and some other metal are called amalgams. Solutions that contain metal solutes and a metal solvent are called alloys. When one substance (solute) dissolves in another (solvent) it is said to be soluble: – salt is soluble in water, – bromine is soluble in methylene chloride. When one substance does not dissolve in another it is said to be insoluble: – oil is insoluble in water.

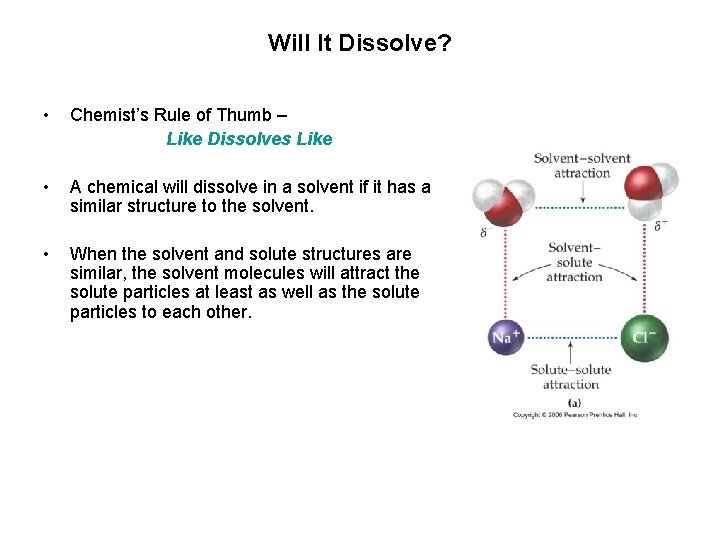
Will It Dissolve? • Chemist’s Rule of Thumb – Like Dissolves Like • A chemical will dissolve in a solvent if it has a similar structure to the solvent. • When the solvent and solute structures are similar, the solvent molecules will attract the solute particles at least as well as the solute particles to each other.

Classifying Solvents Solvent Class Structural Feature Water, H 2 O polar O-H Ethyl Alcohol, C 2 H 5 OH polar O-H Acetone, C 3 H 6 O polar C=O Benzene, C 6 H 6 nonpolar C-C & C-H Hexane, C 6 H 14 nonpolar C-C & C-H Diethyl Ether, C 4 H 10 O nonpolar C-C, C-H & C-O

Will It Dissolve In Water? • Ions are attracted to polar solvents – many ionic compounds dissolve in water. • Polar molecules and ones with a lot of –OH groups are attracted to polar solvents – table sugar, ethyl alcohol and glucose all dissolve well in water. • Nonpolar molecules are attracted to nonpolar solvents – b-carotene, (C 40 H 56), is not water soluble; it dissolves in fatty (nonpolar) tissues. • Many molecules have both polar and nonpolar structures – whether they will dissolve in water depends on the kind, number and location of polar and nonpolar structural features in the molecule.

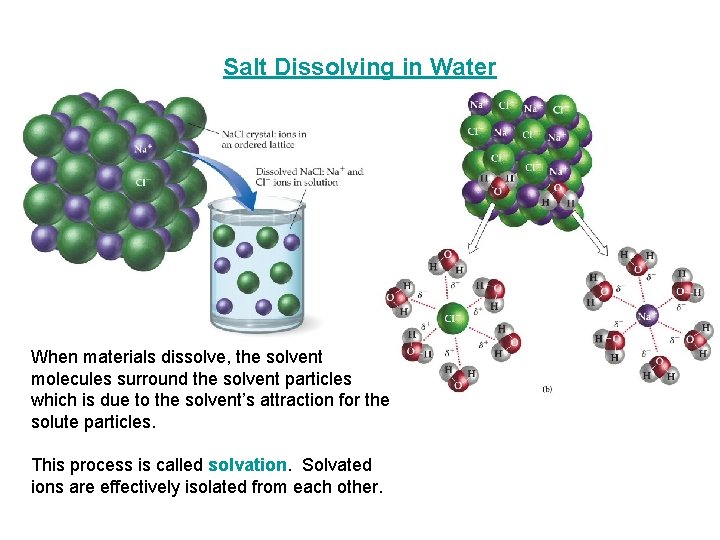
Salt Dissolving in Water When materials dissolve, the solvent molecules surround the solvent particles which is due to the solvent’s attraction for the solute particles. This process is called solvation. Solvated ions are effectively isolated from each other.

Solubility • There is usually a limit to the solubility of one substance in another: – gases are always soluble in each other – two liquids that are mutually soluble are said to be miscible • alcohol and water are miscible • oil and water are immiscible. • The maximum amount of solute that can be dissolved in a given amount of solvent is called the solubility: • Saturated solutions have the maximum amount of solute that will dissolve in that solvent at that temperature; i. e. , colder solvents dissolve less than warm or hot solvents. • Unsaturated solutions can dissolve more solute. • Supersaturated solutions are holding more solute than they should be able to at that temperature and are unstable. When disturbed the amount of solute above the solubility limit comes out of solution.

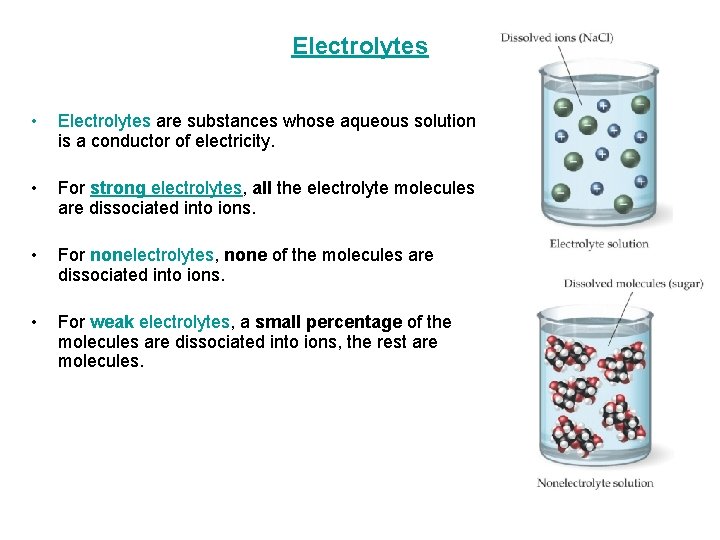
Electrolytes • Electrolytes are substances whose aqueous solution is a conductor of electricity. • For strong electrolytes, all the electrolyte molecules are dissociated into ions. • For nonelectrolytes, none of the molecules are dissociated into ions. • For weak electrolytes, a small percentage of the molecules are dissociated into ions, the rest are molecules.

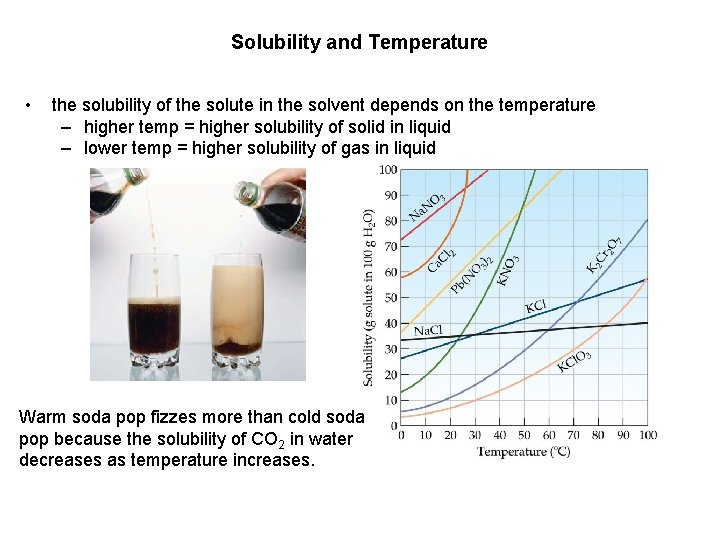
Solubility and Temperature • the solubility of the solute in the solvent depends on the temperature – higher temp = higher solubility of solid in liquid – lower temp = higher solubility of gas in liquid Warm soda pop fizzes more than cold soda pop because the solubility of CO 2 in water decreases as temperature increases.

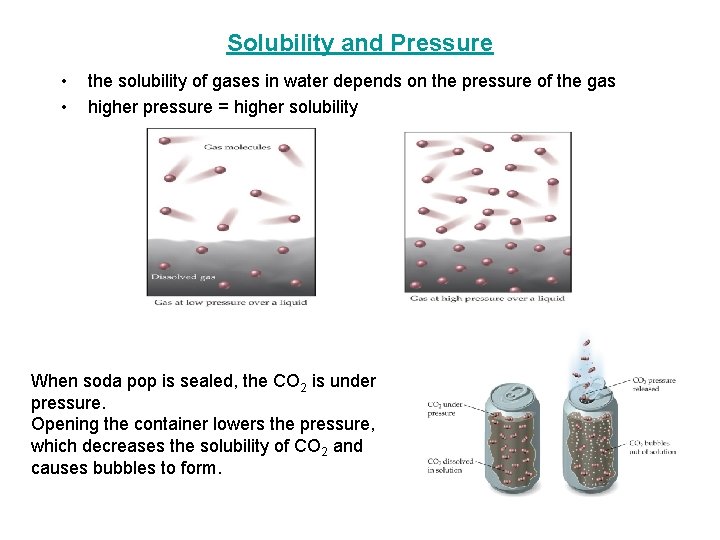
Solubility and Pressure • • the solubility of gases in water depends on the pressure of the gas higher pressure = higher solubility When soda pop is sealed, the CO 2 is under pressure. Opening the container lowers the pressure, which decreases the solubility of CO 2 and causes bubbles to form.

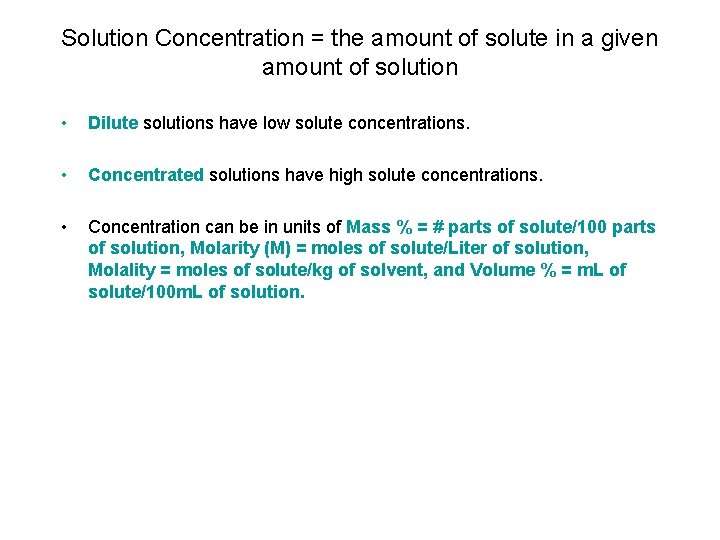
Solution Concentration = the amount of solute in a given amount of solution • Dilute solutions have low solute concentrations. • Concentrated solutions have high solute concentrations. • Concentration can be in units of Mass % = # parts of solute/100 parts of solution, Molarity (M) = moles of solute/Liter of solution, Molality = moles of solute/kg of solvent, and Volume % = m. L of solute/100 m. L of solution.

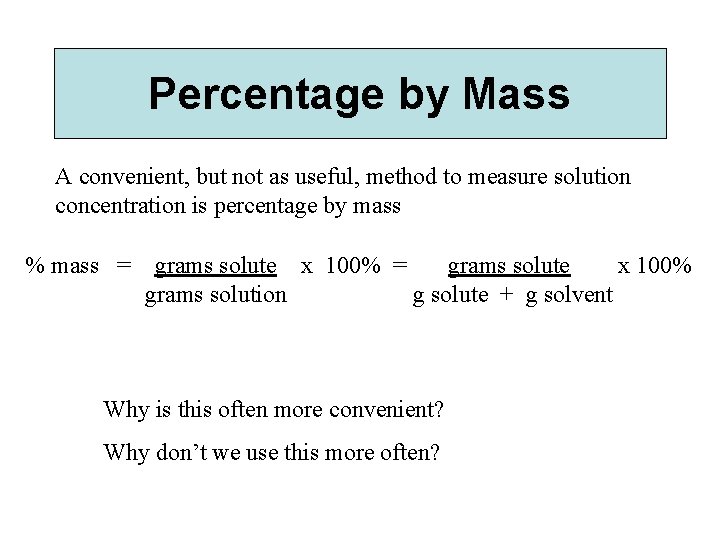
Percentage by Mass A convenient, but not as useful, method to measure solution concentration is percentage by mass % mass = grams solute x 100% grams solution g solute + g solvent Why is this often more convenient? Why don’t we use this more often?

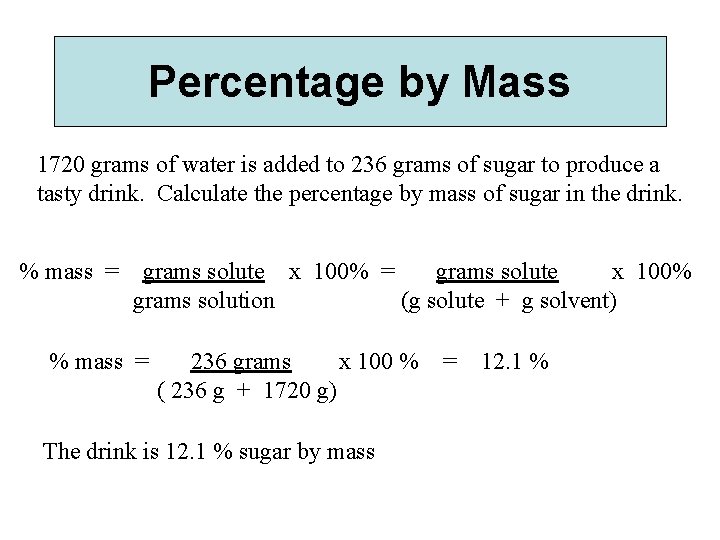
Percentage by Mass 1720 grams of water is added to 236 grams of sugar to produce a tasty drink. Calculate the percentage by mass of sugar in the drink. % mass = grams solute x 100% grams solution (g solute + g solvent) % mass = 236 grams x 100 % ( 236 g + 1720 g) The drink is 12. 1 % sugar by mass = 12. 1 %

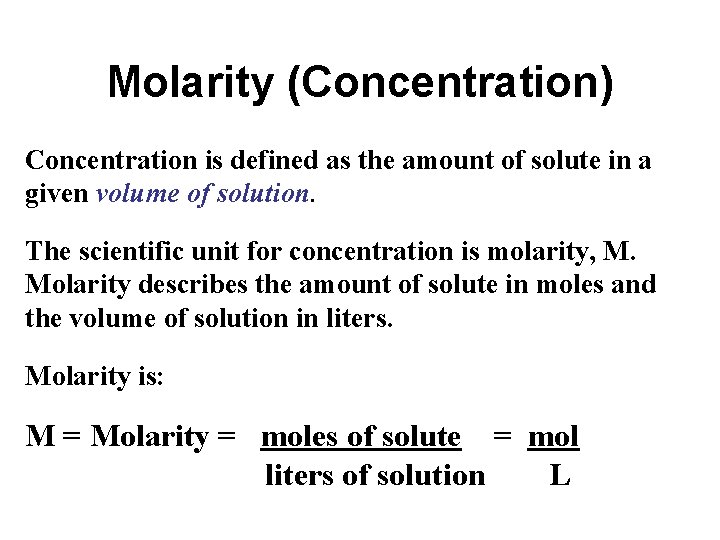
Molarity (Concentration) Concentration is defined as the amount of solute in a given volume of solution. The scientific unit for concentration is molarity, M. Molarity describes the amount of solute in moles and the volume of solution in liters. Molarity is: M = Molarity = moles of solute = mol liters of solution L

Beware!!! Joe adds 30 grams of Na. Cl to 1. 00 L of water to make 1. 03 L of a salt water solution. What is the molarity of the solution? 1. Convert 30 g Na. Cl to moles 30 g Na. Cl x (1 mole Na. Cl) 58. 44 g Na. Cl = 0. 513 moles Na. Cl 2. moles of solute = Molarity = 0. 513 moles Na. Cl = 0. 498 M liters of solution 1. 03 L Note that liters of solution is not necessarily the liters of solvent (water) that is added

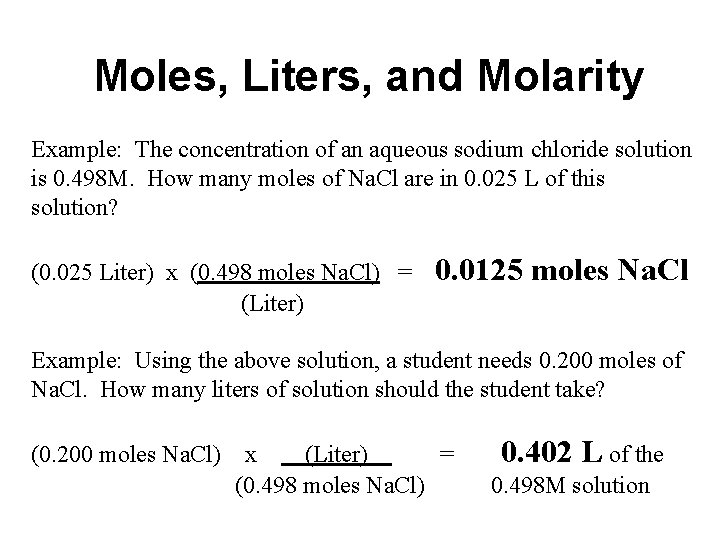
Moles, Liters, and Molarity Example: The concentration of an aqueous sodium chloride solution is 0. 498 M. How many moles of Na. Cl are in 0. 025 L of this solution? (0. 025 Liter) x (0. 498 moles Na. Cl) = (Liter) 0. 0125 moles Na. Cl Example: Using the above solution, a student needs 0. 200 moles of Na. Cl. How many liters of solution should the student take? (0. 200 moles Na. Cl) x (Liter) = (0. 498 moles Na. Cl) 0. 402 L of the 0. 498 M solution

Now Your Turn # Moles Solute # Liters Solution 3. 00 moles 6. 00 L 0. 100 moles 0. 200 L Molarity 0. 050 L 6. 0 M 0. 250 L 0. 100 M 0. 010 moles 0. 500 M 0. 200 moles 0. 050 M

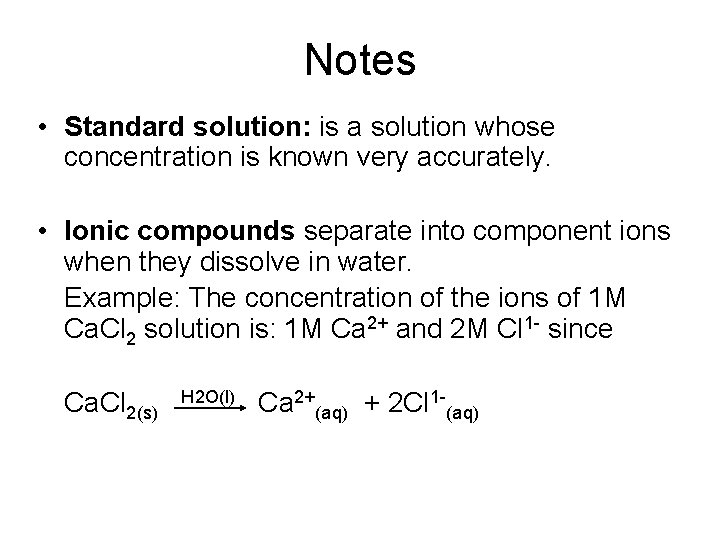
Notes • Standard solution: is a solution whose concentration is known very accurately. • Ionic compounds separate into component ions when they dissolve in water. Example: The concentration of the ions of 1 M Ca. Cl 2 solution is: 1 M Ca 2+ and 2 M Cl 1 - since Ca. Cl 2(s) H 2 O(l) Ca 2+(aq) + 2 Cl 1 -(aq)

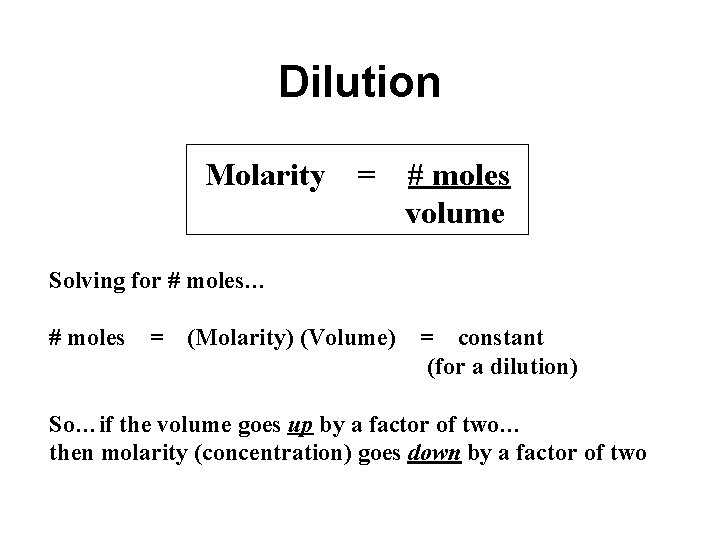
Dilution Molarity = # moles volume Solving for # moles… # moles = (Molarity) (Volume) = constant (for a dilution) So…if the volume goes up by a factor of two… then molarity (concentration) goes down by a factor of two

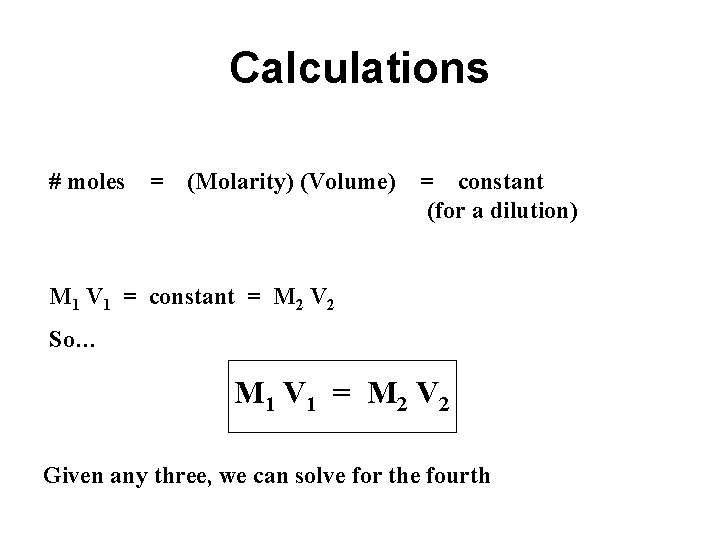
Calculations # moles = (Molarity) (Volume) = constant (for a dilution) M 1 V 1 = constant = M 2 V 2 So… M 1 V 1 = M 2 V 2 Given any three, we can solve for the fourth

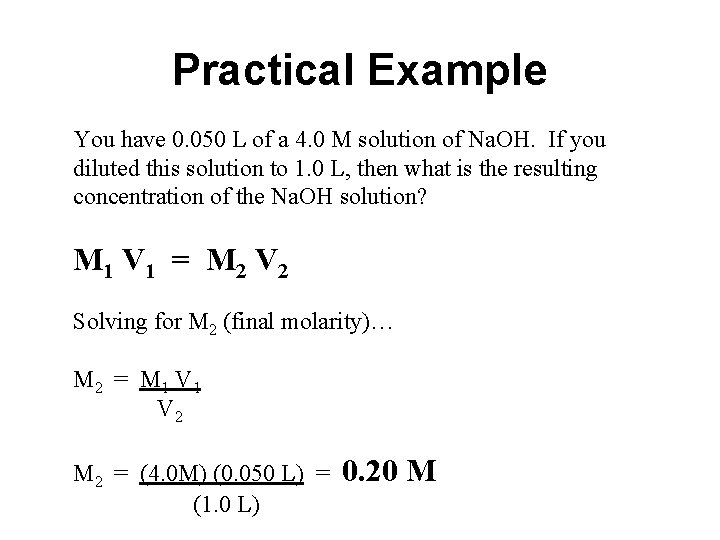
Practical Example You have 0. 050 L of a 4. 0 M solution of Na. OH. If you diluted this solution to 1. 0 L, then what is the resulting concentration of the Na. OH solution? M 1 V 1 = M 2 V 2 Solving for M 2 (final molarity)… M 2 = M 1 V 2 M 2 = (4. 0 M) (0. 050 L) = (1. 0 L) 0. 20 M

Another Example We need to fill a 0. 50 L bottle with 0. 10 M aqueous HCl. In the stockroom, there is a 1. 0 L bottle filled with 6. 0 M HCl. How can we make our desired solution? M 1 V 1 = M 2 V 2 M 1 = 6. 0 M V 1 = ? ? ? M 2 = 0. 10 M V 2 = 0. 500 L V 1 = M 2 V 2 = (0. 10 M) (0. 500 L) = 0. 0083 L = 8. 3 m. L M 1 (6. 0 M) So, 8. 3 m. L of the 6. 0 M HCl is put in the bottle and water is added until the 0. 50 L bottle is filled.

Making a Solution by Dilution M 1 x V 1 = M 2 x V 2 M 1 = 12. 0 M V 1 = ? L M 2 = 1. 50 M V 2 = 5. 00 L dilute 0. 625 L of 12. 0 M solution to 5. 00 L

Now Your Turn How many liters of concentrated H 2 SO 4 (18 M) should be used to prepare 2. 0 L of 1. 0 M H 2 SO 4? (Hint: M 1, M 2, and V 2 are given, solve for V 1) How many liters of concentrated HCl (12 M) should be used to prepare 1. 0 L of 2. 0 M HCl? If 0. 20 L of concentrated H 2 SO 4 (18 M) is diluted with water to 4 L, then what is the concentration (molarity) of the resulting solution?