Solution Chemistry Notes Definition of a Solution q





- Slides: 24

Solution Chemistry Notes

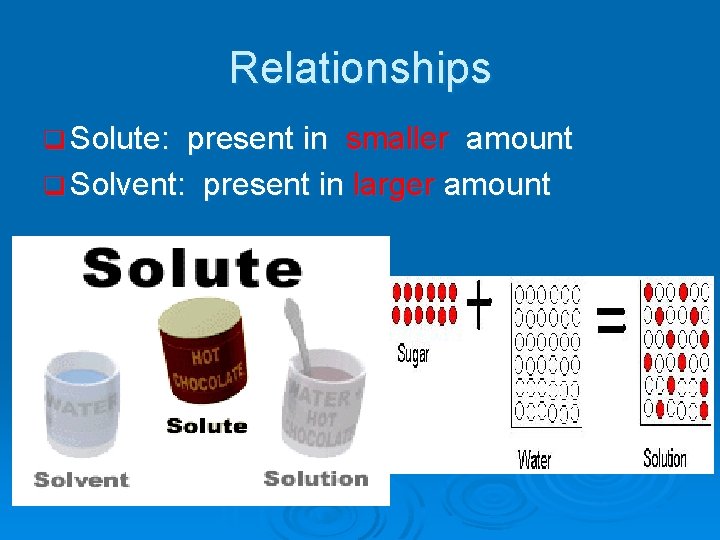
Definition of a “Solution” q A solution is a homogeneous (uniform) mixture of a solute and a solvent. q Everyday solutions: CO 2, sugar dissolved in water Salt dissolved in water

Relationships q Solute: present in smaller amount q Solvent: present in larger amount

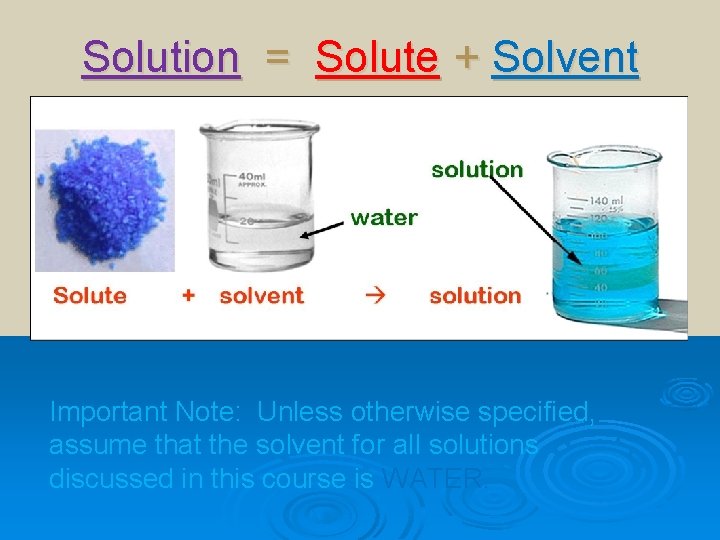
Solution = Solute + Solvent Important Note: Unless otherwise specified, assume that the solvent for all solutions discussed in this course is WATER.

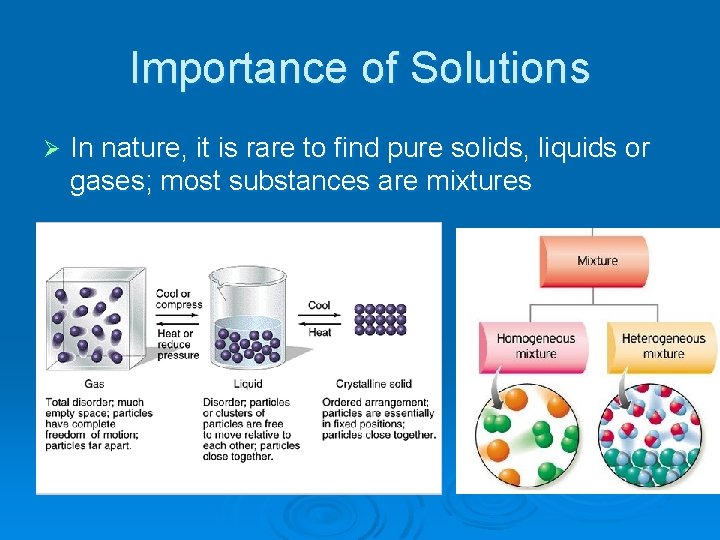
Importance of Solutions Ø In nature, it is rare to find pure solids, liquids or gases; most substances are mixtures

Using Solutions to Speed Up Chemical Reactions Ø Most elements and most compounds of interest are solids at RT; however the rxn rate between solids is very slow due to low surface area which limits contacts between reacting particles Ø In the lab, most chemical reactions are carried out by dissolving solids in liquid solvents to increase the # of collisions and hence the rxn rates

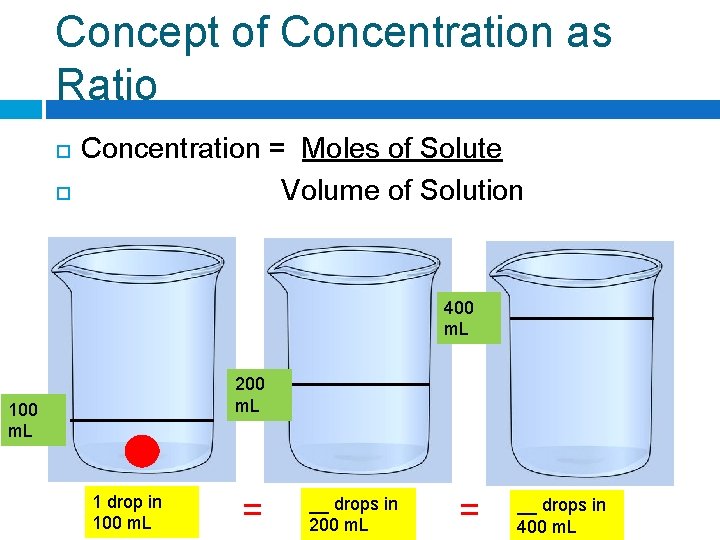
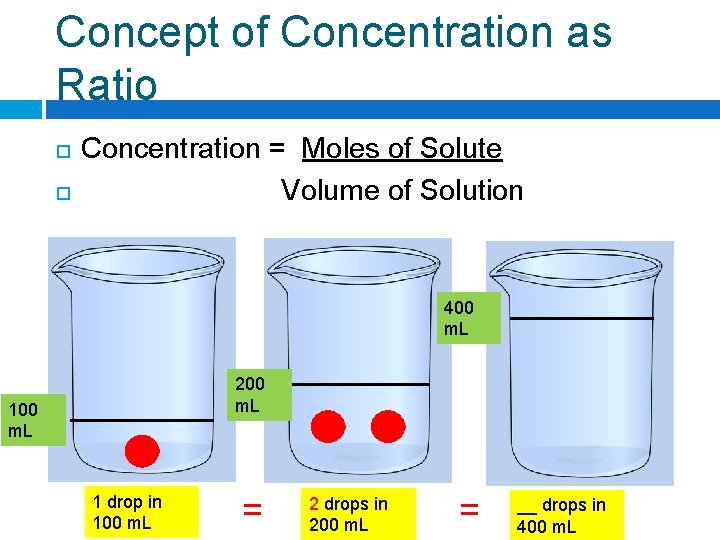
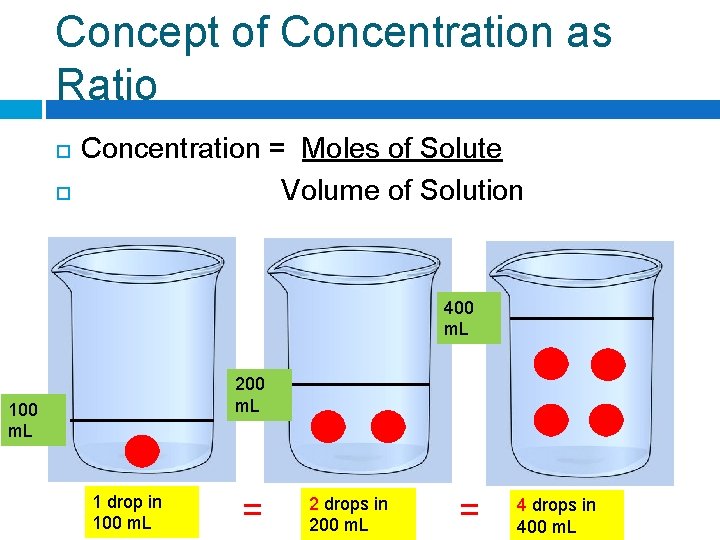
Concept of Concentration as Ratio Concentration = Moles of Solute Volume of Solution 400 m. L 200 m. L 1 drop in 100 m. L = __ drops in 200 m. L = __ drops in 400 m. L

Concept of Concentration as Ratio Concentration = Moles of Solute Volume of Solution 400 m. L 200 m. L 1 drop in 100 m. L = 2 drops in 200 m. L = __ drops in 400 m. L

Concept of Concentration as Ratio Concentration = Moles of Solute Volume of Solution 400 m. L 200 m. L 1 drop in 100 m. L = 2 drops in 200 m. L = 4 drops in 400 m. L

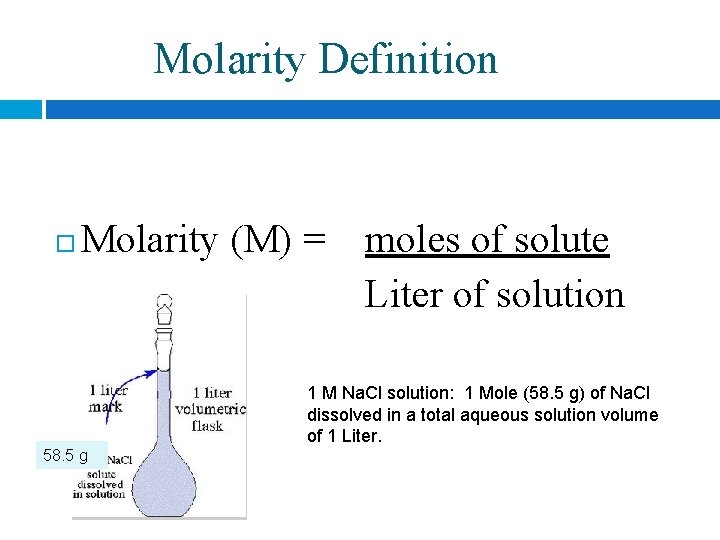
Molarity Definition Molarity (M) = moles of solute Liter of solution 1 M Na. Cl solution: 1 Mole (58. 5 g) of Na. Cl dissolved in a total aqueous solution volume of 1 Liter. 58. 5 g

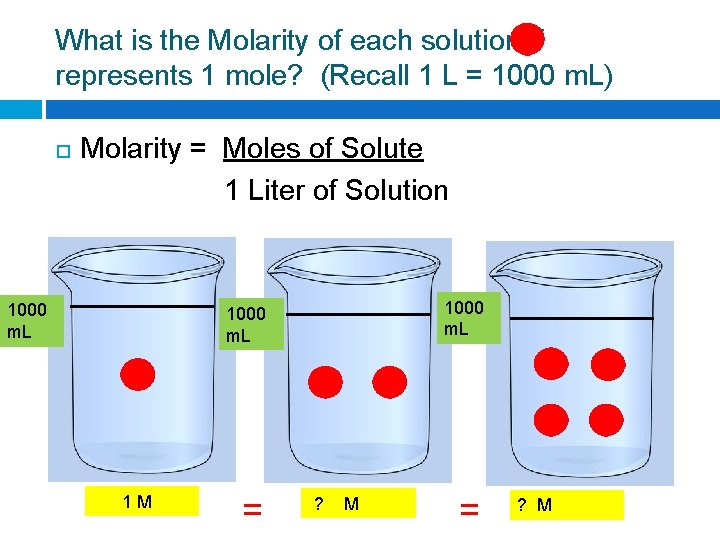
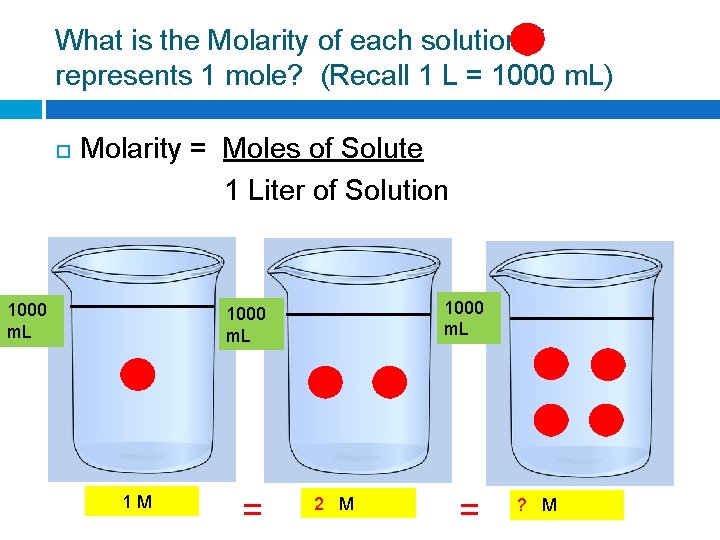
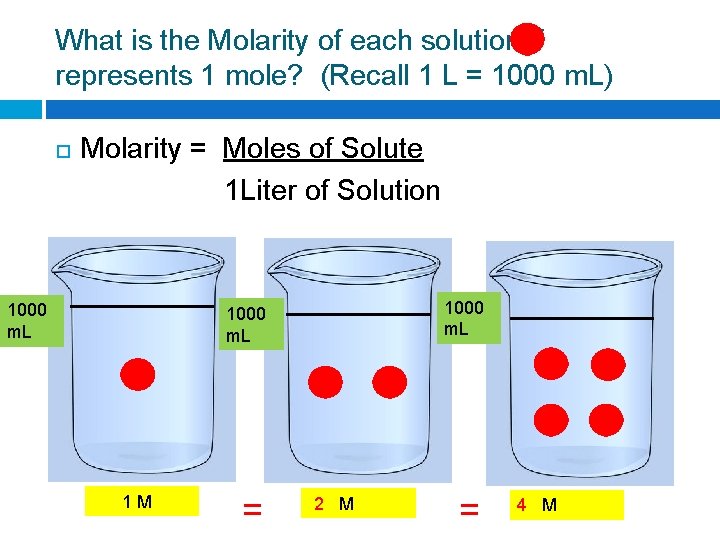
What is the Molarity of each solution if represents 1 mole? (Recall 1 L = 1000 m. L) Molarity = Moles of Solute 1 Liter of Solution 1000 m. L 1 M = ? M

What is the Molarity of each solution if represents 1 mole? (Recall 1 L = 1000 m. L) Molarity = Moles of Solute 1 Liter of Solution 1000 m. L 1 M = 2 M = ? M

What is the Molarity of each solution if represents 1 mole? (Recall 1 L = 1000 m. L) Molarity = Moles of Solute 1 Liter of Solution 1000 m. L 1 M = 2 M = 4 M

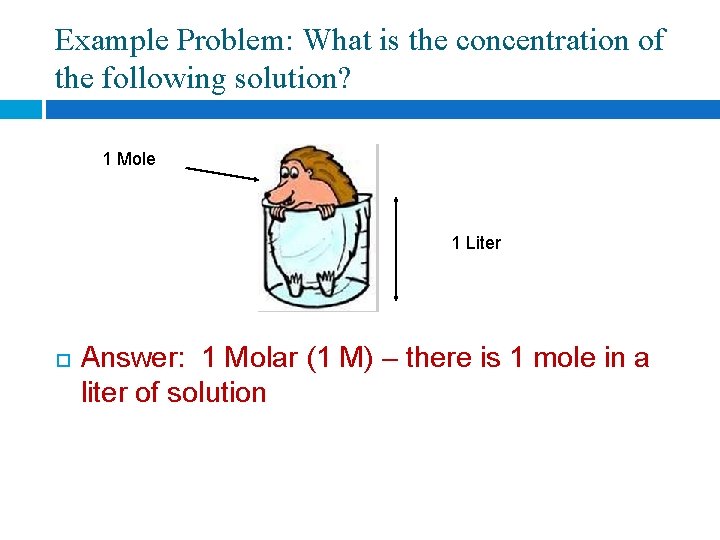
Example Problem: What is the concentration of the following solution? 1 Mole 1 Liter Answer: 1 Molar (1 M) – there is 1 mole in a liter of solution

Although the definition of Molarity is the moles of solute in 1 liter of solution, as long as the ratio of moles to volume is unchanged any volume of solution at the same concentration can be prepared.

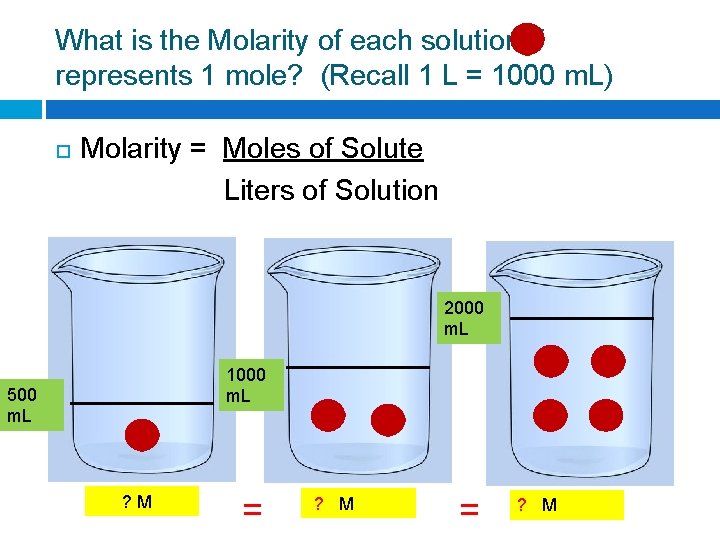
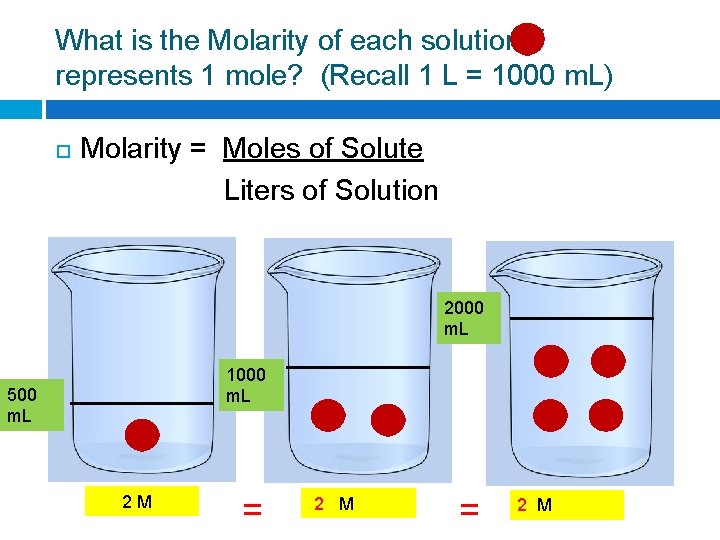
What is the Molarity of each solution if represents 1 mole? (Recall 1 L = 1000 m. L) Molarity = Moles of Solute Liters of Solution 2000 m. L 1000 m. L 500 m. L ? M = ? M

What is the Molarity of each solution if represents 1 mole? (Recall 1 L = 1000 m. L) Molarity = Moles of Solute Liters of Solution 2000 m. L 1000 m. L 500 m. L 2 M = 2 M

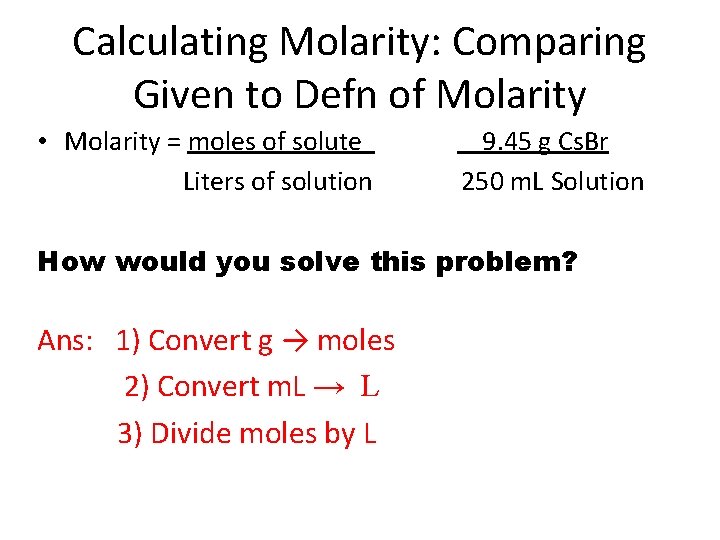
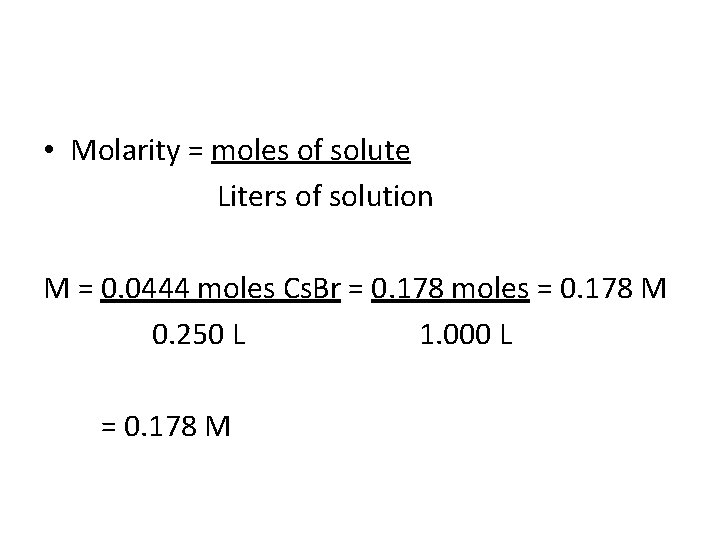
Example: Calculating Molarity If 9. 45 g of Cs. Br is dissolved in enough water to give 250. m. L of solution, what is the molarity of the solution? Molarity = moles of solute Liters of solution What is the solute, solvent and solution in this problem? Ans: solute = Cs. Br, solvent = water, solution = Cs. Br dissolved in water

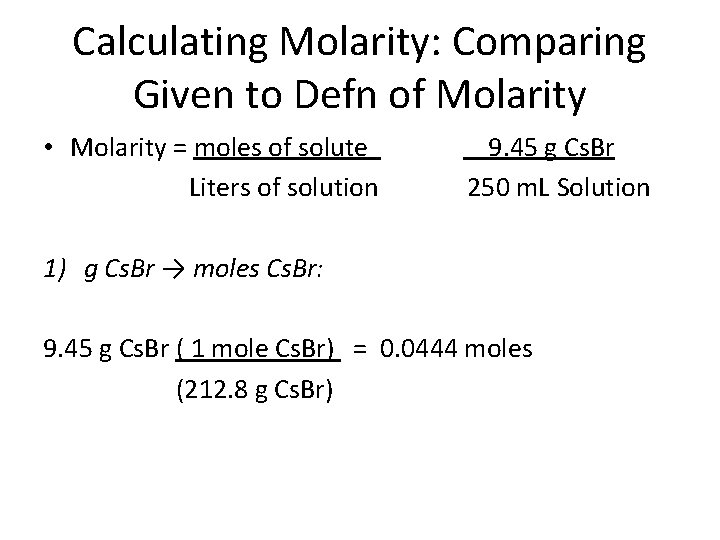
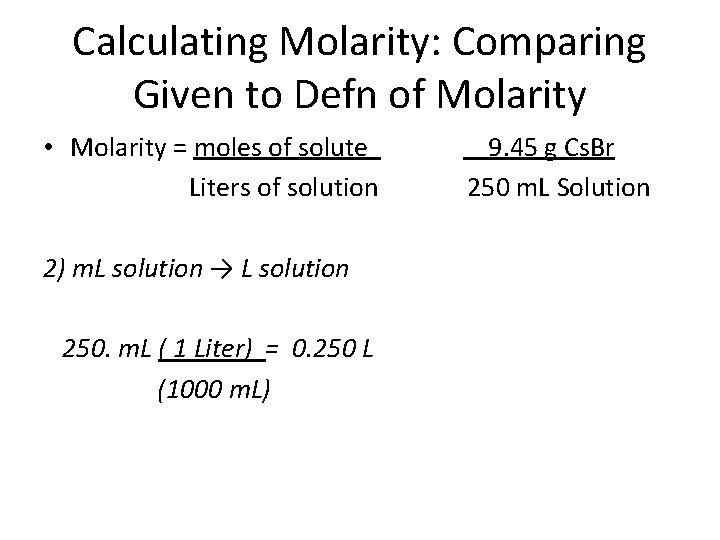
Calculating Molarity: Comparing Given to Defn of Molarity • Molarity = moles of solute Liters of solution 9. 45 g Cs. Br 250 m. L Solution How would you solve this problem? Ans: 1) Convert g → moles 2) Convert m. L → L 3) Divide moles by L

Calculating Molarity: Comparing Given to Defn of Molarity • Molarity = moles of solute Liters of solution 9. 45 g Cs. Br 250 m. L Solution 1) g Cs. Br → moles Cs. Br: 9. 45 g Cs. Br ( 1 mole Cs. Br) = 0. 0444 moles (212. 8 g Cs. Br)

Calculating Molarity: Comparing Given to Defn of Molarity • Molarity = moles of solute Liters of solution 2) m. L solution → L solution 250. m. L ( 1 Liter) = 0. 250 L (1000 m. L) 9. 45 g Cs. Br 250 m. L Solution

• Molarity = moles of solute Liters of solution M = 0. 0444 moles Cs. Br = 0. 178 moles = 0. 178 M 0. 250 L 1. 000 L = 0. 178 M

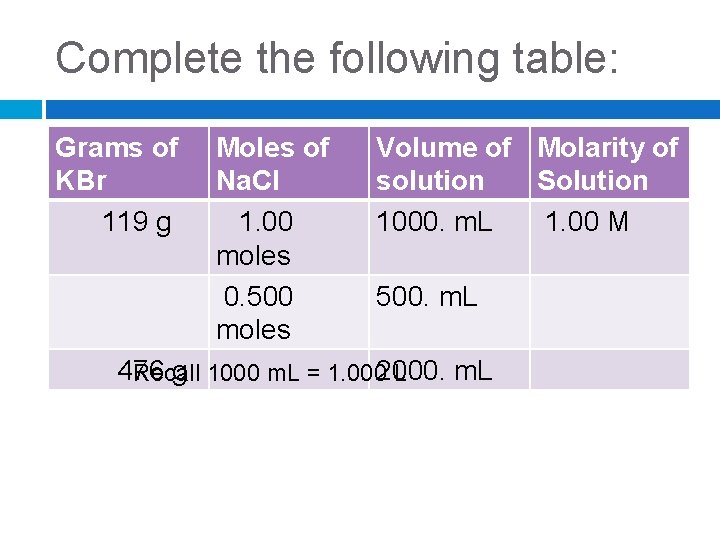
Complete the following table: Grams of KBr 119 g Moles of Na. Cl 1. 00 moles 0. 500 moles Volume of Molarity of solution Solution 1000. m. L 1. 00 M 500. m. L 476 g 1000 m. L = 1. 0002000. m. L Recall L

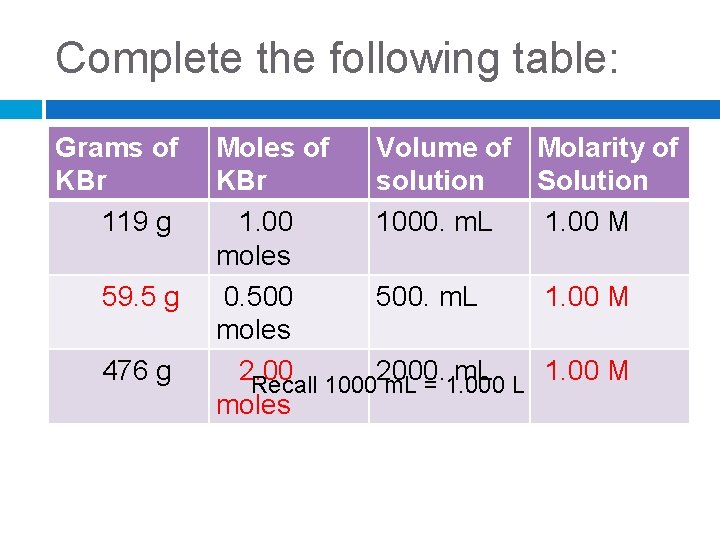
Complete the following table: Grams of KBr 119 g 59. 5 g 476 g Moles of Volume of KBr solution 1. 00 1000. m. L moles 0. 500. m. L moles 2. 00 2000. m. L Recall 1000 m. L = 1. 000 L moles Molarity of Solution 1. 00 M