Types of Chemical Reactions Predicting Products from the

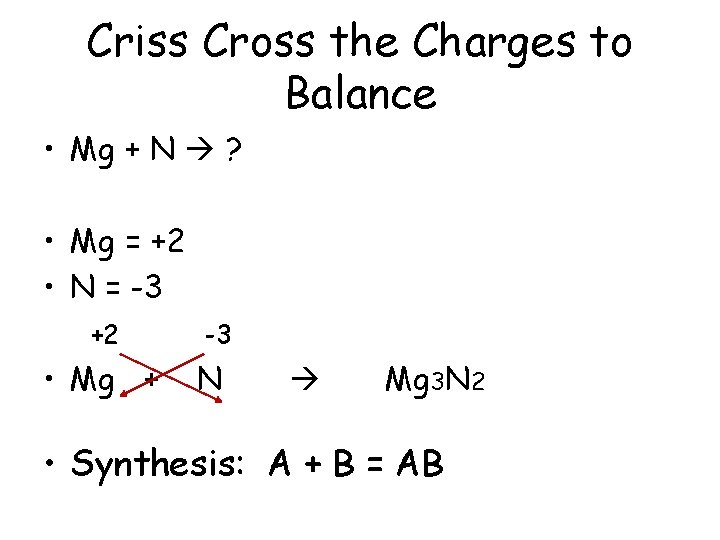
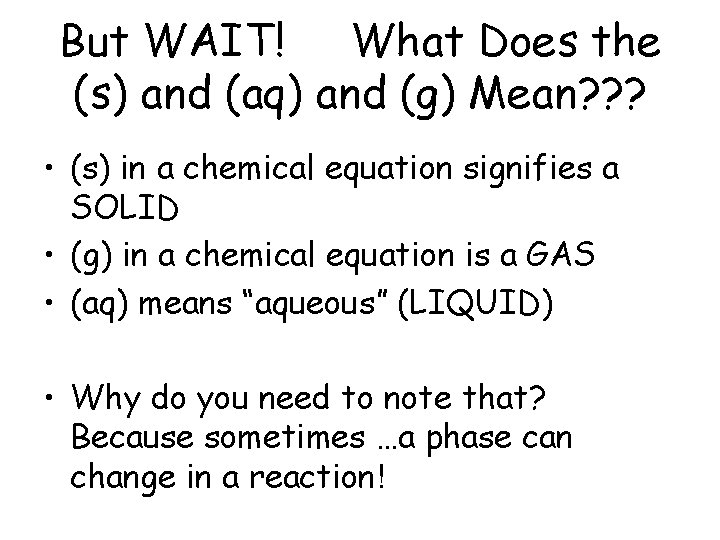

- Slides: 19

Types of Chemical Reactions Predicting Products from the Reactants

Types of Reactions 1. 2. 3. 4. 5. Synthesis reactions Decomposition reactions Single displacement reactions Double displacement reactions Combustion reactions You need to be able to identify each

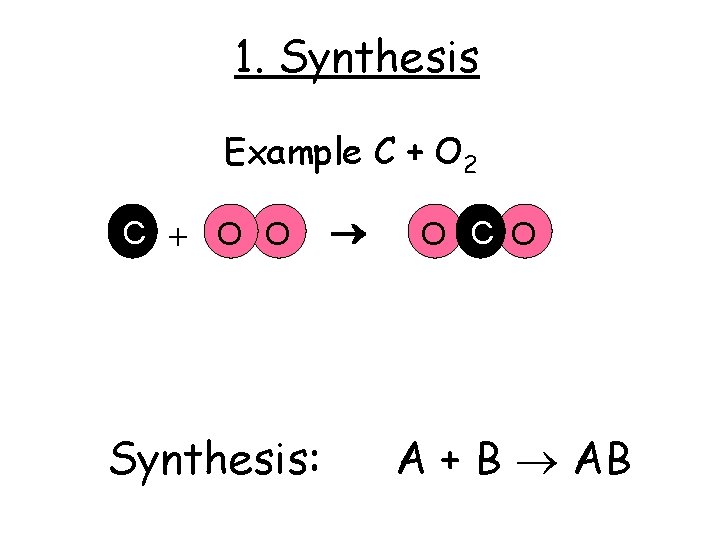
1. Synthesis Example C + O 2 C + O O Synthesis: O C O A + B AB
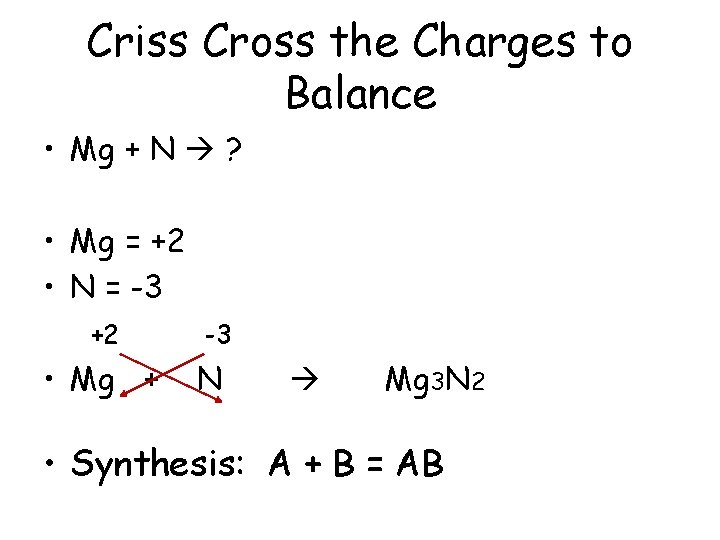
Criss Cross the Charges to Balance • Mg + N ? • Mg = +2 • N = -3 +2 • Mg + -3 N Mg 3 N 2 • Synthesis: A + B = AB

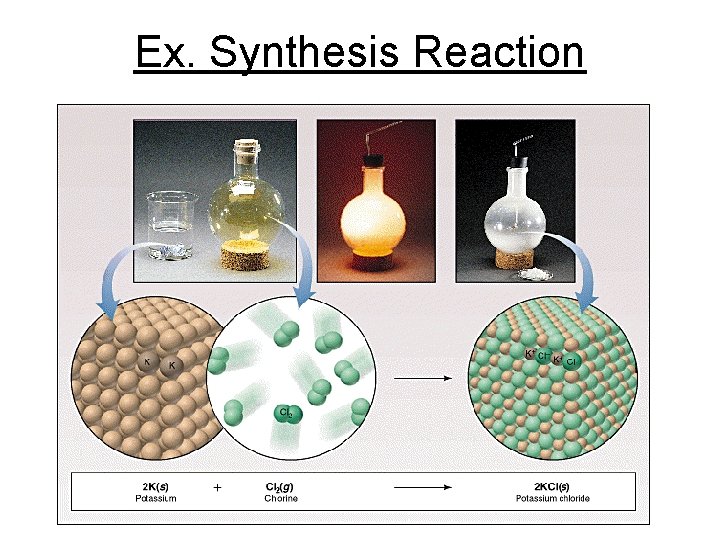
Ex. Synthesis Reaction

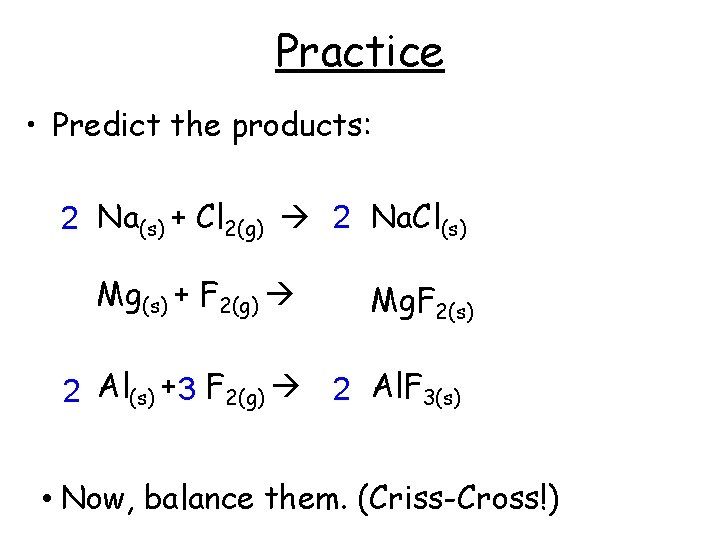
Practice • Predict the products: 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Mg(s) + F 2(g) Mg. F 2(s) 2 Al(s) + 3 F 2(g) 2 Al. F 3(s) • Now, balance them. (Criss-Cross!)
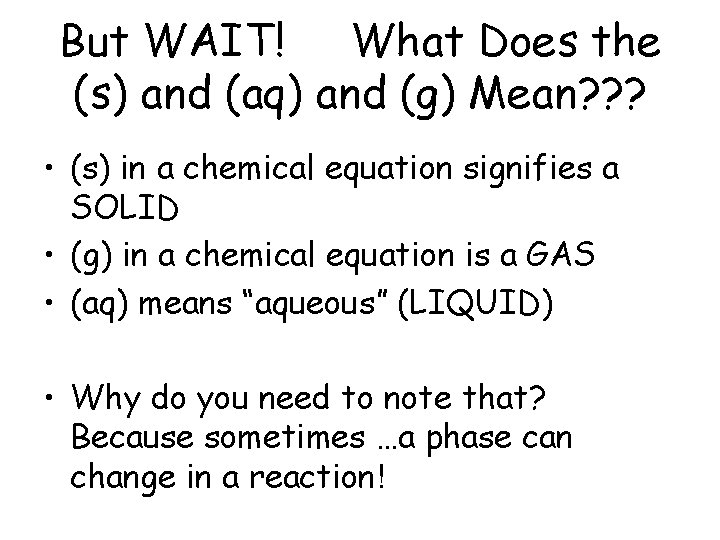
But WAIT! What Does the (s) and (aq) and (g) Mean? ? ? • (s) in a chemical equation signifies a SOLID • (g) in a chemical equation is a GAS • (aq) means “aqueous” (LIQUID) • Why do you need to note that? Because sometimes …a phase can change in a reaction!

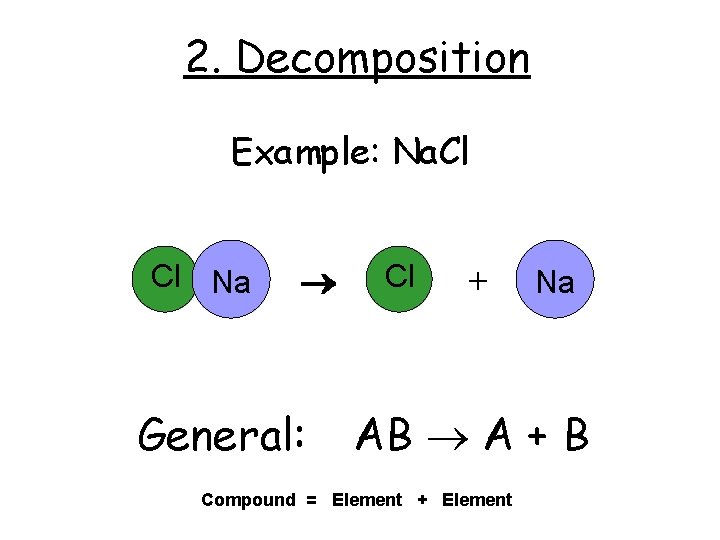
2. Decomposition Example: Na. Cl Cl Na General: Cl + Na AB A + B Compound = Element + Element

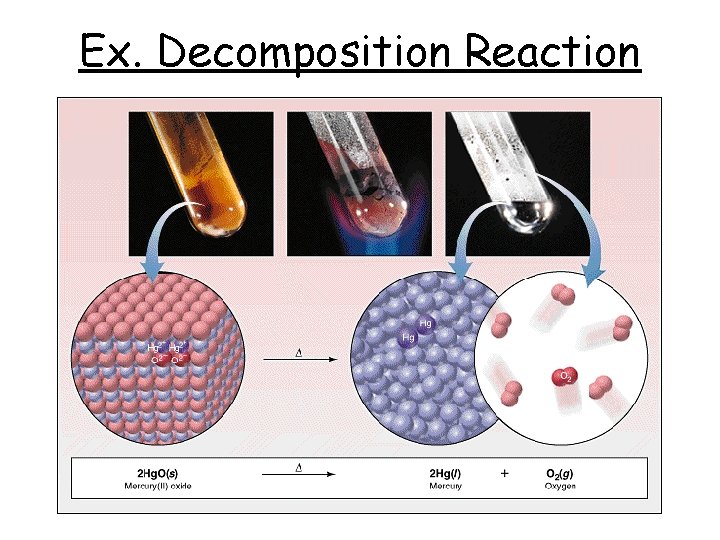
Ex. Decomposition Reaction

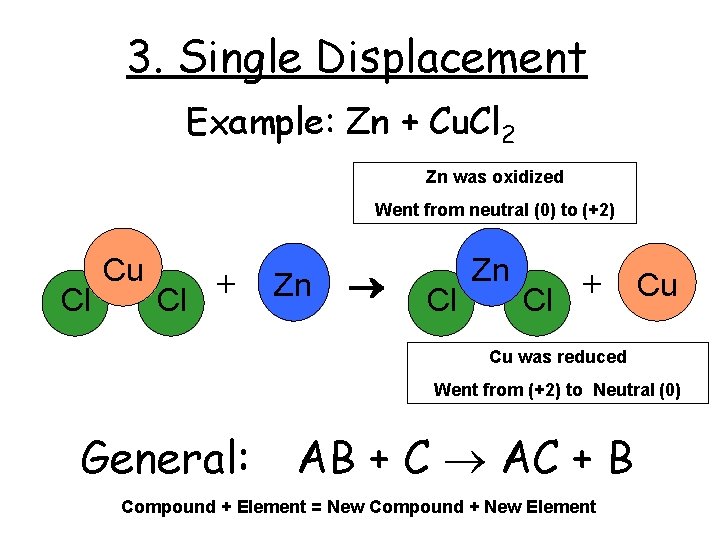
3. Single Displacement Example: Zn + Cu. Cl 2 Zn was oxidized Went from neutral (0) to (+2) Cl Cu + Cl Zn + Cu Cl Cu was reduced Went from (+2) to Neutral (0) General: AB + C AC + B Compound + Element = New Compound + New Element

But WAIT! What do “oxidized” and ”reduced” Mean? • • Remember? LEO the lion says GER Lose electrons = oxidation = LEO Gain electrons = reduction = GER • Metals LOSE electrons • Non-metals GAIN electrons

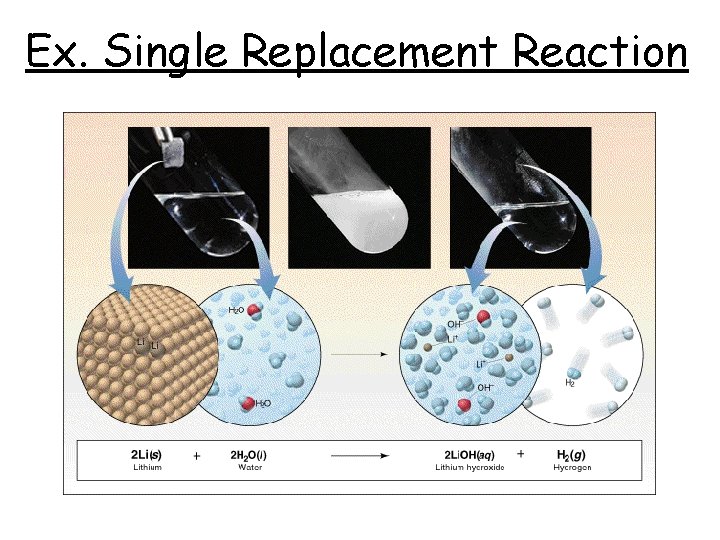
Ex. Single Replacement Reaction

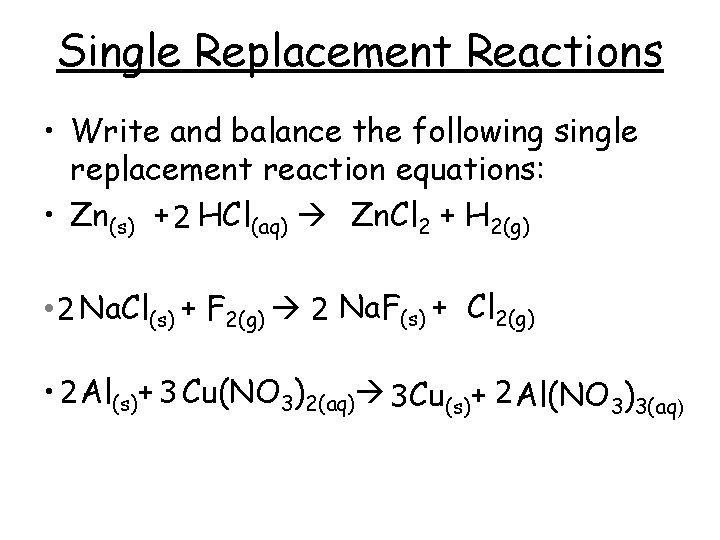
Single Replacement Reactions • Write and balance the following single replacement reaction equations: • Zn(s) + 2 HCl(aq) Zn. Cl 2 + H 2(g) • 2 Na. Cl(s) + F 2(g) 2 Na. F(s) + Cl 2(g) • 2 Al(s)+ 3 Cu(NO 3)2(aq) 3 Cu(s)+ 2 Al(NO 3)3(aq)

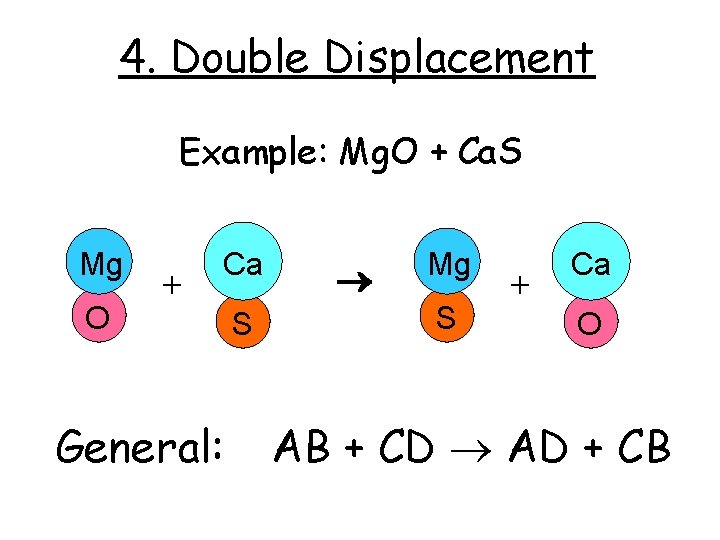
4. Double Displacement Example: Mg. O + Ca. S Mg O + Ca General: S Mg S + Ca O AB + CD AD + CB

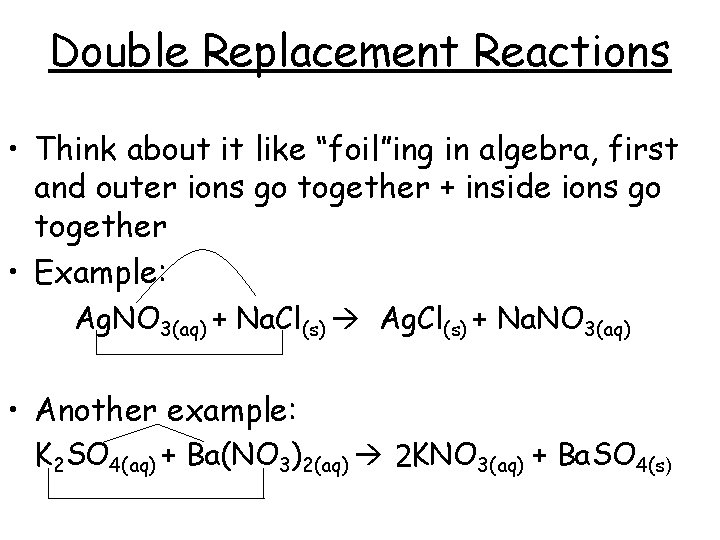
Double Replacement Reactions • Think about it like “foil”ing in algebra, first and outer ions go together + inside ions go together • Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) • Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) 2 KNO 3(aq) + Ba. SO 4(s)

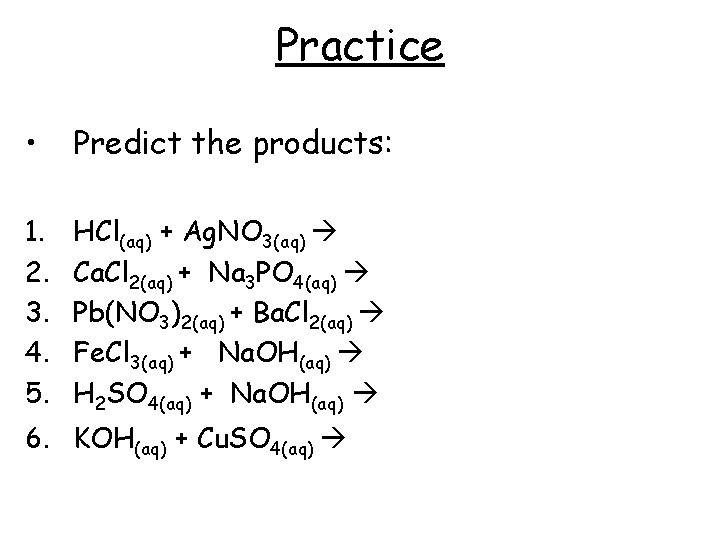
Practice • Predict the products: 1. 2. 3. 4. 5. HCl(aq) + Ag. NO 3(aq) Ca. Cl 2(aq) + Na 3 PO 4(aq) Pb(NO 3)2(aq) + Ba. Cl 2(aq) Fe. Cl 3(aq) + Na. OH(aq) H 2 SO 4(aq) + Na. OH(aq) 6. KOH(aq) + Cu. SO 4(aq)

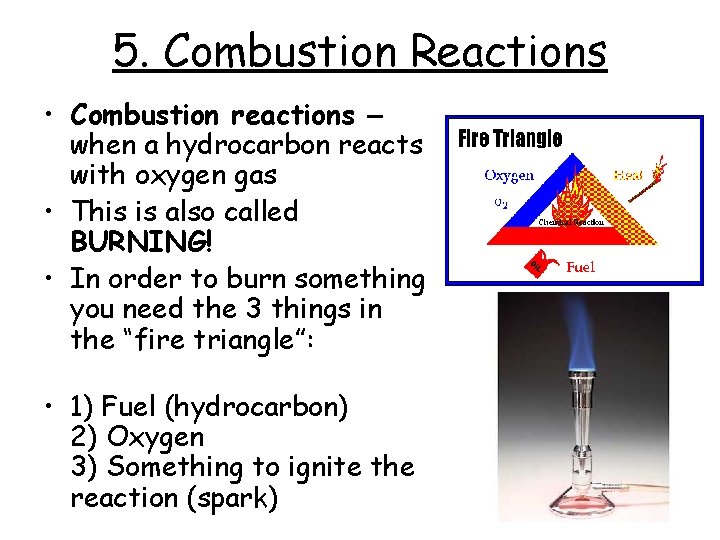
5. Combustion Reactions • Combustion reactions – when a hydrocarbon reacts with oxygen gas • This is also called BURNING! • In order to burn something you need the 3 things in the “fire triangle”: • 1) Fuel (hydrocarbon) 2) Oxygen 3) Something to ignite the reaction (spark)

Combustion Reactions • In general: Cx. Hy + O 2 CO 2 + H 2 O • Products are ALWAYS CARBON DIOXIDE AND WATER! • Combustion is used to heat homes and run automobiles (octane, as in gasoline, is a hydrocarbon: C 8 H 18 )

Mixed Practice • State the type of reaction & predict the products (try to balance the equation!) 1. 2. 3. 4. 5. Ba. Cl 2 + H 2 SO 4 C 6 H 12 + O 2 Zn + Cu. SO 4 Cs + Br 2 Fe. CO 3
 Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Synthesis reaction predicting products
Synthesis reaction predicting products Combination reaction equation
Combination reaction equation Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Reactants and products
Reactants and products Predicting single replacement reactions
Predicting single replacement reactions How to identify a precipitate
How to identify a precipitate How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Single displacement activity series
Single displacement activity series Redox table
Redox table Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Predicting products
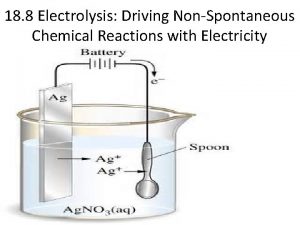
Predicting products Predicting products of electrolysis
Predicting products of electrolysis Types of redox reactions
Types of redox reactions Identify types of reactions
Identify types of reactions 4 types of chemical reactions
4 types of chemical reactions