Unit 5 Chemical Reactions I Intro to Reactions









- Slides: 38

Unit 5 – Chemical Reactions I. Intro to Reactions I II IV

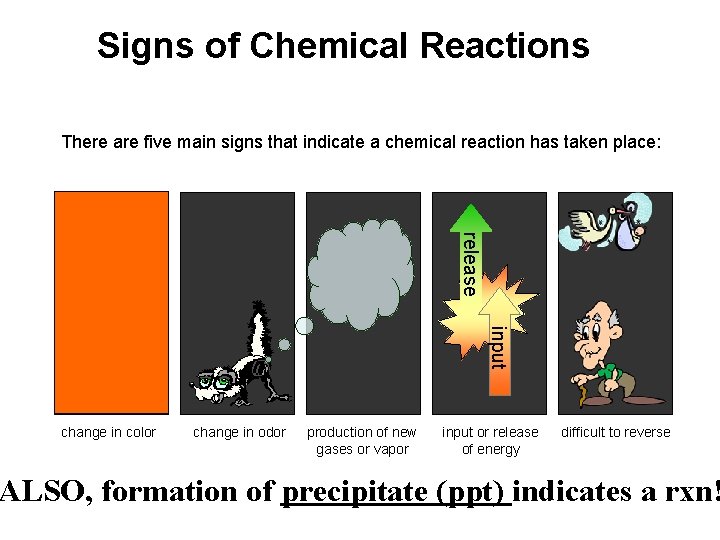
Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse ALSO, formation of precipitate (ppt) indicates a rxn!

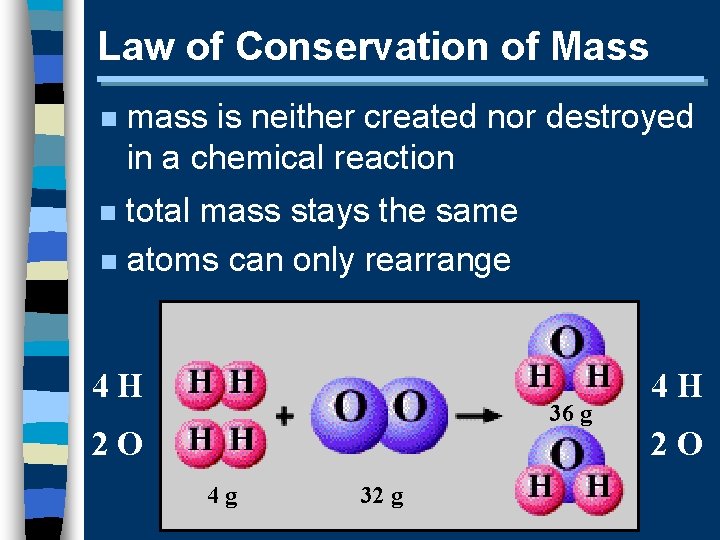
Law of Conservation of Mass n mass is neither created nor destroyed in a chemical reaction total mass stays the same n atoms can only rearrange n 4 H 36 g 2 O 4 g 32 g 4 H 2 O

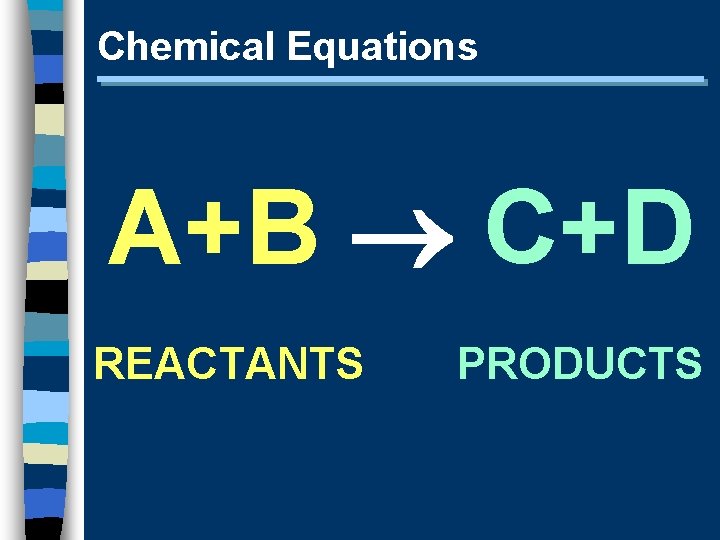
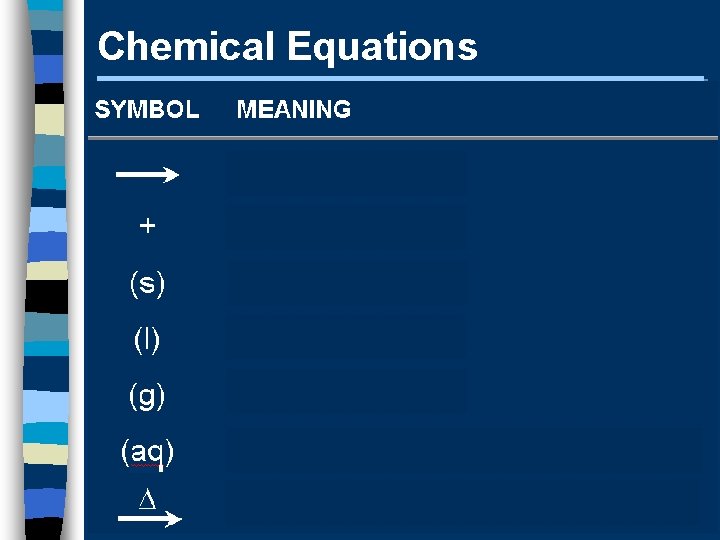
Chemical Equations A+B C+D REACTANTS PRODUCTS

Chemical Equations

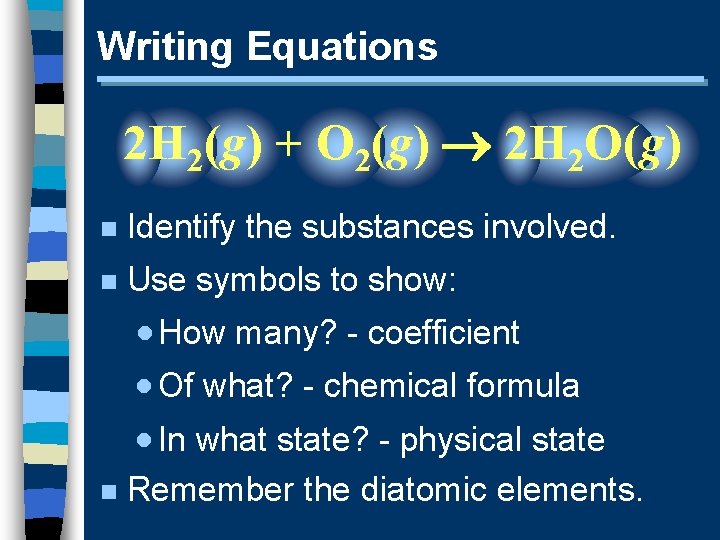
Writing Equations 2 H 2(g) + O 2(g) 2 H 2 O(g) n Identify the substances involved. n Use symbols to show: · How many? - coefficient · Of what? - chemical formula · In what state? - physical state n Remember the diatomic elements.

Writing Equations Two atoms of aluminum react with three units of aqueous copper(II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. • How many? • Of what? • In what state? 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq)

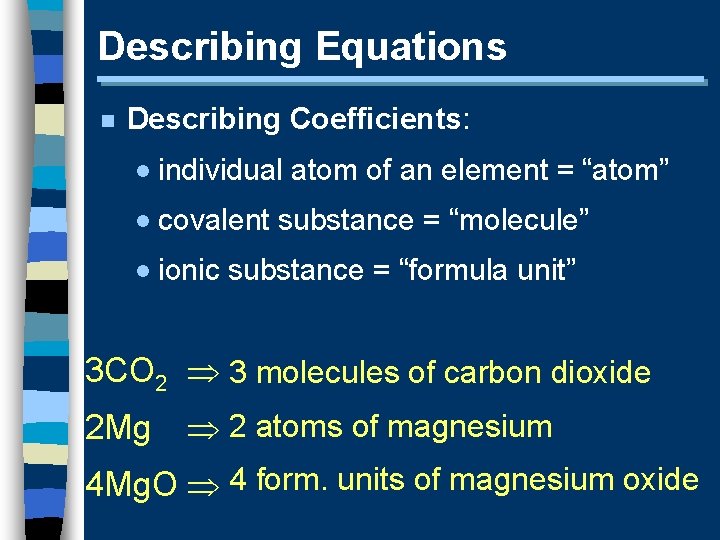
Describing Equations n Describing Coefficients: · individual atom of an element = “atom” · covalent substance = “molecule” · ionic substance = “formula unit” 3 CO 2 3 molecules of carbon dioxide 2 Mg 2 atoms of magnesium 4 Mg. O 4 form. units of magnesium oxide

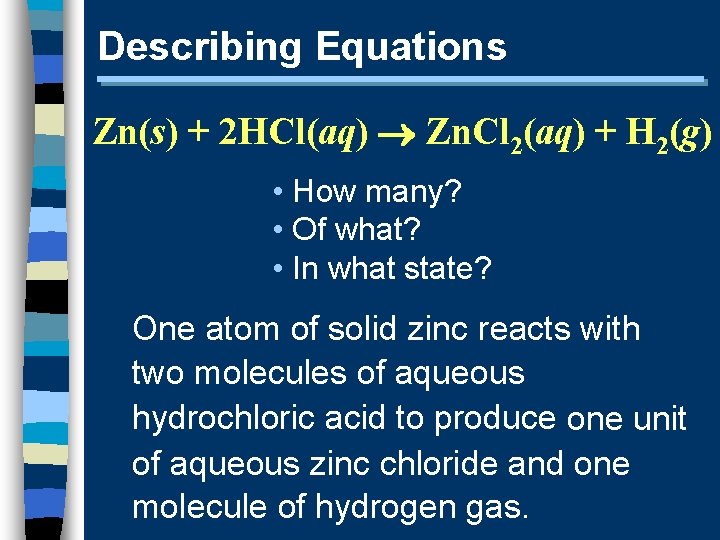
Describing Equations Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? • Of what? • In what state? One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas.

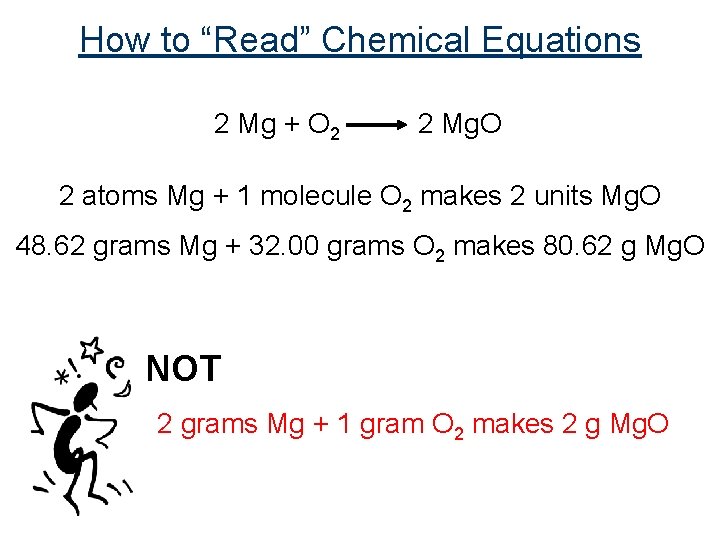
How to “Read” Chemical Equations 2 Mg + O 2 2 Mg. O 2 atoms Mg + 1 molecule O 2 makes 2 units Mg. O 48. 62 grams Mg + 32. 00 grams O 2 makes 80. 62 g Mg. O NOT 2 grams Mg + 1 gram O 2 makes 2 g Mg. O

Practice n Introduction to Reactions WS

Unit 5 – Chemical Reactions II. Balancing Equations I II IV

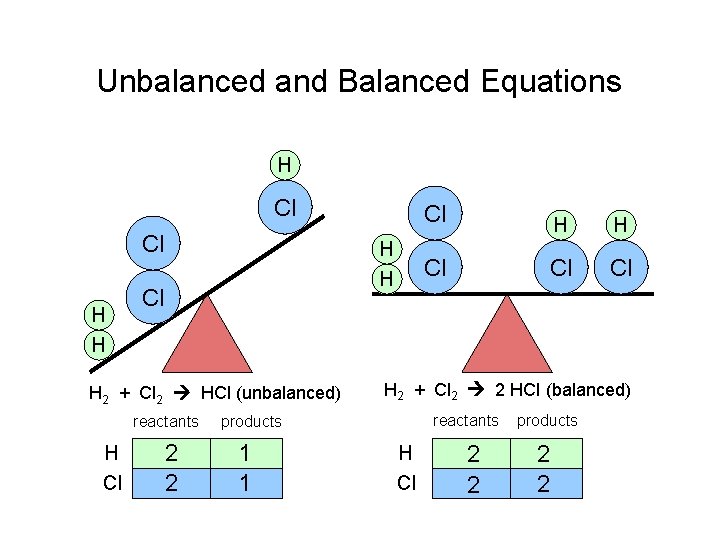
Unbalanced and Balanced Equations H Cl Cl H H Cl H 2 + Cl 2 HCl (unbalanced) reactants H Cl 2 2 H H Cl Cl Cl H 2 + Cl 2 2 HCl (balanced) reactants products 1 1 Cl H Cl 2 2 products 2 2

Balancing Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #’s equal on both sides of the equation. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

Helpful Tips Balance one element at a time. n Update ALL atom counts after adding a coefficient. n Balance polyatomic ions as single units. · “ 1 SO 4” instead of “ 1 S” and “ 4 O” n MINOH Technique! n

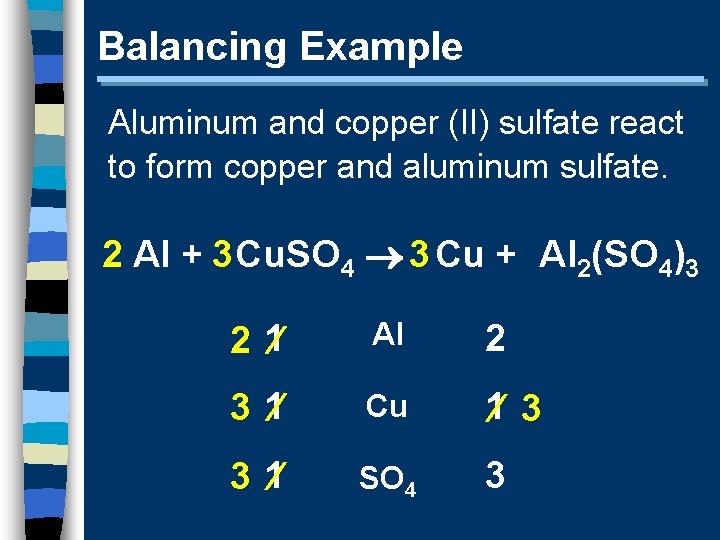
Balancing Example Aluminum and copper (II) sulfate react to form copper and aluminum sulfate. 2 Al + 3 Cu. SO 4 3 Cu + Al 2(SO 4)3 2 1 Al 2 3 1 Cu 1 3 3 1 SO 4 3

Practice n Balancing Chemical Equations WS

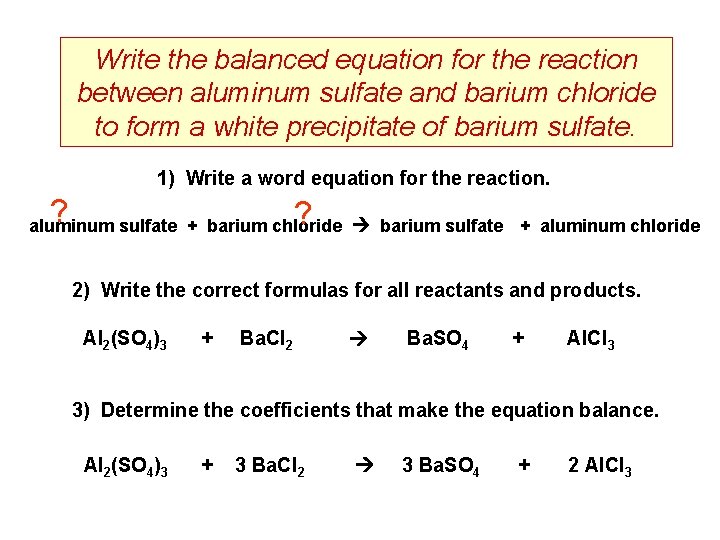
Write the balanced equation for the reaction between aluminum sulfate and barium chloride to form a white precipitate of barium sulfate. 1) Write a word equation for the reaction. ? ? aluminum sulfate + barium chloride barium sulfate + aluminum chloride 2) Write the correct formulas for all reactants and products. Al 2(SO 4)3 + Ba. Cl 2 Ba. SO 4 + Al. Cl 3 3) Determine the coefficients that make the equation balance. Al 2(SO 4)3 + 3 Ba. Cl 2 3 Ba. SO 4 + 2 Al. Cl 3

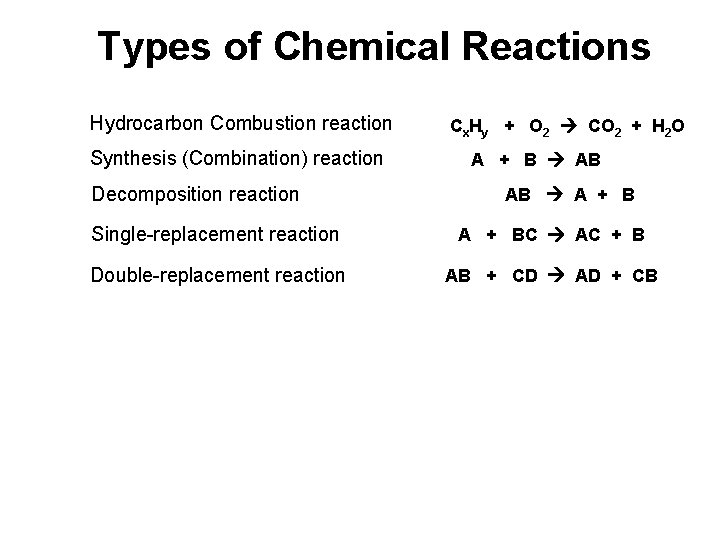
Types of Chemical Reactions Hydrocarbon Combustion reaction Synthesis (Combination) reaction Decomposition reaction Cx. Hy + O 2 CO 2 + H 2 O A + B AB AB A + B Single-replacement reaction A + BC AC + B Double-replacement reaction AB + CD AD + CB

Unit 5 – Chemical Reactions III. Types of Chemical Reactions I II IV

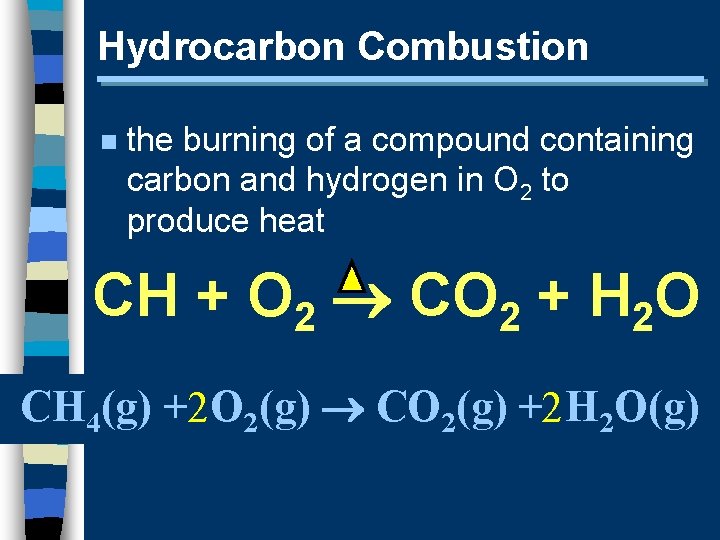
Hydrocarbon Combustion n the burning of a compound containing carbon and hydrogen in O 2 to produce heat CH + O 2 CO 2 + H 2 O CH 4(g) +2 O 2(g) CO 2(g) +2 H 2 O(g)

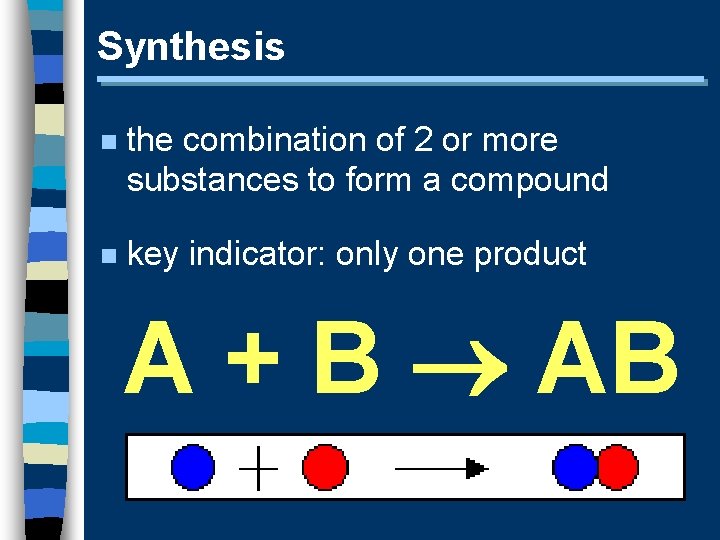
Synthesis n the combination of 2 or more substances to form a compound n key indicator: only one product A + B AB

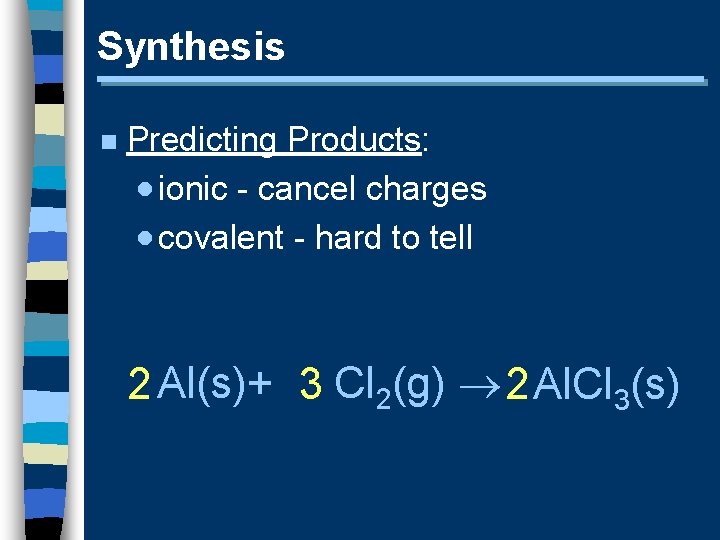
Synthesis n Predicting Products: · ionic - cancel charges · covalent - hard to tell 2 Al(s)+ 3 Cl 2(g) 2 Al. Cl 3(s)

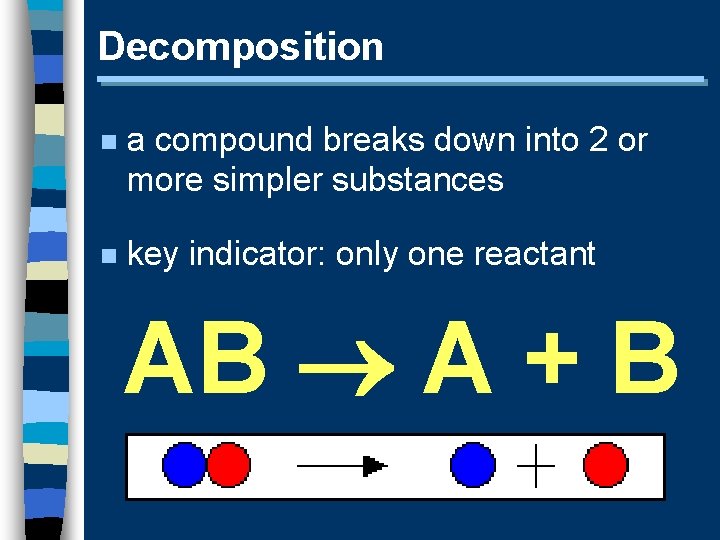
Decomposition n a compound breaks down into 2 or more simpler substances n key indicator: only one reactant AB A + B

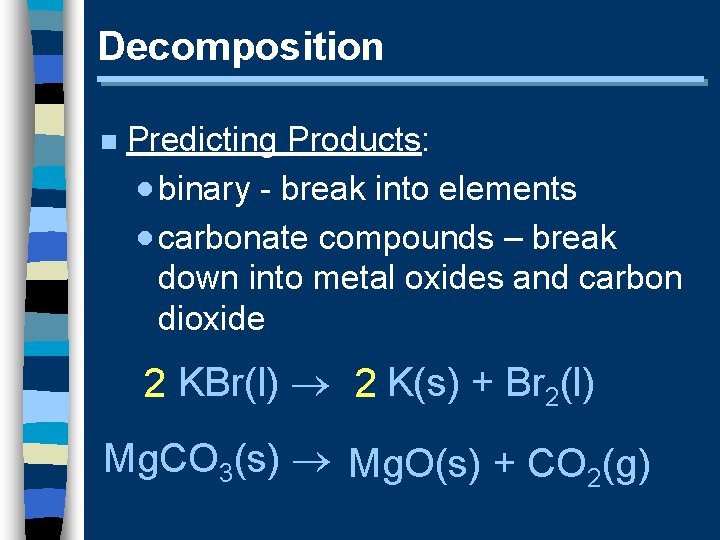
Decomposition n Predicting Products: · binary - break into elements · carbonate compounds – break down into metal oxides and carbon dioxide 2 KBr(l) 2 K(s) + Br 2(l) Mg. CO 3(s) Mg. O(s) + CO 2(g)

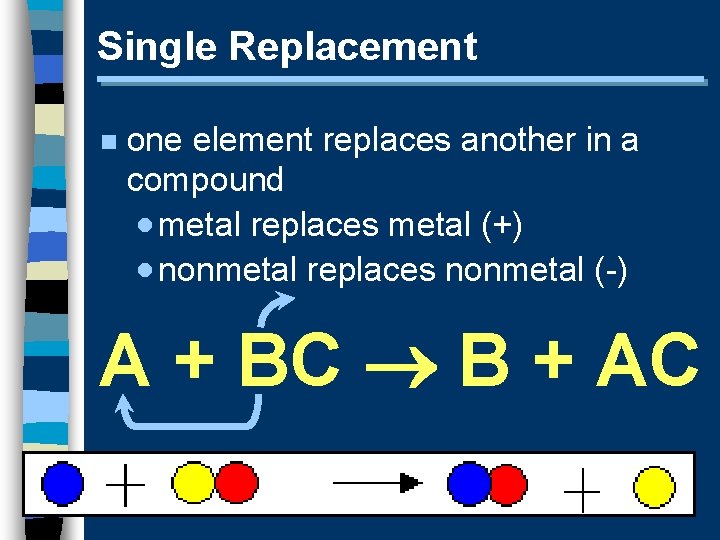
Single Replacement n one element replaces another in a compound · metal replaces metal (+) · nonmetal replaces nonmetal (-) A + BC B + AC

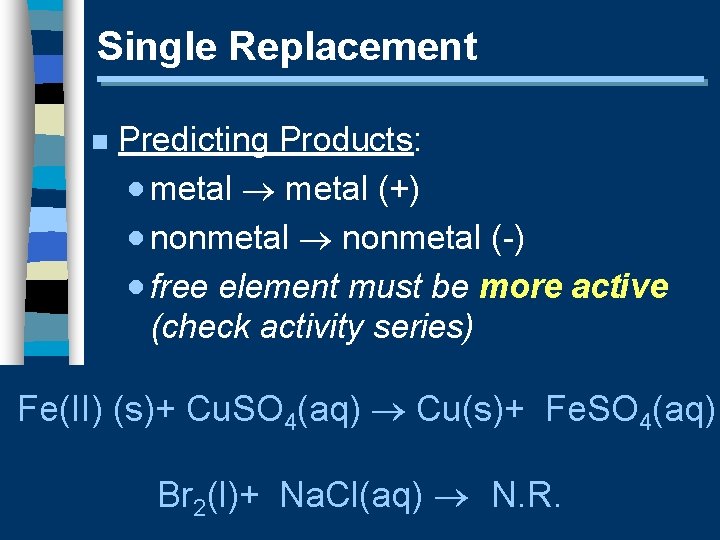
Single Replacement n Predicting Products: · metal (+) · nonmetal (-) · free element must be more active (check activity series) Fe(II) (s)+ Cu. SO 4(aq) Cu(s)+ Fe. SO 4(aq) Br 2(l)+ Na. Cl(aq) N. R.

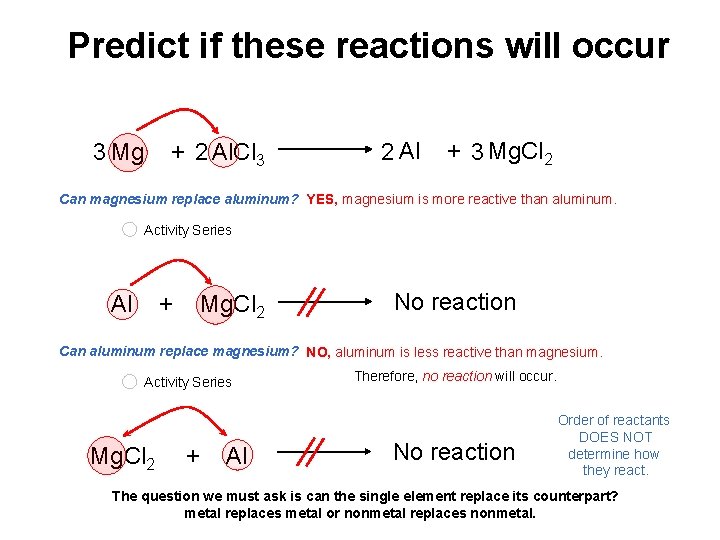
Predict if these reactions will occur 3 Mg + 2 Al. Cl 3 2 Al + 3 Mg. Cl 2 Can magnesium replace aluminum? YES, magnesium is more reactive than aluminum. Activity Series Al + Mg. Cl 2 No reaction Can aluminum replace magnesium? NO, aluminum is less reactive than magnesium. Activity Series Mg. Cl 2 + Al Therefore, no reaction will occur. No reaction Order of reactants DOES NOT determine how they react. The question we must ask is can the single element replace its counterpart? metal replaces metal or nonmetal replaces nonmetal.

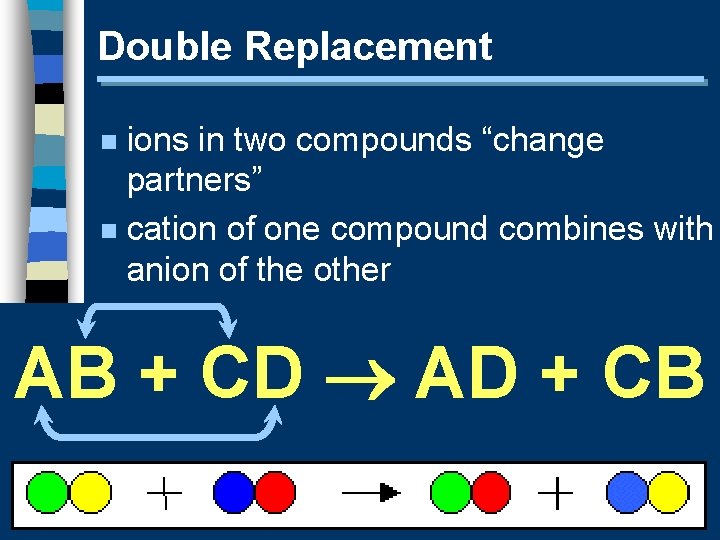
Double Replacement ions in two compounds “change partners” n cation of one compound combines with anion of the other n AB + CD AD + CB

Double Replacement n Predicting Products: · switch negative ions - “outside-outside, inside-inside” · one product must be a solid, liquid, or gas (check solubility table)

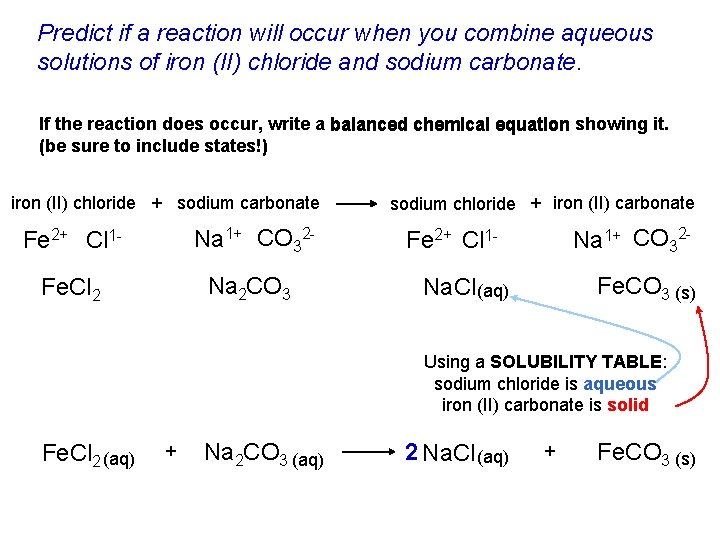
Predict if a reaction will occur when you combine aqueous solutions of iron (II) chloride and sodium carbonate. If the reaction does occur, write a balanced chemical equation showing it. (be sure to include states!) iron (II) chloride + sodium carbonate Fe 2+ Cl 1 - Na 1+ CO 32 - Fe. Cl 2 Na 2 CO 3 sodium chloride + iron (II) carbonate Na 1+ CO 32 - Fe 2+ Cl 1 - Fe. CO 3 (s) Na. Cl (aq) Using a SOLUBILITY TABLE: sodium chloride is aqueous iron (II) carbonate is solid Fe. Cl 2 (aq) + Na 2 CO 3 (aq) 2 Na. Cl (aq) + Fe. CO 3 (s)

Practice Classify reactions on Balancing Chemical Equations WS n Predicting Products WS n

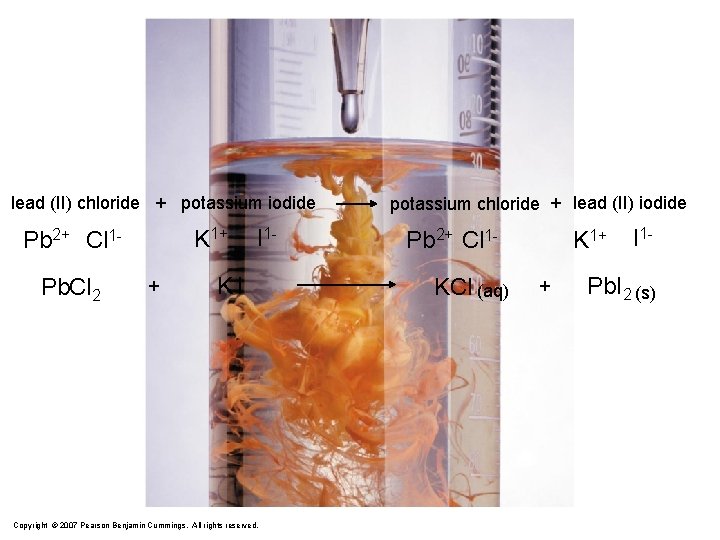
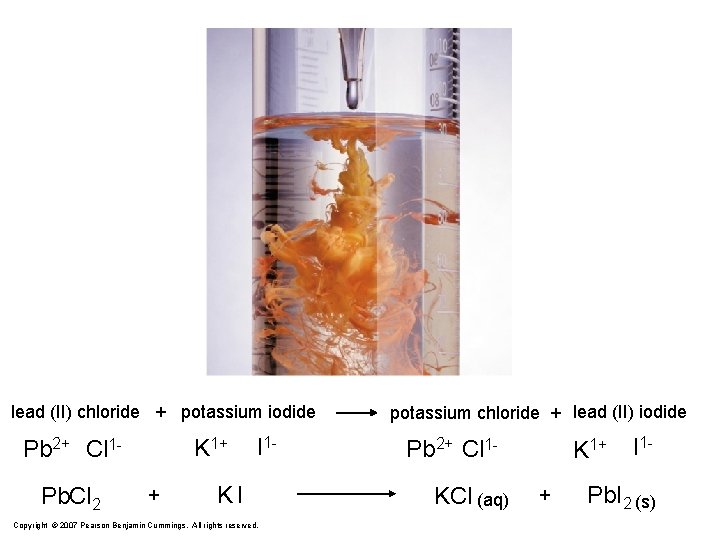
lead (II) chloride + potassium iodide K 1+ Pb 2+ Cl 1 Pb. Cl 2 + I 1 - KI Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. potassium chloride + lead (II) iodide Pb 2+ Cl 1 KCl (aq) K 1+ + I 1 - Pb. I 2 (s)

lead (II) chloride + potassium iodide K 1+ Pb 2+ Cl 1 Pb. Cl 2 + I 1 - KI Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. potassium chloride + lead (II) iodide Pb 2+ Cl 1 KCl (aq) K 1+ + I 1 - Pb. I 2 (s)

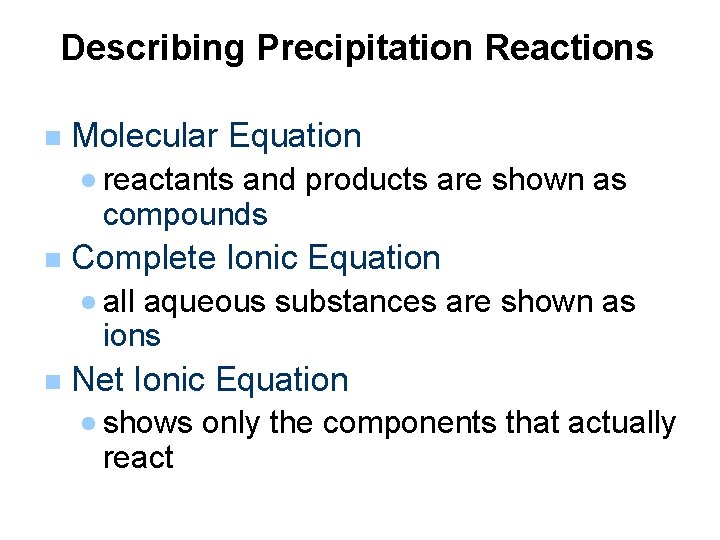
Describing Precipitation Reactions n Molecular Equation · reactants and products are shown as compounds n Complete Ionic Equation · all aqueous substances are shown as ions n Net Ionic Equation · shows only the components that actually react

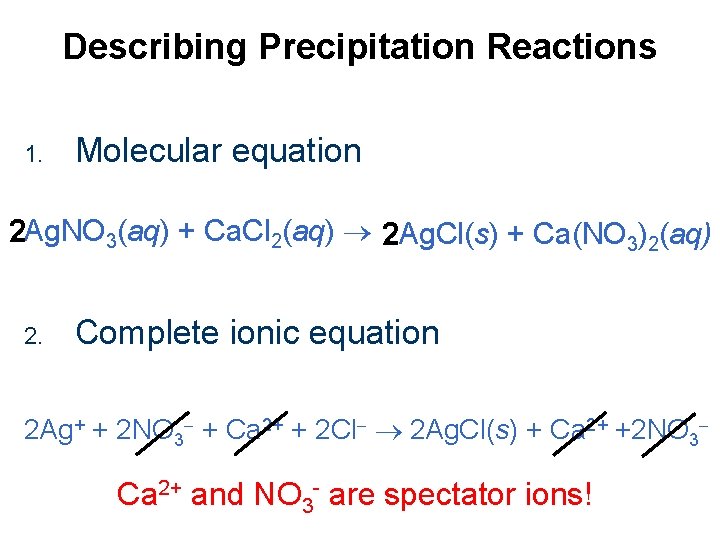
Describing Precipitation Reactions 1. Molecular equation 2 Ag. NO 3(aq) + Ca. Cl 2(aq) 2 Ag. Cl(s) + Ca(NO 3)2(aq) 2. Complete ionic equation 2 Ag+ + 2 NO 3 + Ca 2+ + 2 Cl 2 Ag. Cl(s) + Ca 2+ +2 NO 3 Ca 2+ and NO 3 - are spectator ions!

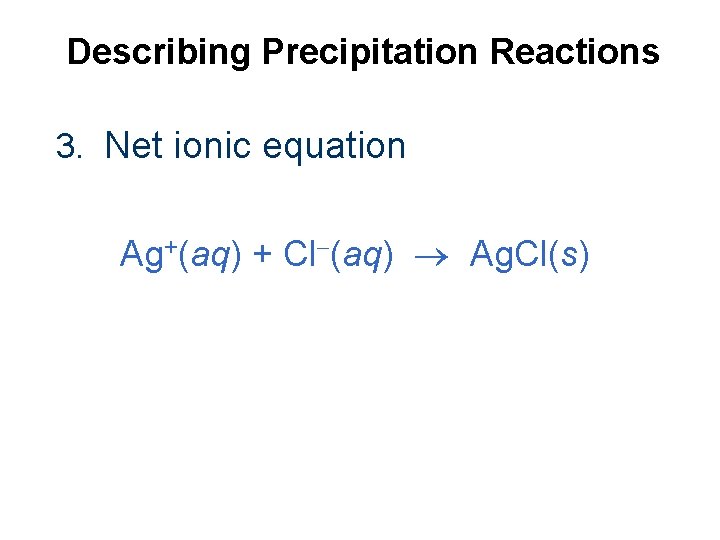
Describing Precipitation Reactions 3. Net ionic equation Ag+(aq) + Cl (aq) Ag. Cl(s)

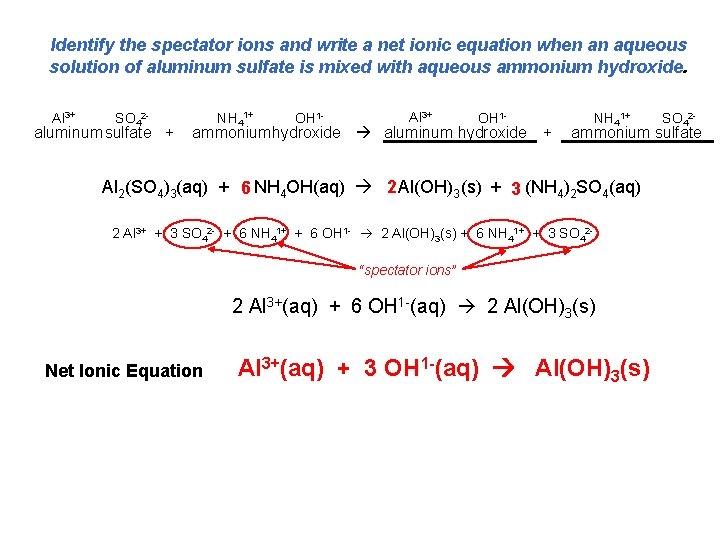
Identify the spectator ions and write a net ionic equation when an aqueous solution of aluminum sulfate is mixed with aqueous ammonium hydroxide. Al 3+ SO 42 - aluminum sulfate + NH 41+ OH 1 - Al 3+ OH 1 - ammoniumhydroxide aluminum hydroxide + NH 41+ ammonium sulfate Al 2(SO 4)3(aq) + 6 NH 4 OH(aq) 2 Al(OH)3 (s) + 3 (NH 4)2 SO 4(aq) 2 Al 3+ + 3 SO 42 - + 6 NH 41+ + 6 OH 1 - 2 Al(OH)3(s) + 6 NH 41+ + 3 SO 42“spectator ions” 2 Al 3+(aq) + 6 OH 1 -(aq) 2 Al(OH)3(s) Net Ionic Equation SO 42 - Al 3+(aq) + 3 OH 1 -(aq) Al(OH)3(s)
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Balancing chemical equations definition
Balancing chemical equations definition Unit 11 chemical reactions
Unit 11 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions 10 examples of redox reaction
10 examples of redox reaction What is bivariate data
What is bivariate data Unit 1 government study guide
Unit 1 government study guide Stoichiometry mole island diagram
Stoichiometry mole island diagram Types of chemical reactions redox
Types of chemical reactions redox Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Ouchterlony
Ouchterlony Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Combination reaction example
Combination reaction example Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Four types of chemical reactions
Four types of chemical reactions Biology-roots.com
Biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Five chemical changes
Five chemical changes Reactants and products
Reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Equilibrium of chemical reactions
Equilibrium of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions




























































