Chemical reactions reactants products A chemical reaction is
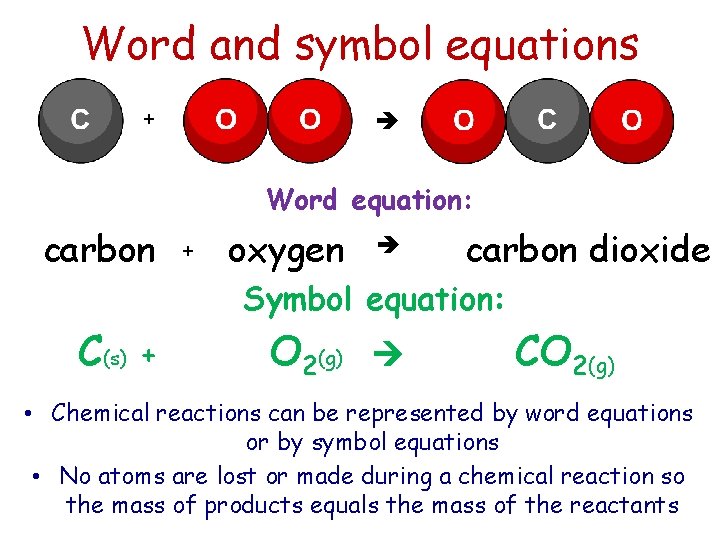

- Slides: 12

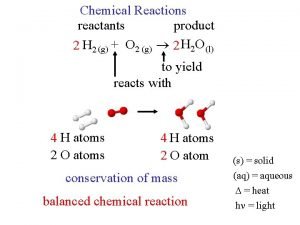
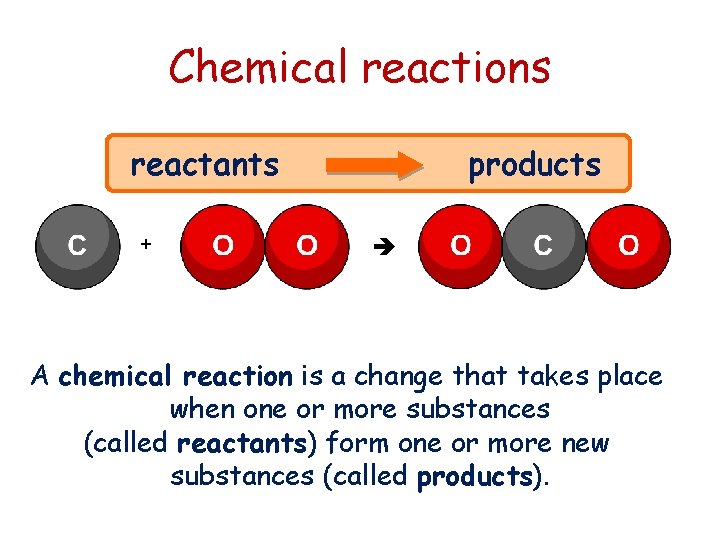
Chemical reactions reactants + products A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products).

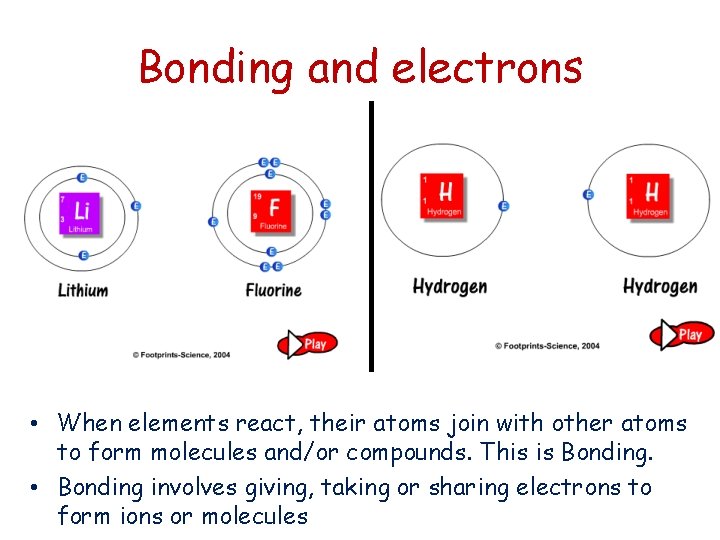
Bonding and electrons • When elements react, their atoms join with other atoms to form molecules and/or compounds. This is Bonding. • Bonding involves giving, taking or sharing electrons to form ions or molecules

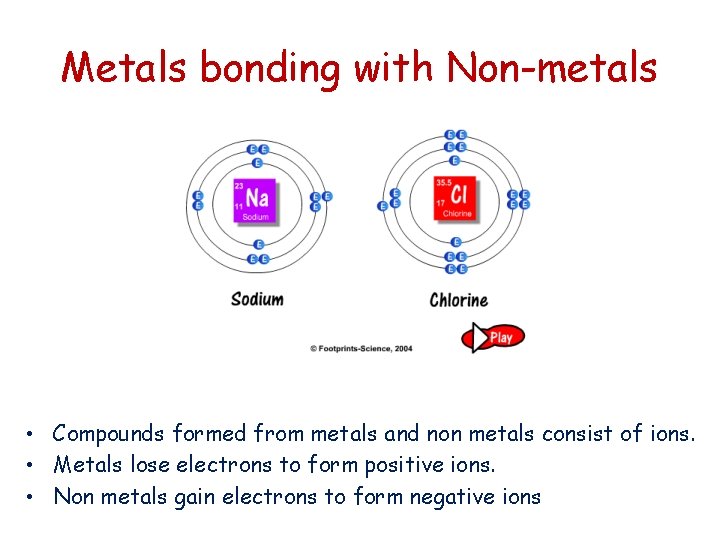
Metals bonding with Non-metals • Compounds formed from metals and non metals consist of ions. • Metals lose electrons to form positive ions. • Non metals gain electrons to form negative ions
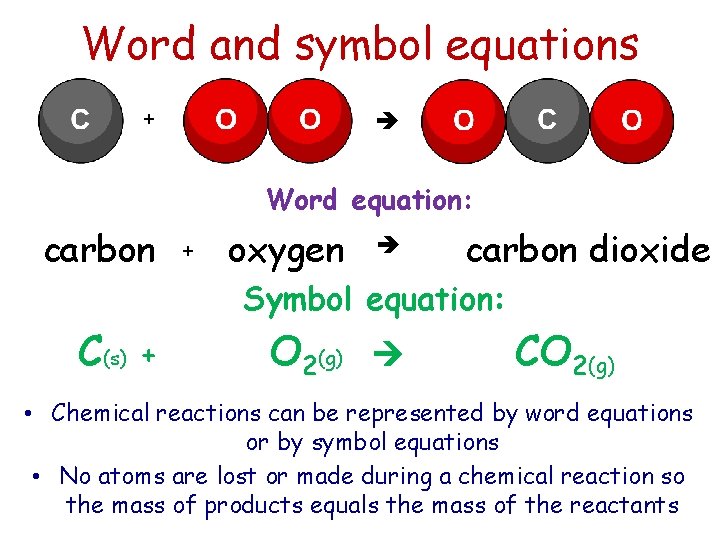
Word and symbol equations + Word equation: carbon + oxygen carbon dioxide Symbol equation: C(s) + O 2(g) CO 2(g) • Chemical reactions can be represented by word equations or by symbol equations • No atoms are lost or made during a chemical reaction so the mass of products equals the mass of the reactants

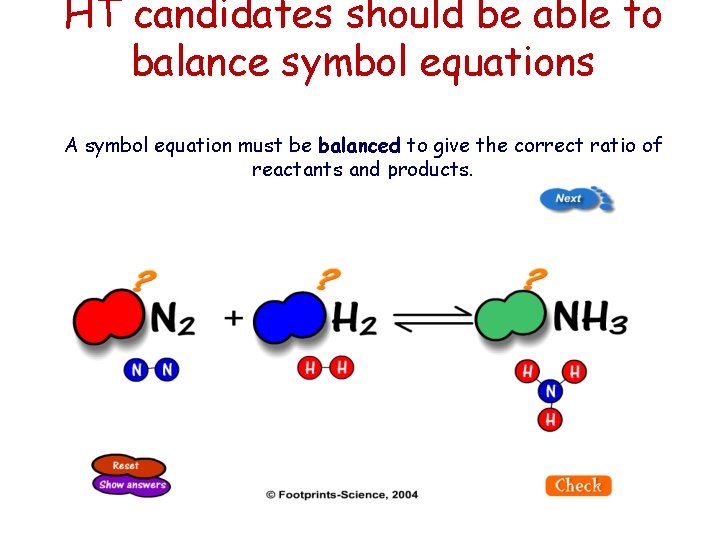
HT candidates should be able to balance symbol equations A symbol equation must be balanced to give the correct ratio of reactants and products.

Limestone = calcium carbonate • Limestone is mainly composed of the compound calcium carbonate (Ca. CO 3) • Limestone is quarried (dug up) and can be used as a building material

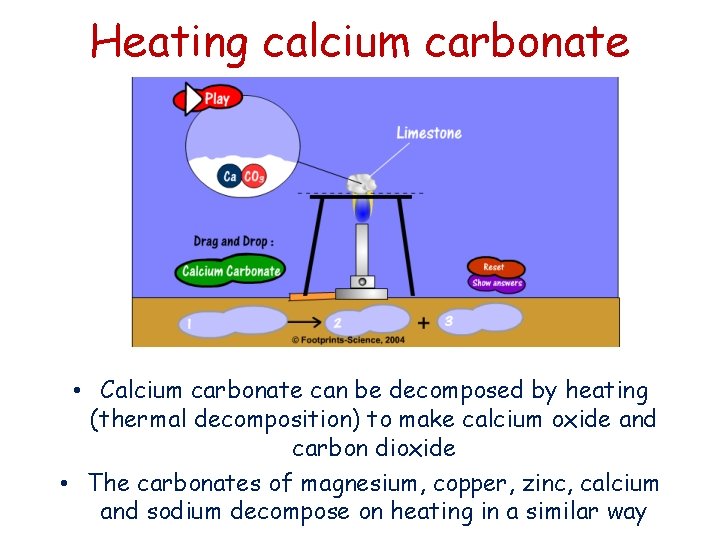
Heating calcium carbonate • Calcium carbonate can be decomposed by heating (thermal decomposition) to make calcium oxide and carbon dioxide • The carbonates of magnesium, copper, zinc, calcium and sodium decompose on heating in a similar way

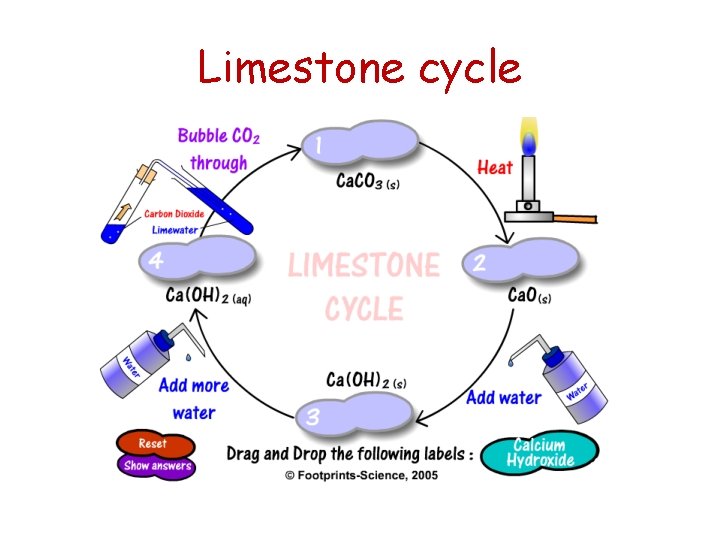
Limestone cycle

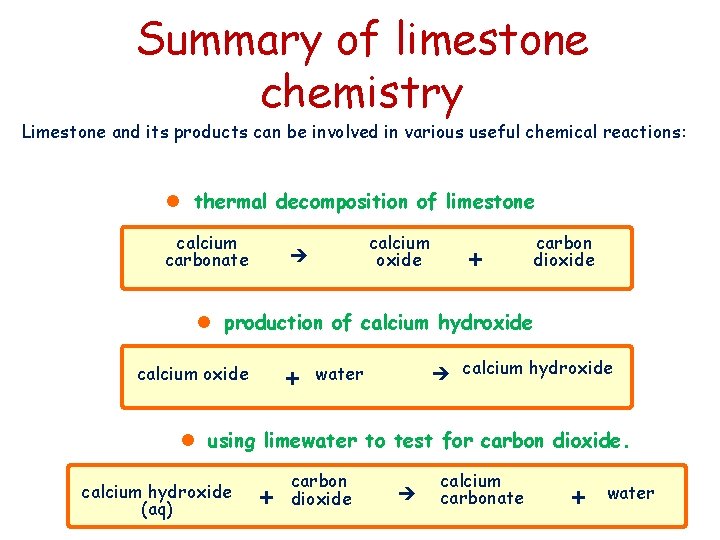
Summary of limestone chemistry Limestone and its products can be involved in various useful chemical reactions: l thermal decomposition of limestone calcium carbonate calcium oxide + carbon dioxide l production of calcium hydroxide + calcium oxide calcium hydroxide water l using limewater to test for carbon dioxide. calcium hydroxide (aq) + carbon dioxide calcium carbonate + water

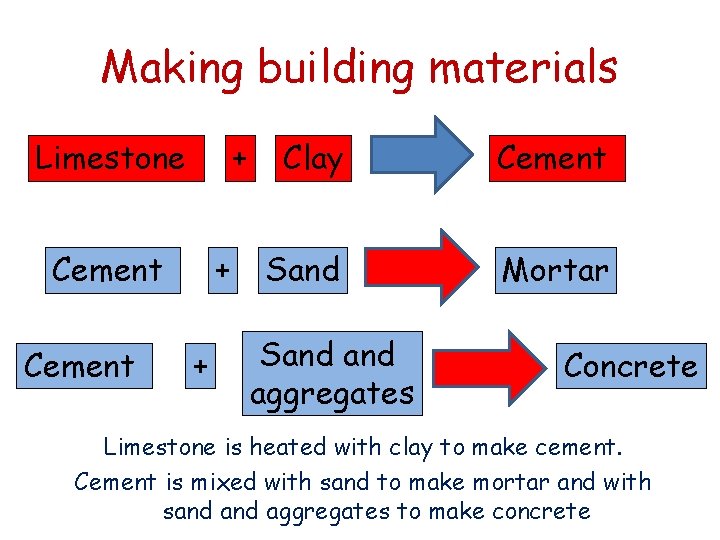
Making building materials Limestone + Cement + + Clay Cement Sand Mortar Sand aggregates Concrete Limestone is heated with clay to make cement. Cement is mixed with sand to make mortar and with sand aggregates to make concrete

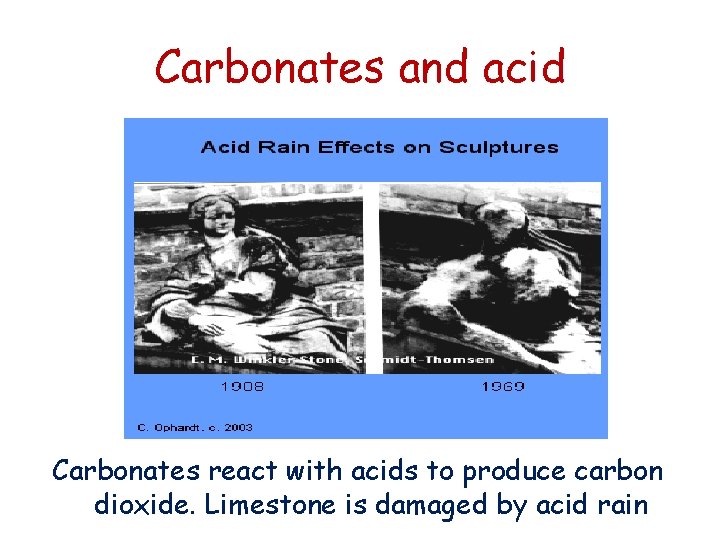
Carbonates and acid Carbonates react with acids to produce carbon dioxide. Limestone is damaged by acid rain

Pros and cons of quarrying
 Chemical reactions reactants and products
Chemical reactions reactants and products Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Reactants and products
Reactants and products Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting synthesis reactions
Predicting synthesis reactions Activity series of metals
Activity series of metals Stoichiometry is the study of
Stoichiometry is the study of Phet reactants products and leftovers
Phet reactants products and leftovers Reactants - products enthalpy
Reactants - products enthalpy Chemical equation symbols
Chemical equation symbols