Solution Chemistry Solution Chemistry Chemical reactions that occur









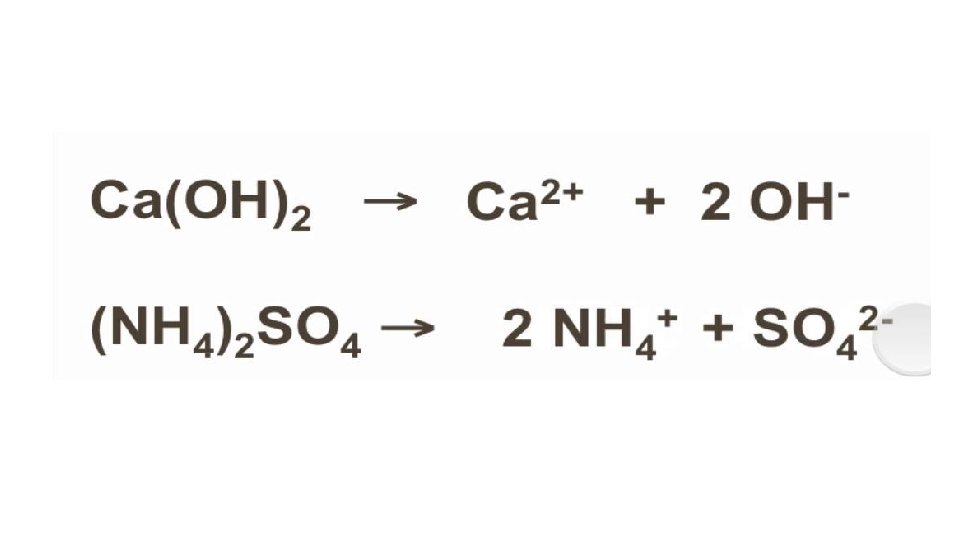
- Slides: 32

Solution Chemistry

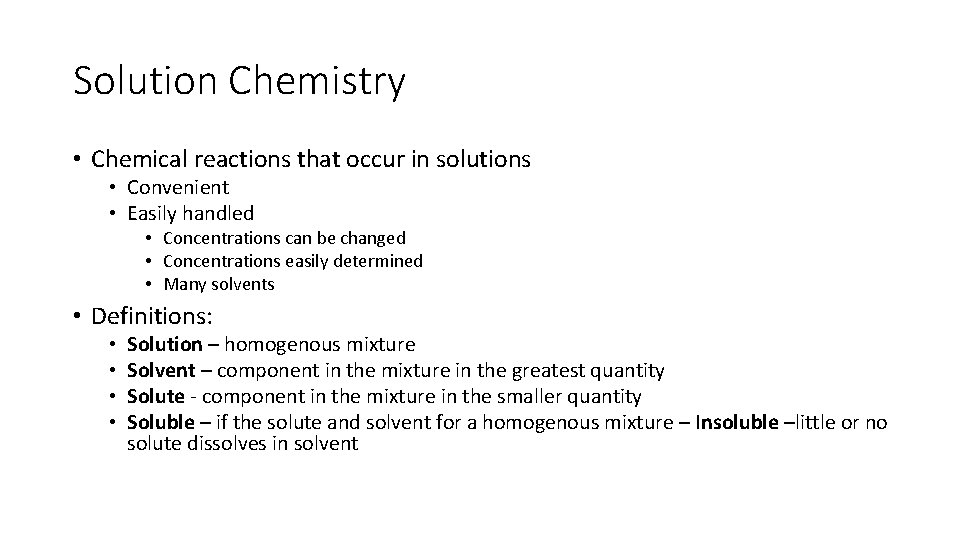
Solution Chemistry • Chemical reactions that occur in solutions • Convenient • Easily handled • Concentrations can be changed • Concentrations easily determined • Many solvents • Definitions: • • Solution – homogenous mixture Solvent – component in the mixture in the greatest quantity Solute - component in the mixture in the smaller quantity Soluble – if the solute and solvent for a homogenous mixture – Insoluble –little or no solute dissolves in solvent

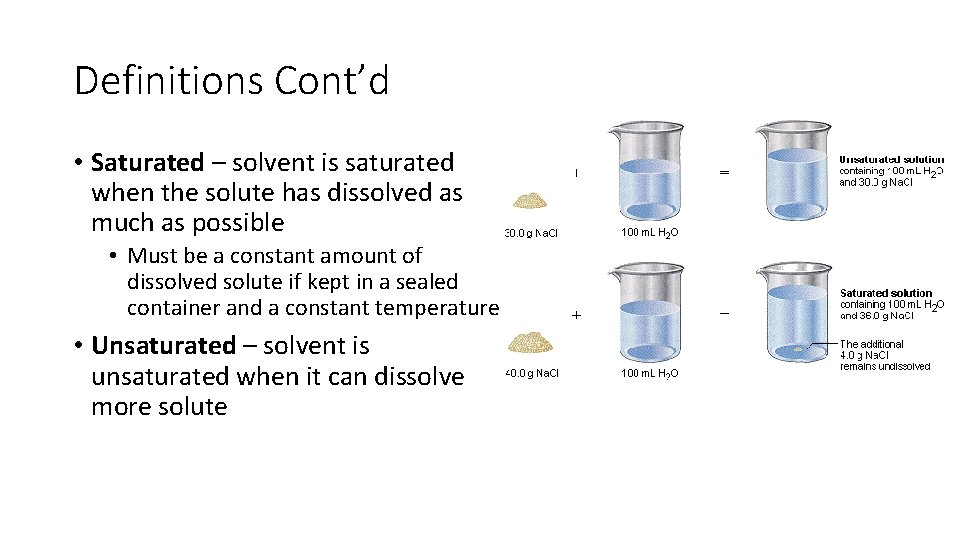
Definitions Cont’d • Saturated – solvent is saturated when the solute has dissolved as much as possible • Must be a constant amount of dissolved solute if kept in a sealed container and a constant temperature • Unsaturated – solvent is unsaturated when it can dissolve more solute

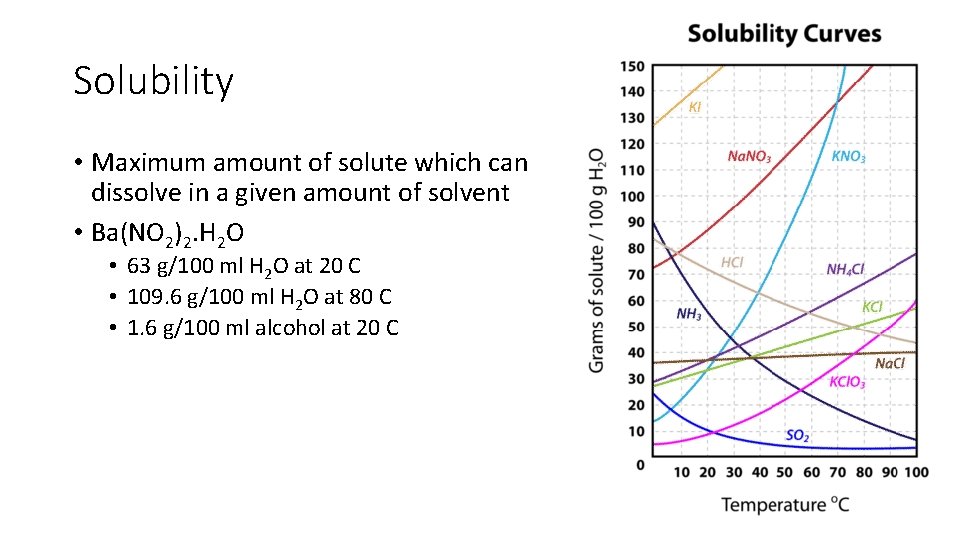
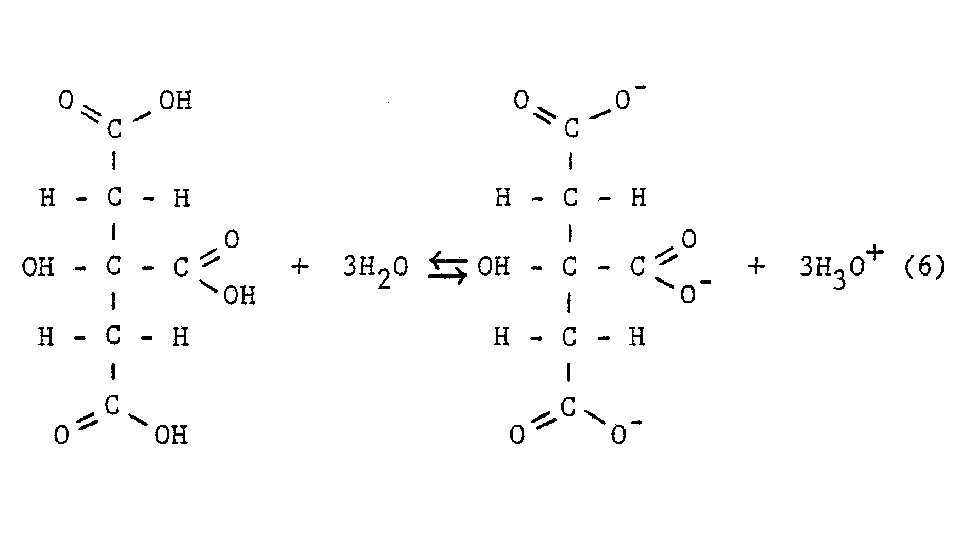
Solubility • Maximum amount of solute which can dissolve in a given amount of solvent • Ba(NO 2)2. H 2 O • 63 g/100 ml H 2 O at 20 C • 109. 6 g/100 ml H 2 O at 80 C • 1. 6 g/100 ml alcohol at 20 C

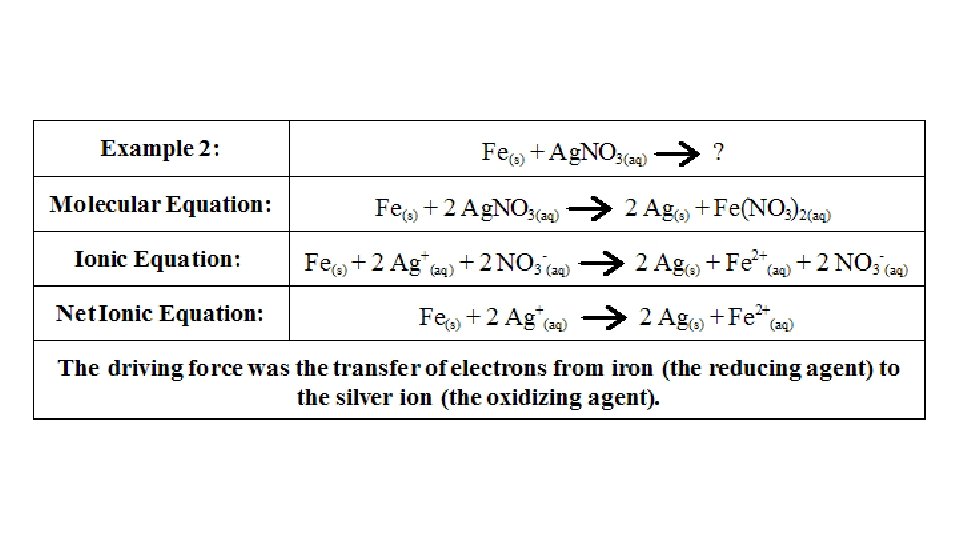
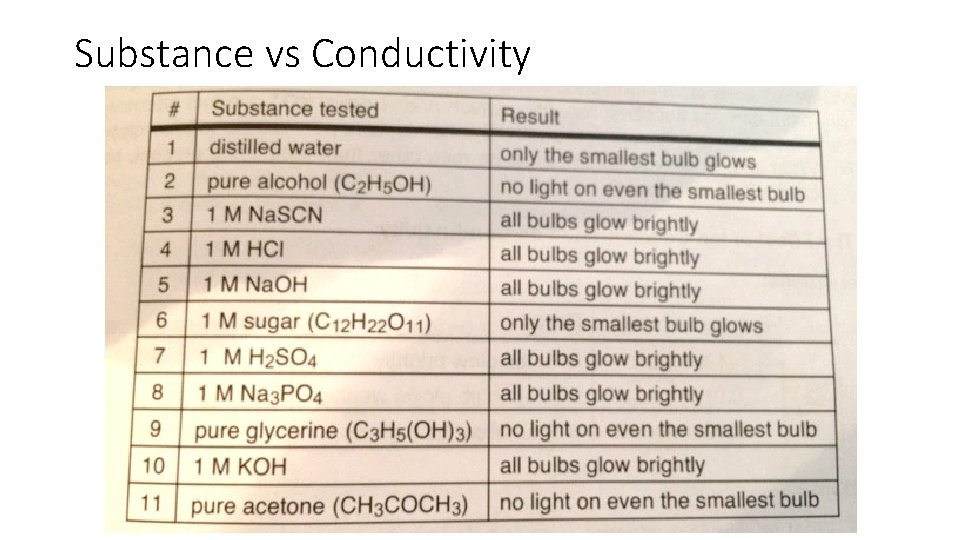
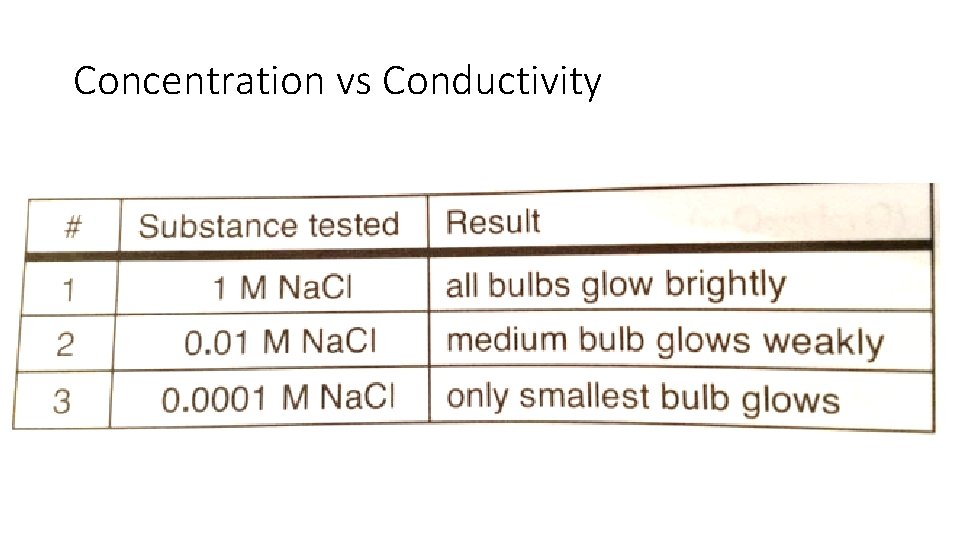
Conductivity of Aqueous Solutions • Ions – have electric charge • Ionic solution conduct electricity • Generally: • Conducting solutions contain ions, the greater the concentration the more conductivity • Nonmetal and nonmetal solution will not form an ionic solution. Little to no conductivity • Carbon compounds do not form ionic solutions in water. Except organic acids


Substance vs Conductivity

State vs Conductivity

Concentration vs Conductivity


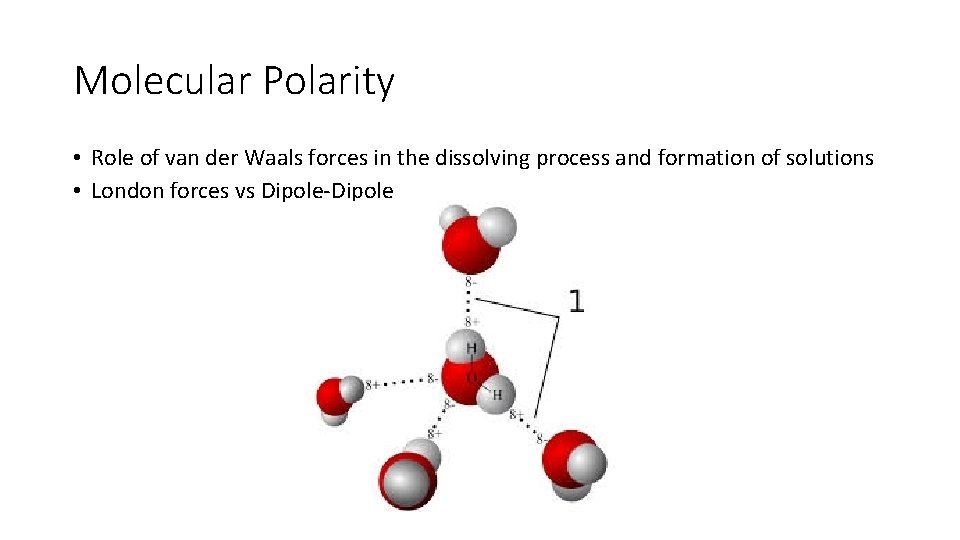
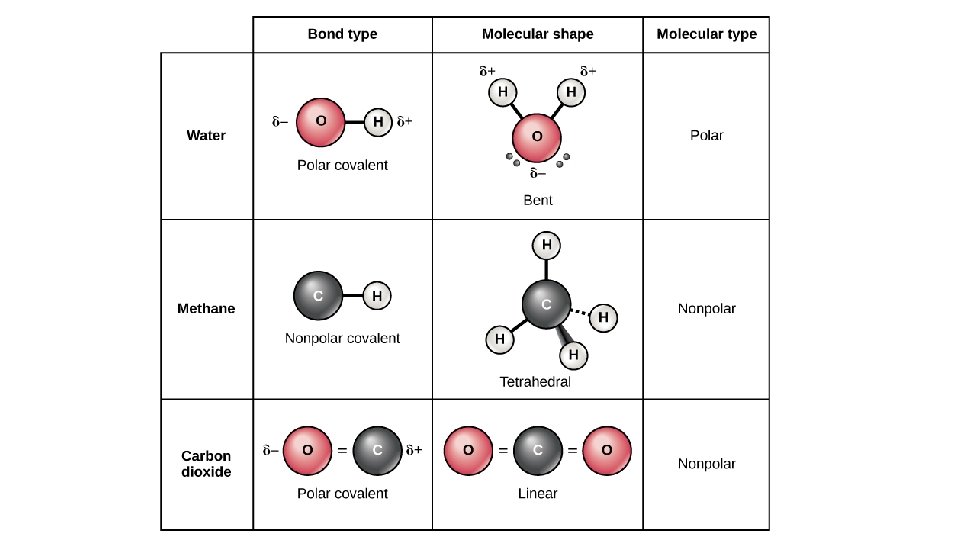
Molecular Polarity • Role of van der Waals forces in the dissolving process and formation of solutions • London forces vs Dipole-Dipole

London Forces • Weak attractive forces as a result of temporary attractions between neighboring molecules • If permanent dipole is absent then only van der Waals present

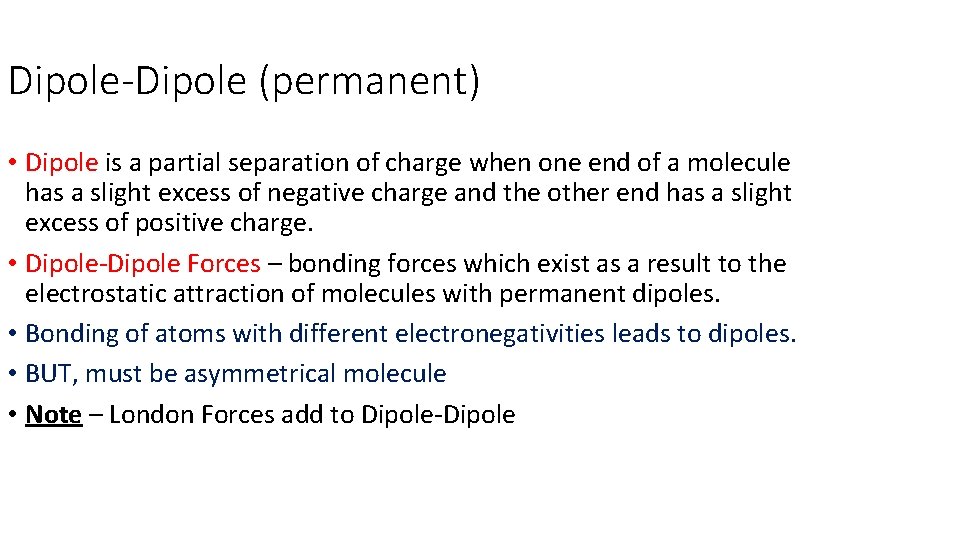
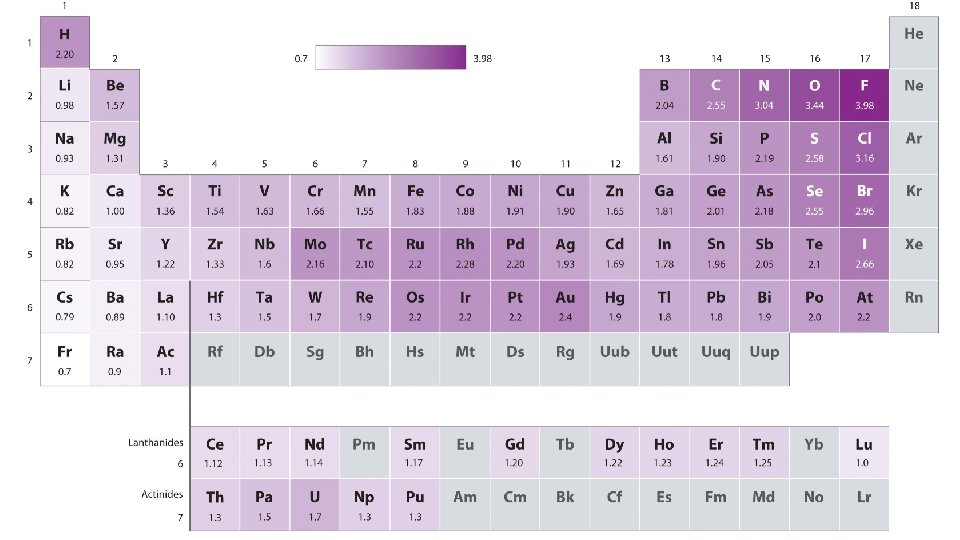
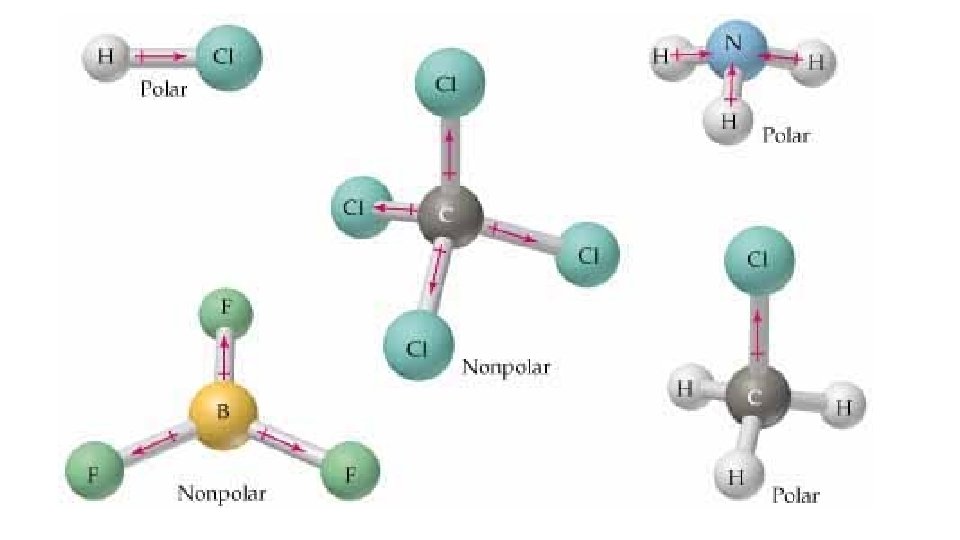
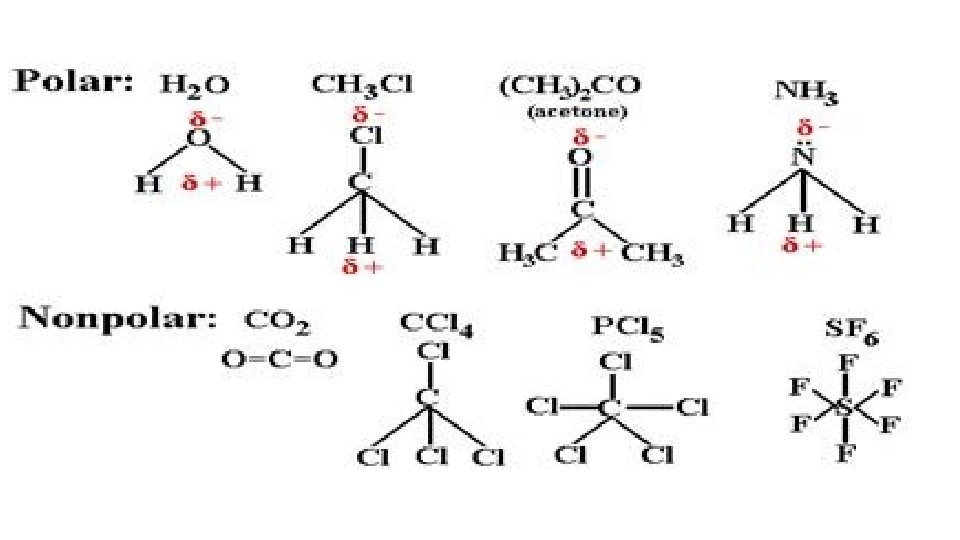
Dipole-Dipole (permanent) • Dipole is a partial separation of charge when one end of a molecule has a slight excess of negative charge and the other end has a slight excess of positive charge. • Dipole-Dipole Forces – bonding forces which exist as a result to the electrostatic attraction of molecules with permanent dipoles. • Bonding of atoms with different electronegativities leads to dipoles. • BUT, must be asymmetrical molecule • Note – London Forces add to Dipole-Dipole





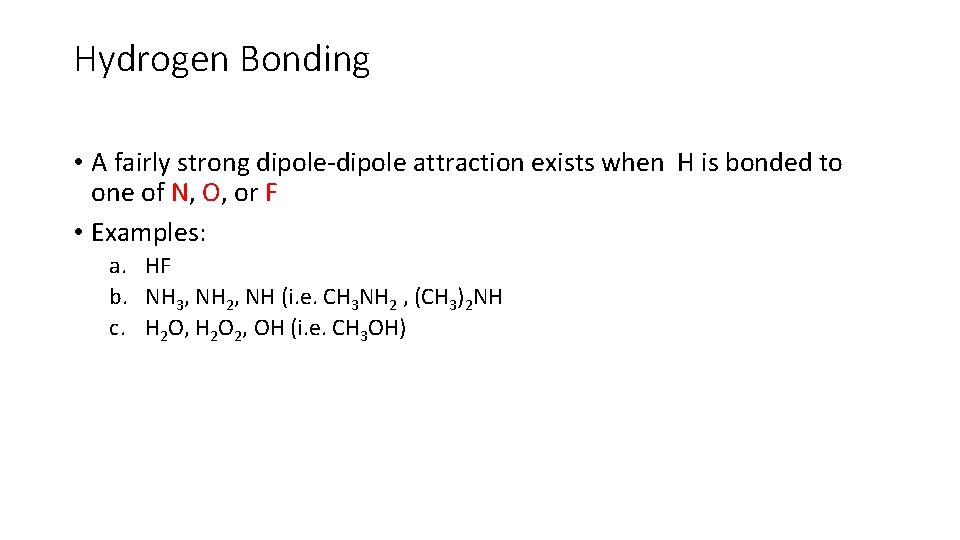
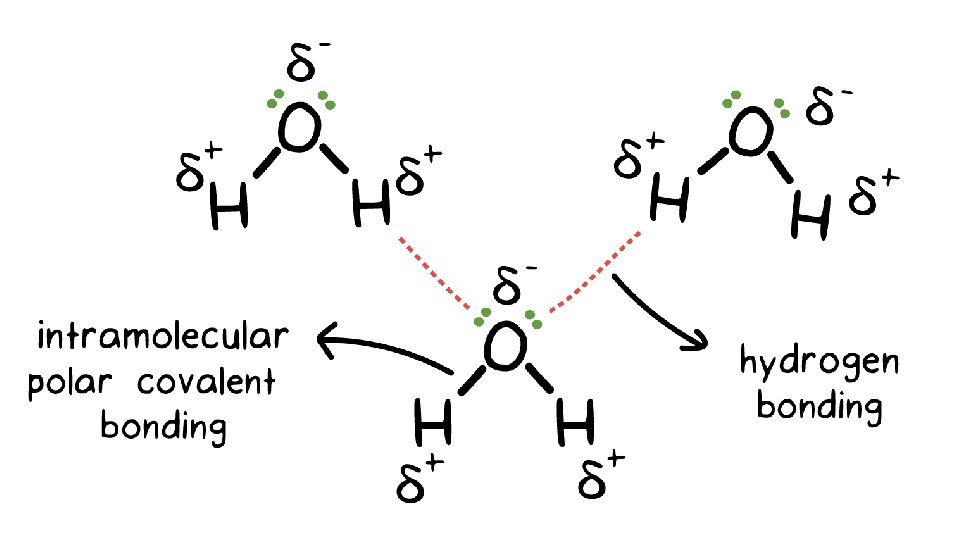
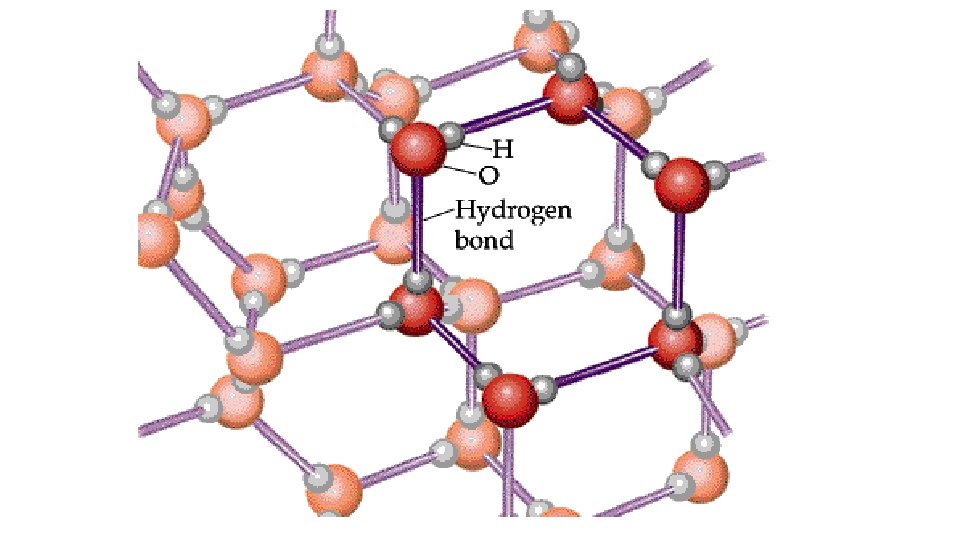
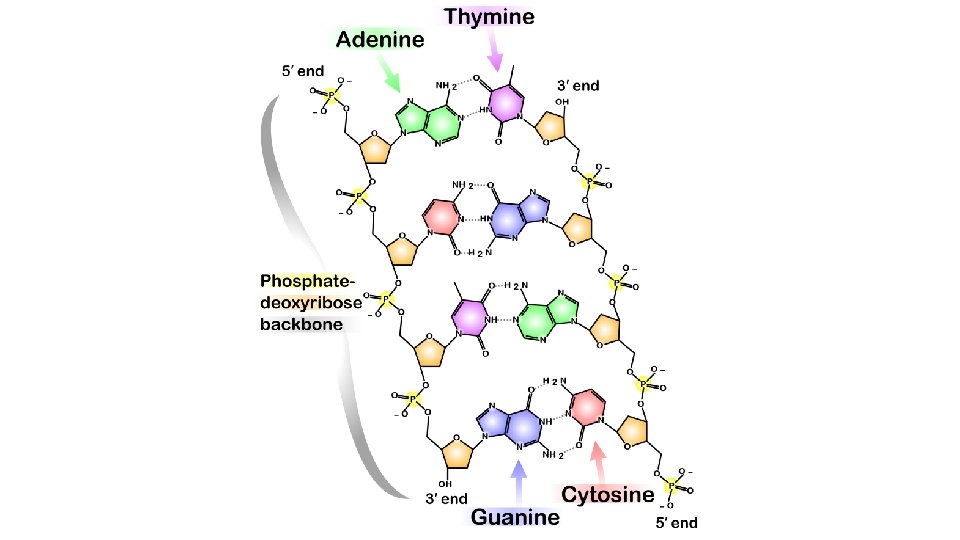
Hydrogen Bonding • A fairly strong dipole-dipole attraction exists when H is bonded to one of N, O, or F • Examples: a. HF b. NH 3, NH 2, NH (i. e. CH 3 NH 2 , (CH 3)2 NH c. H 2 O, H 2 O 2, OH (i. e. CH 3 OH)




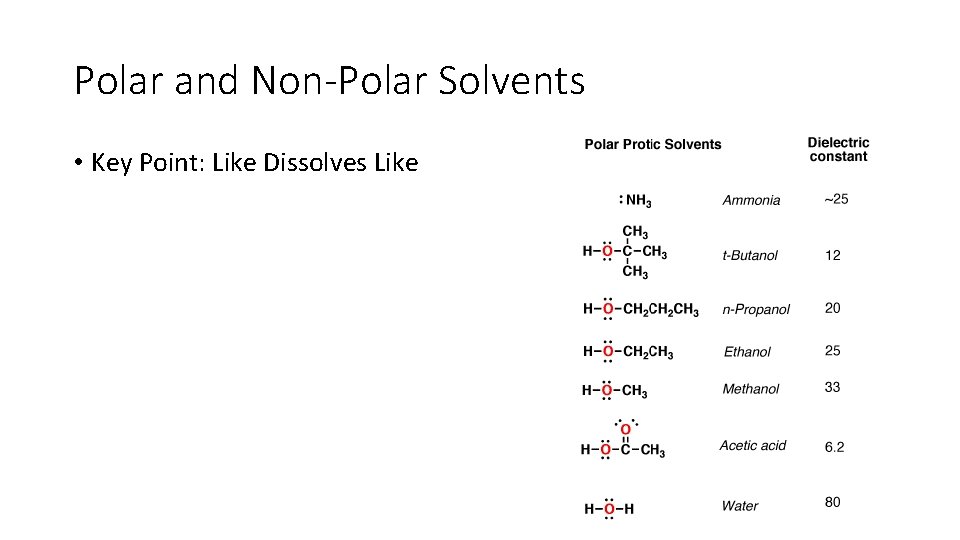
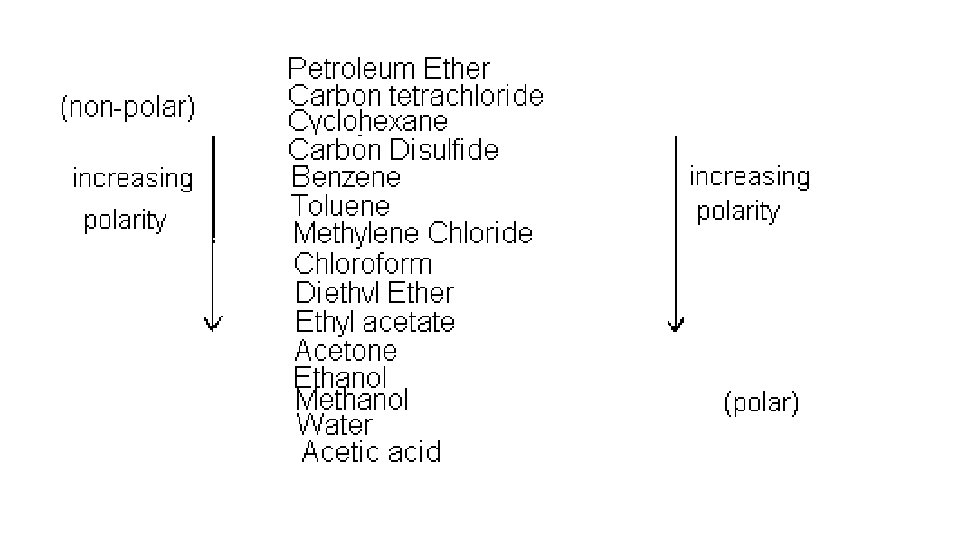
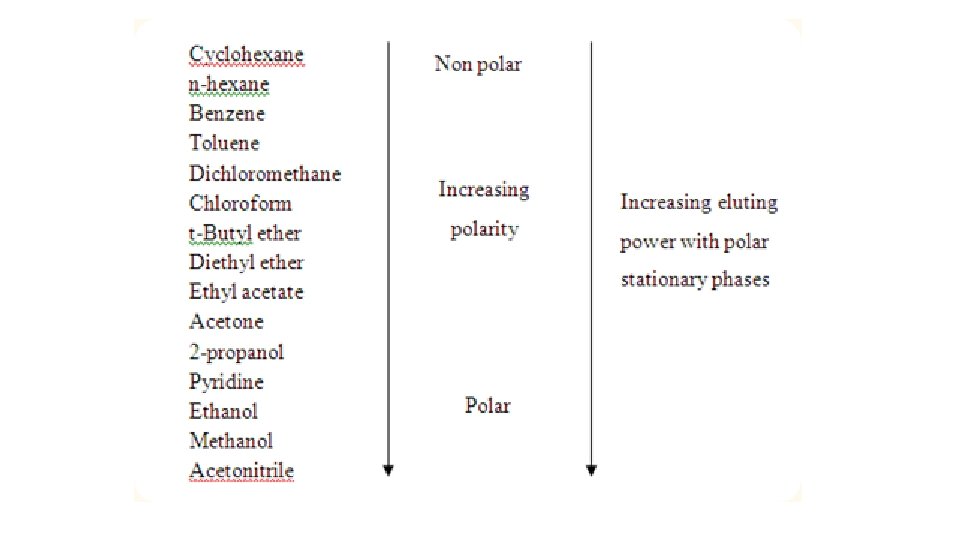
Polar and Non-Polar Solvents • Key Point: Like Dissolves Like



Why Like Dissolves Like: Polar • Ionic compounds - + and – ions bound together strongly as a solid • Three types of attractions • Attraction of solvent-solvent molecules • Attraction of solvent-solute particles • Attraction of solute-solute particle • To break bonds, energy is needed and energy is released when a bond forms • If the solvent forms strong enough bond with the solute then a solute can separate. • Solvent-solvent attraction allows solvent-solute to mix in the general population of solvent molecules • Conclusion: polar and ionic solutes have low solubility in non-polar solvents

Solubility of Non-Polar Solutes • Non-polar solvents do not possess charges – no polar attraction of polar solute = no dipoles • Only attracted to solutes by London Forces • Non-polar solvent London forces over come the weak London forces of solute = dissolves

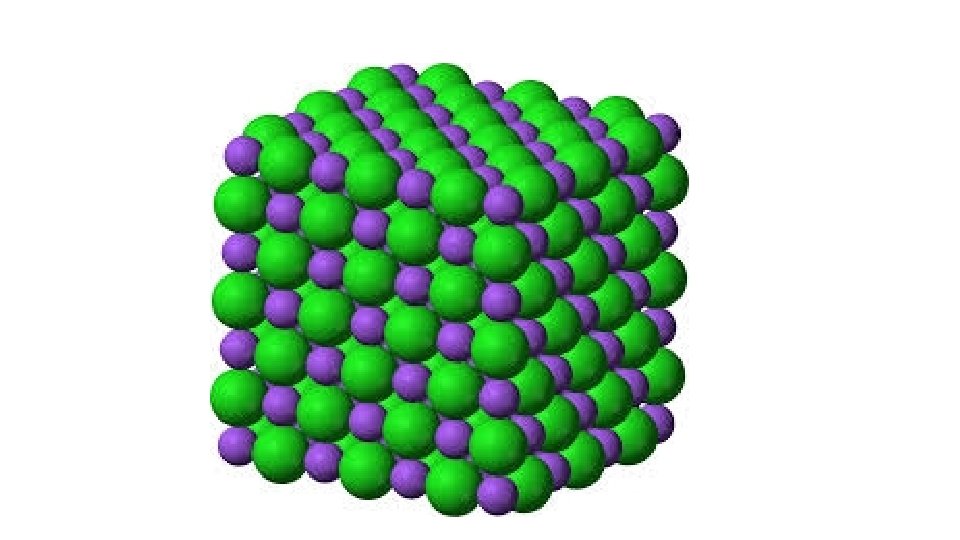
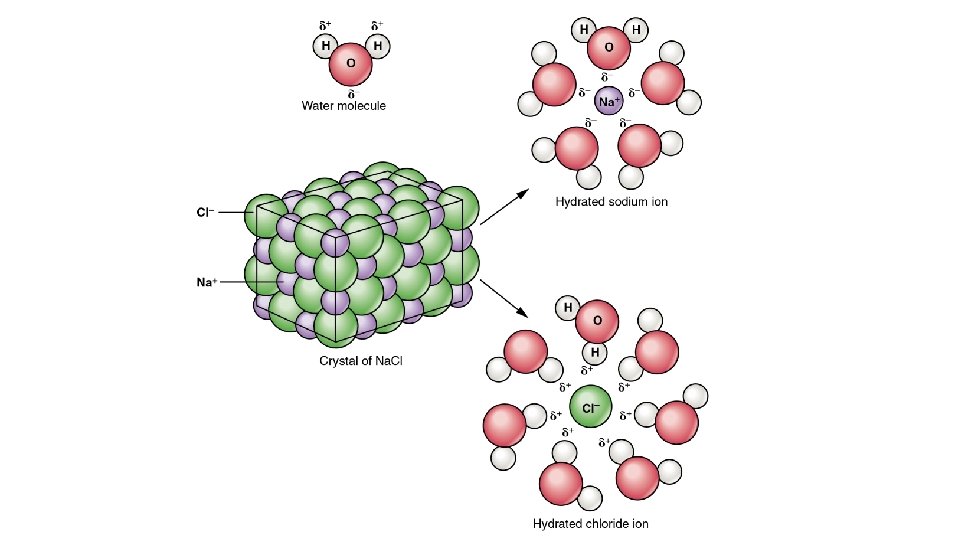
Solution of Ions • Complex! • Definitions: • Solvation – interaction between solute and solvent • Ionic Solid – solid whose crystal structure is made up of ions • Molecular Solid – a solid whose crystal structure is made up of neutral ions



Definitions cont’d • Dissociation – • Separation of previously existing ions as ionic solid • Ions are already ions in which the solvent separates them from each other • Ionization Reaction – • Ions do not exist until the solvent is able to react with the molecules to break it apart into ions
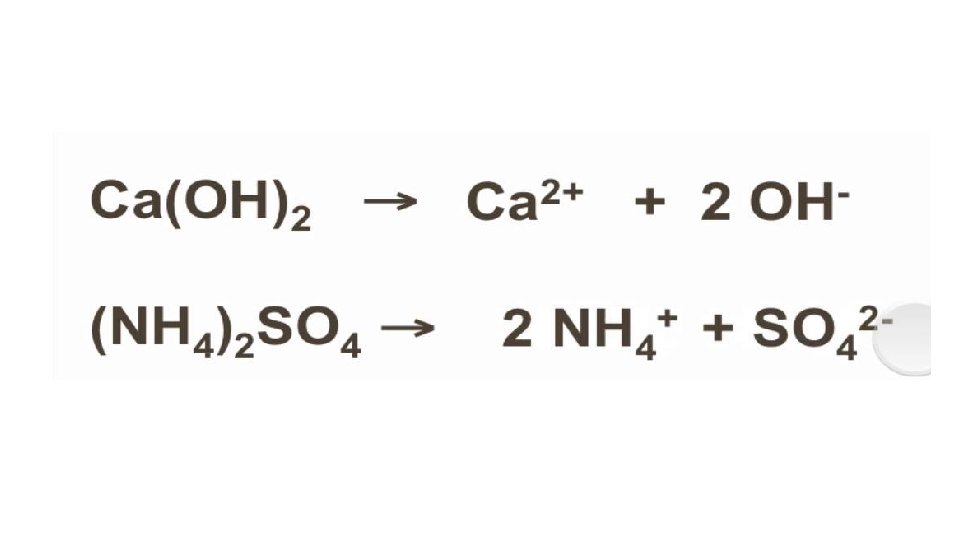

 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Reduction half reaction
Reduction half reaction Phân độ lown
Phân độ lown Premature atrial contraction
Premature atrial contraction Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Did a chemical reaction occur
Did a chemical reaction occur Did a chemical reaction occur
Did a chemical reaction occur Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of redox reactions
Types of redox reactions Identify types of reactions
Identify types of reactions 4 types of chemical reactions
4 types of chemical reactions Types of reaction
Types of reaction Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions