Predicting Products of Chemical Reactions Synthesis Reactions In



- Slides: 8

Predicting Products of Chemical Reactions

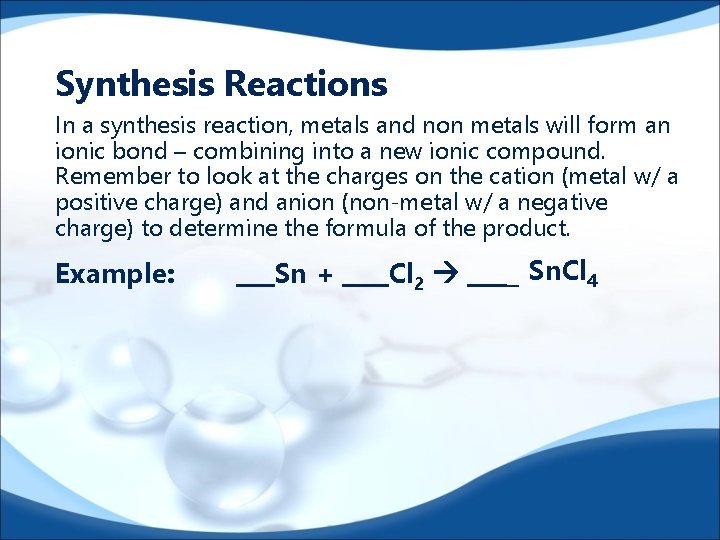
Synthesis Reactions In a synthesis reaction, metals and non metals will form an ionic bond – combining into a new ionic compound. Remember to look at the charges on the cation (metal w/ a positive charge) and anion (non-metal w/ a negative charge) to determine the formula of the product. Example: Sn + Cl 2 _ Sn. Cl 4

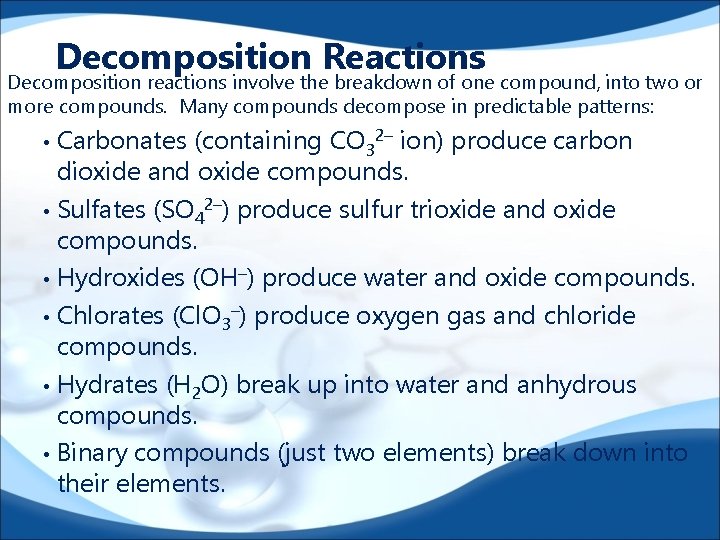
Decomposition Reactions Decomposition reactions involve the breakdown of one compound, into two or more compounds. Many compounds decompose in predictable patterns: Carbonates (containing CO 32– ion) produce carbon dioxide and oxide compounds. • Sulfates (SO 42–) produce sulfur trioxide and oxide compounds. • Hydroxides (OH–) produce water and oxide compounds. • Chlorates (Cl. O 3–) produce oxygen gas and chloride compounds. • Hydrates (H 2 O) break up into water and anhydrous compounds. • Binary compounds (just two elements) break down into their elements. •

Decomposition Reactions • • • Carbonates (containing CO 32– ion) produce carbon dioxide and oxide compounds. Sulfates (SO 42–) produce sulfur trioxide and oxide compounds. Hydroxides (OH–) produce water and oxide compounds. Chlorates (Cl. O 3–) produce oxygen gas and chloride compounds. Hydrates (H 2 O) break up into water and anhydrous compounds. Binary compounds (just two elements) break down into their elements. 1. _____NH 3 ____N 2 + _____H 2 3. _____H 2 SO 4 ____SO 3 + _____H 2 O 8. ____Mg(Cl. O 3)2 ____O 2 + _____Mg. Cl 2

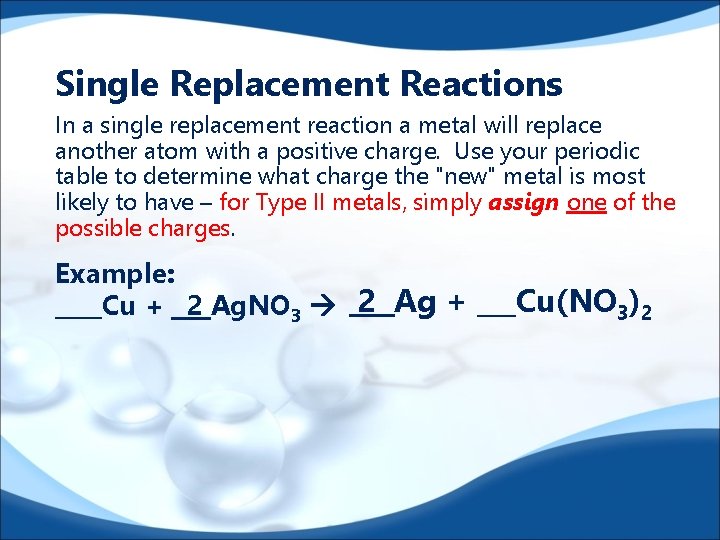
Single Replacement Reactions In a single replacement reaction a metal will replace another atom with a positive charge. Use your periodic table to determine what charge the "new" metal is most likely to have – for Type II metals, simply assign one of the possible charges. Example: ____Cu + 2 Ag. NO 3 2 Ag + ___Cu(NO 3)2

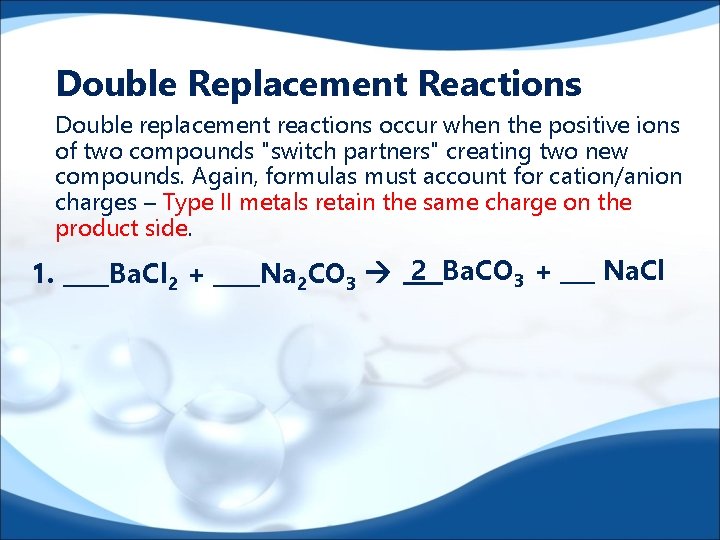
Double Replacement Reactions Double replacement reactions occur when the positive ions of two compounds "switch partners" creating two new compounds. Again, formulas must account for cation/anion charges – Type II metals retain the same charge on the product side. 1. ____Ba. Cl 2 + ____Na 2 CO 3 2 Ba. CO 3 + ___ Na. Cl

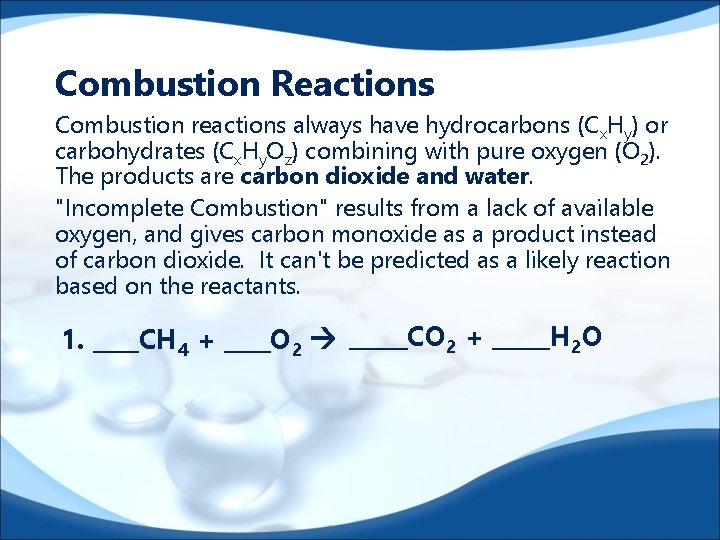
Combustion Reactions Combustion reactions always have hydrocarbons (C x. Hy) or carbohydrates (Cx. Hy. Oz) combining with pure oxygen (O 2). The products are carbon dioxide and water. "Incomplete Combustion" results from a lack of available oxygen, and gives carbon monoxide as a product instead of carbon dioxide. It can't be predicted as a likely reaction based on the reactants. 1. ____CH 4 + ____O 2 _____CO 2 + _____H 2 O

Identify the most likely reaction type; predict the products; and, balance the following equations. 1. ____Al + ____O 2 2. ____Ba. SO 4 + ____Mg. Cl 2 3. ____ Zn + ____H 2 SO 4 4. ____Cr. Cl 3 6. ____Ca(OH)2 7. ____Cu(Cl. O 3)2
 Chemistry predicting products
Chemistry predicting products Combination reaction equation
Combination reaction equation Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions reactants and products
Chemical reactions reactants and products Stoichiometry predicting amounts in reactions
Stoichiometry predicting amounts in reactions















