Real Solutions Lecture 7 How does free energy












- Slides: 24

Real Solutions Lecture 7

How does free energy change in an ideal solution at various temperatures? Recall: The change due to solution is simply the second term on the right:

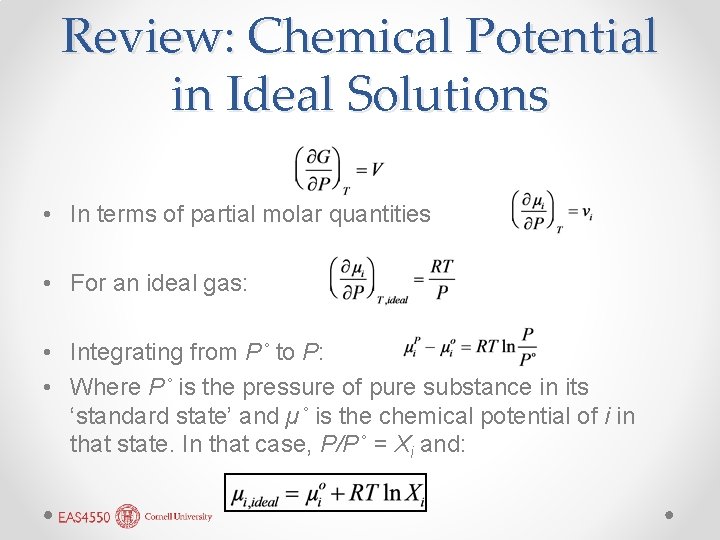
Review: Chemical Potential in Ideal Solutions • In terms of partial molar quantities • For an ideal gas: • Integrating from P˚ to P: • Where P˚ is the pressure of pure substance in its ‘standard state’ and µ˚ is the chemical potential of i in that state. In that case, P/P˚ = Xi and:

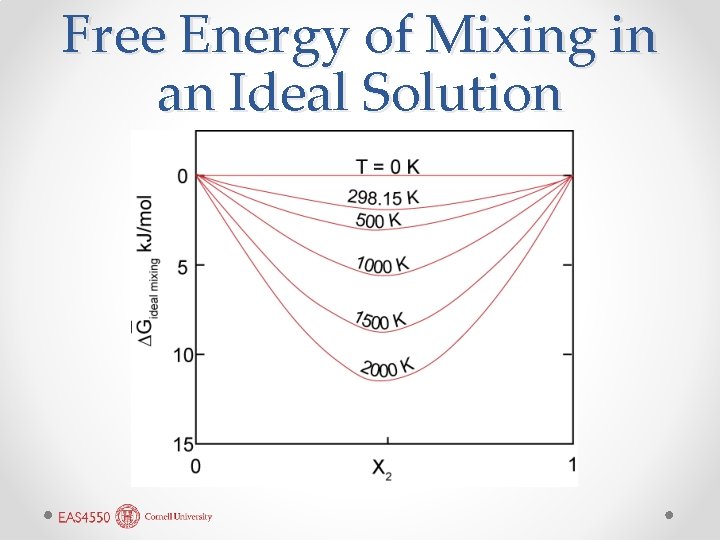
Free Energy of Mixing in an Ideal Solution

Mixing and Mixture • We are calling the reduction in free energy due only to dissolving one component in another ∆Gideal mixing. • Once again, mixtures are not solutions. • In our equation: • The first term on the right is the free energy of the mixture: it is simply the sum of the molar free energies (or equivalently the chemical potentials) of the pure components weighted by their mole fractions. • Again, the second term arises from the increase in entropy that occurs when we create a true solution.

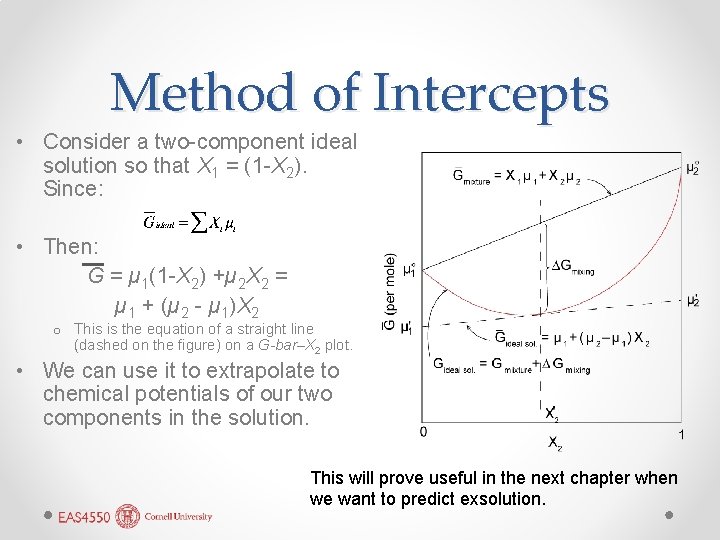
Method of Intercepts • Consider a two-component ideal solution so that X 1 = (1 -X 2). Since: • Then: G = µ 1(1 -X 2) +µ 2 X 2 = µ 1 + (µ 2 - µ 1)X 2 o This is the equation of a straight line (dashed on the figure) on a G-bar–X 2 plot. • We can use it to extrapolate to chemical potentials of our two components in the solution. This will prove useful in the next chapter when we want to predict exsolution.

Henry’s Law • In dilute solutions, most substances exhibit Henry’s Law behavior as their mole fractions approach 0 (Xi � 0). • Henry’s Law is: P i = h i. X i o where hi is Henry’s Law ‘constant’. It can be (usually is) a function of T and P and the nature of the solution, but is independent of the concentration of i. • In a way analogous to our derivation of chemical potential for ideal solutions, we can express Henry’s Law as: µi =µi˚ + RT ln hi. Xi

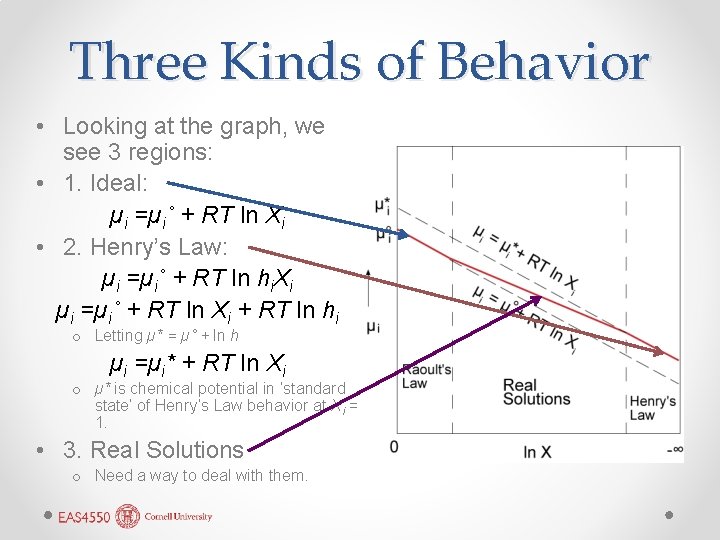
Three Kinds of Behavior • Looking at the graph, we see 3 regions: • 1. Ideal: µi =µi˚ + RT ln Xi • 2. Henry’s Law: µi =µi˚ + RT ln hi. Xi µi =µi˚ + RT ln Xi + RT ln hi o Letting µ* = µ˚ + ln h µi =µi* + RT ln Xi o µ* is chemical potential in ‘standard state’ of Henry’s Law behavior at Xi = 1. • 3. Real Solutions o Need a way to deal with them.

We want to find a way to deal with real solutions within the framework we have already constructed for ideal ones.

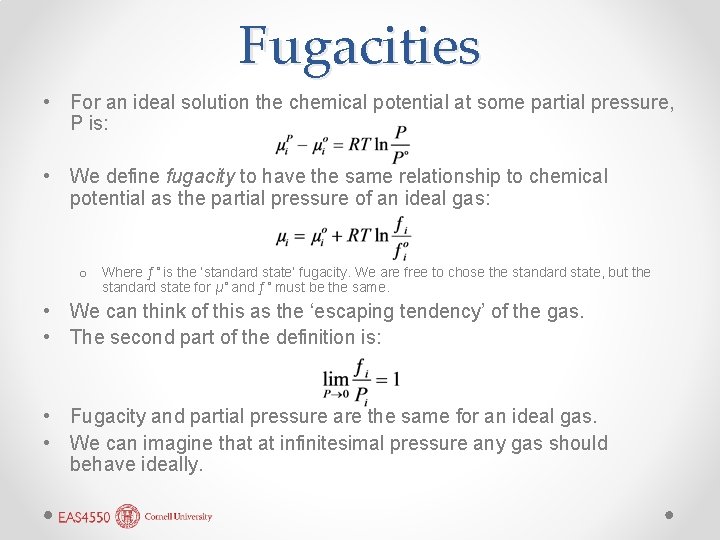
Fugacities • For an ideal solution the chemical potential at some partial pressure, P is: • We define fugacity to have the same relationship to chemical potential as the partial pressure of an ideal gas: o Where ƒ˚ is the ‘standard state’ fugacity. We are free to chose the standard state, but the standard state for µ˚ and ƒ˚ must be the same. • We can think of this as the ‘escaping tendency’ of the gas. • The second part of the definition is: • Fugacity and partial pressure are the same for an ideal gas. • We can imagine that at infinitesimal pressure any gas should behave ideally.

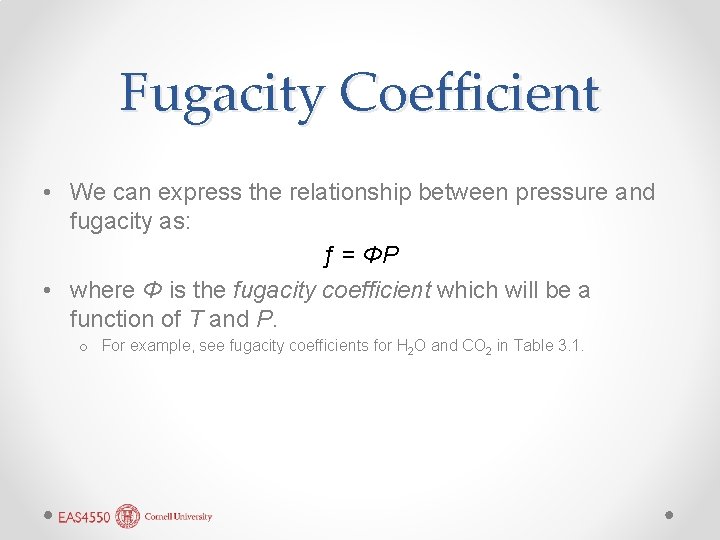
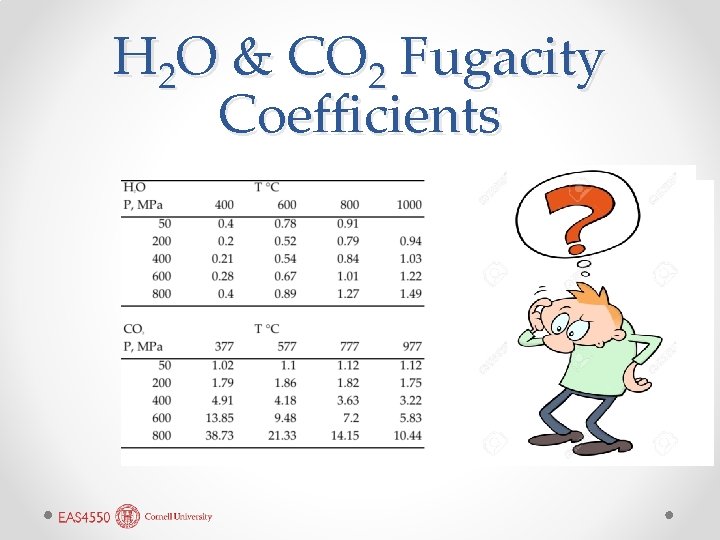
Fugacity Coefficient • We can express the relationship between pressure and fugacity as: ƒ = ΦP • where Φ is the fugacity coefficient which will be a function of T and P. o For example, see fugacity coefficients for H 2 O and CO 2 in Table 3. 1.

H 2 O & CO 2 Fugacity Coefficients

Activities • Fugacities are useful for gases such as H 2 O and CO 2, but we can extent the concept to calculate chemical potentials in real liquid and solid solutions. • Recalling: • We define the activity as: • Hence o Same equation as for an ideal solution, except that ai replaces Xi. • We have retained our ideal solution formulation and stuffed all non-ideal behavior into the activity. • Activity can be thought of as the effective concentration.

Activity Coefficients • We’ll express the relationship between activity and mole fraction as: a i = λ i. X i • The activity coefficient is a function of temperature, pressure, and composition (including Xi). o Now we have stuffed that non-ideal behavior into the activity coefficient! • For an ideal solution, ai = Xi and λi = 1.

Rational and Practical Activity Coefficients • The rational activity coefficient, λ, relates activity to mole fraction. • Although mole fraction is the natural thermodynamic concentration unit, other units, such as moles (of a solute) per kilogram (molality) or liter (molarity) or solution are more commonly used (because they are easily measured). • In those units, we use the practical activity coefficient, γ.

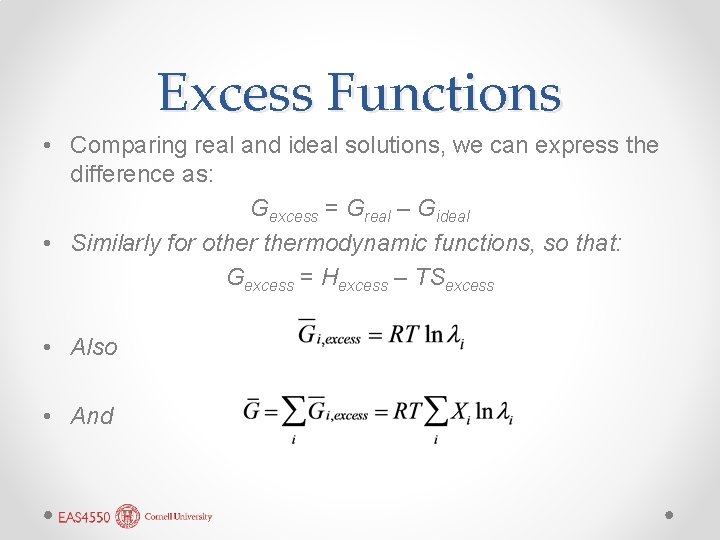
Excess Functions • Comparing real and ideal solutions, we can express the difference as: Gexcess = Greal – Gideal • Similarly for othermodynamic functions, so that: Gexcess = Hexcess – TSexcess • Also • And

Water and Electrolyte Solutions

Electrolyte solutions are solutions in which the solute dissociates to form ions, which facilitate electric conduction. Seawater and all natural waters are electrolyte solutions. These solutions are of enormous importance in many geologic processes. Solvent is the substance present in greatest abundance. Solute refers to the remaining substances present in solution.

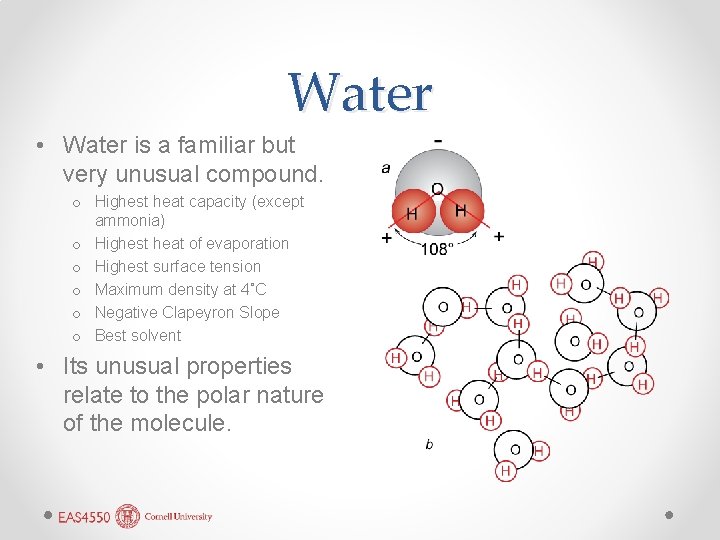
Water • Water is a familiar but very unusual compound. o Highest heat capacity (except ammonia) o Highest heat of evaporation o Highest surface tension o Maximum density at 4˚C o Negative Clapeyron Slope o Best solvent • Its unusual properties relate to the polar nature of the molecule.

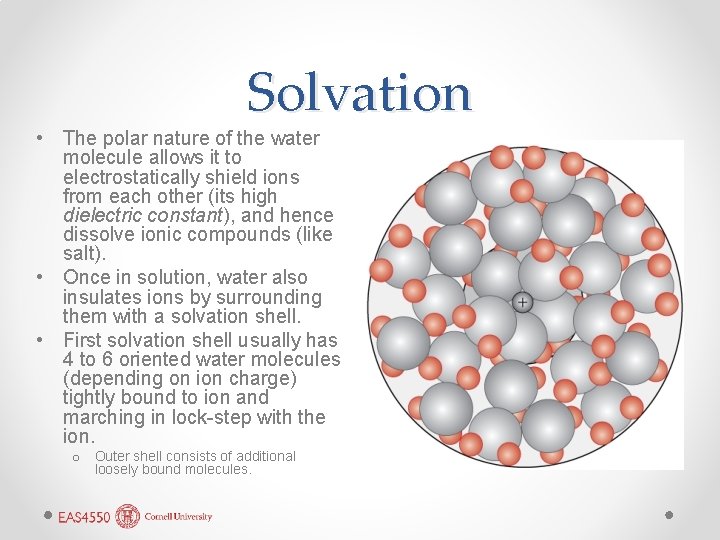
Solvation • The polar nature of the water molecule allows it to electrostatically shield ions from each other (its high dielectric constant), and hence dissolve ionic compounds (like salt). • Once in solution, water also insulates ions by surrounding them with a solvation shell. • First solvation shell usually has 4 to 6 oriented water molecules (depending on ion charge) tightly bound to ion and marching in lock-step with the ion. o Outer shell consists of additional loosely bound molecules.

Solvation Effects • Enhances solubility • Electrostriction: water molecules in solvation shell more tightly packed, reducing volume of the solution. • Causes partial collapse of the H-bonded structure of water. • Non-ideal behavior

Aside: Back to Rum ‘n Coke • Some covalently bonded substances like sugars and ethanol will readily dissolve in water because, like water, they contain polar hydrogen-oxygen bonds. These substances are called hydrophilic. • Other covalently bonded substances, such as hydrocarbons and lipids (fats, oils) do not dissolve in water because their bonds are not polar. These are known as hydrophobic substances.

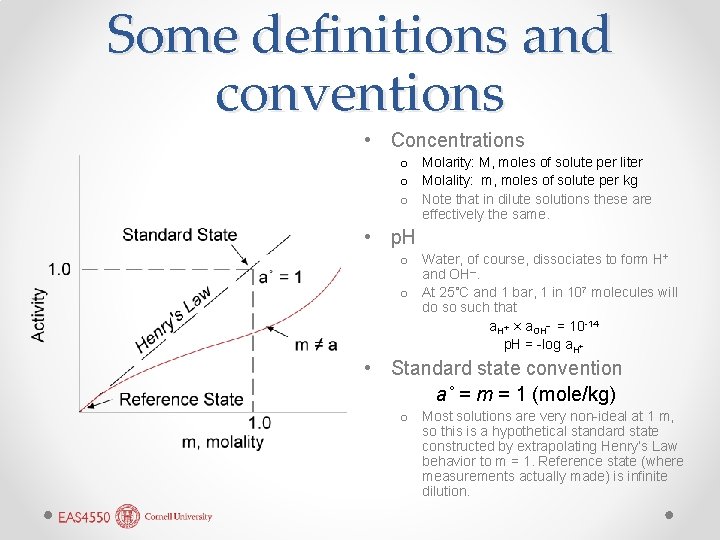
Some definitions and conventions • Concentrations o Molarity: M, moles of solute per liter o Molality: m, moles of solute per kg o Note that in dilute solutions these are effectively the same. • p. H o Water, of course, dissociates to form H+ and OH–. o At 25˚C and 1 bar, 1 in 107 molecules will do so such that a. H+ × a. OH– = 10 -14 p. H = -log a. H+ • Standard state convention a˚ = m = 1 (mole/kg) o Most solutions are very non-ideal at 1 m, so this is a hypothetical standard state constructed by extrapolating Henry’s Law behavior to m = 1. Reference state (where measurements actually made) is infinite dilution.

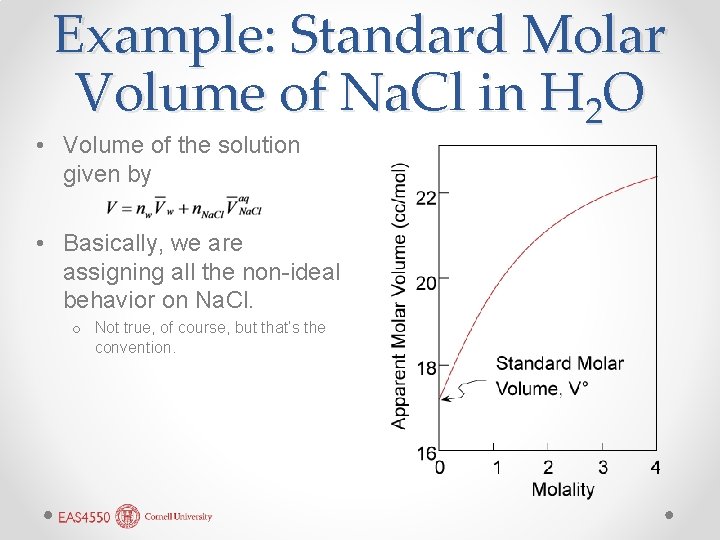
Example: Standard Molar Volume of Na. Cl in H 2 O • Volume of the solution given by • Basically, we are assigning all the non-ideal behavior on Na. Cl. o Not true, of course, but that’s the convention.