18 8 Electrolysis Driving NonSpontaneous Chemical Reactions with






- Slides: 11

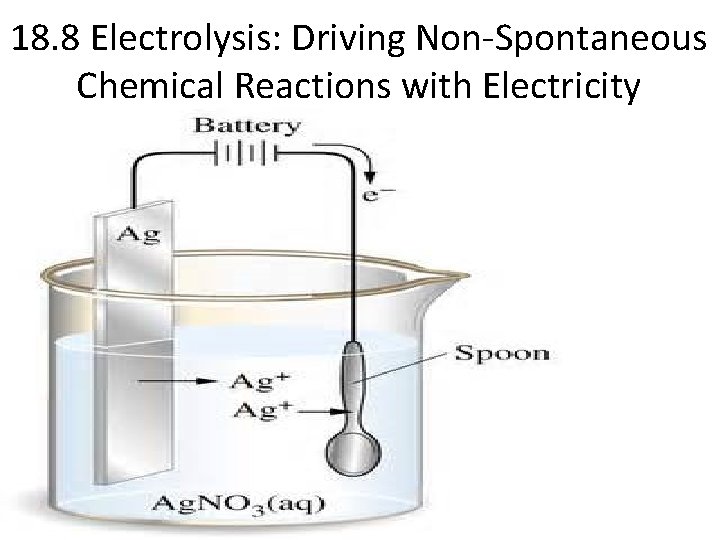
18. 8 Electrolysis: Driving Non-Spontaneous Chemical Reactions with Electricity

Electrolytic Cell: The Process of Electrolysis • An electrolytic cell differs from a voltaic (galvanic) cell in that in an electrolytic cell, an external power source must be used to force a non-spontaneous reaction. • In an electrolytic cell, electrical energy is needed to produce chemical energy (the exact opposite of what happens in a voltaic cell). • The process of driving a non-spontaneous reaction using electrical current is known as electrolysis.

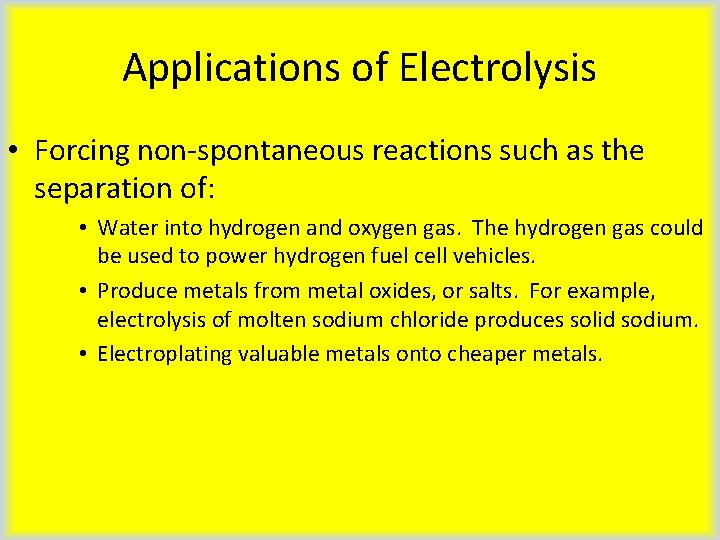
Applications of Electrolysis • Forcing non-spontaneous reactions such as the separation of: • Water into hydrogen and oxygen gas. The hydrogen gas could be used to power hydrogen fuel cell vehicles. • Produce metals from metal oxides, or salts. For example, electrolysis of molten sodium chloride produces solid sodium. • Electroplating valuable metals onto cheaper metals.

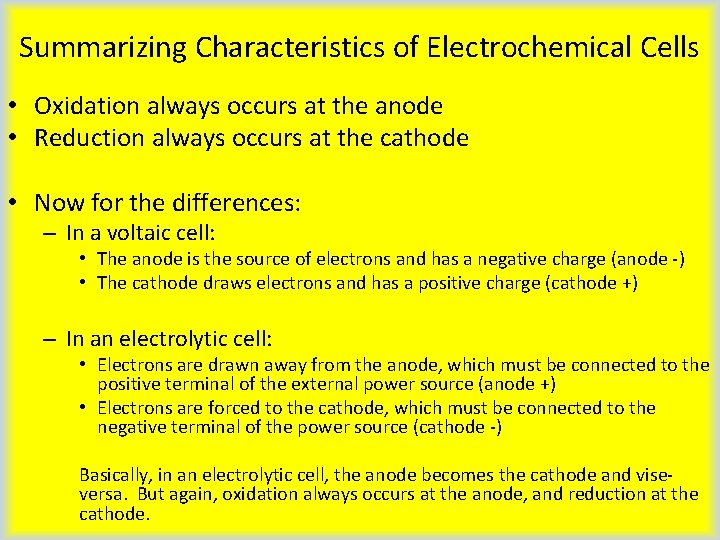
Summarizing Characteristics of Electrochemical Cells • Oxidation always occurs at the anode • Reduction always occurs at the cathode • Now for the differences: – In a voltaic cell: • The anode is the source of electrons and has a negative charge (anode -) • The cathode draws electrons and has a positive charge (cathode +) – In an electrolytic cell: • Electrons are drawn away from the anode, which must be connected to the positive terminal of the external power source (anode +) • Electrons are forced to the cathode, which must be connected to the negative terminal of the power source (cathode -) Basically, in an electrolytic cell, the anode becomes the cathode and viseversa. But again, oxidation always occurs at the anode, and reduction at the cathode.

Predicting Products of Electrolysis of: • Molten salts: – During the electrolysis of a molten salt the _______ would be oxidized and the _______ would be reduced. (Choices are: cation and anion). Anion would be oxidized and the cation would be reduced. (remember, the only species present when a salt is molten are ions. ) • Mixtures of cations and anions: – What if a mixture molten salts is present, so that there may be more than one cation and more than one anion…which one would be oxidized and which would be reduced? • To answer this question, we need to look at the table of standard electrode potentials. The cation with the more positive (higher) voltage will be more easily reduced, and the anion with the more negative (lower) voltage would be more easily oxidized. • If there was a mixture of molten Na. Cl(l) and KCl(l) which cation would be reduced at the electrode? – Na, because it has a more positive (or less negative) electrode potential. • If there was a mixture of molten Na. Cl(l) and Na. Br(l), which anion would be more easily oxidized at the electrode? – Br, because it has a lower cell potential. – N. I. O. and P. I. R. (Negative is oxidized and positive is reduced)

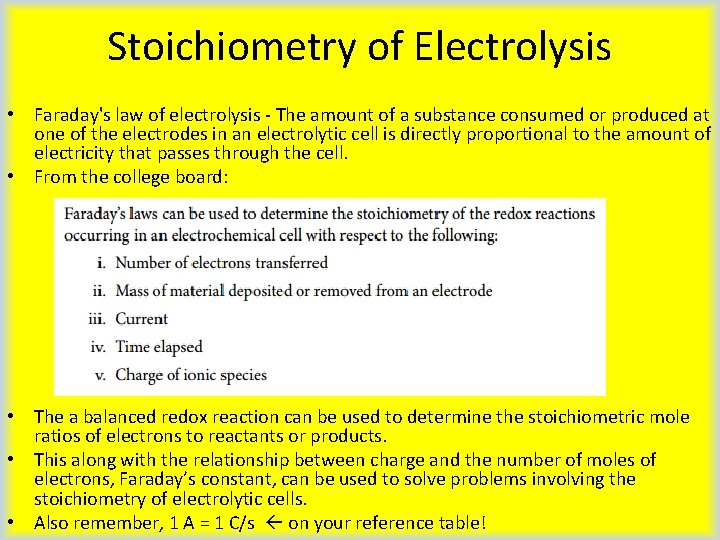
Stoichiometry of Electrolysis • Faraday's law of electrolysis - The amount of a substance consumed or produced at one of the electrodes in an electrolytic cell is directly proportional to the amount of electricity that passes through the cell. • From the college board: • The a balanced redox reaction can be used to determine the stoichiometric mole ratios of electrons to reactants or products. • This along with the relationship between charge and the number of moles of electrons, Faraday’s constant, can be used to solve problems involving the stoichiometry of electrolytic cells. • Also remember, 1 A = 1 C/s on your reference table!

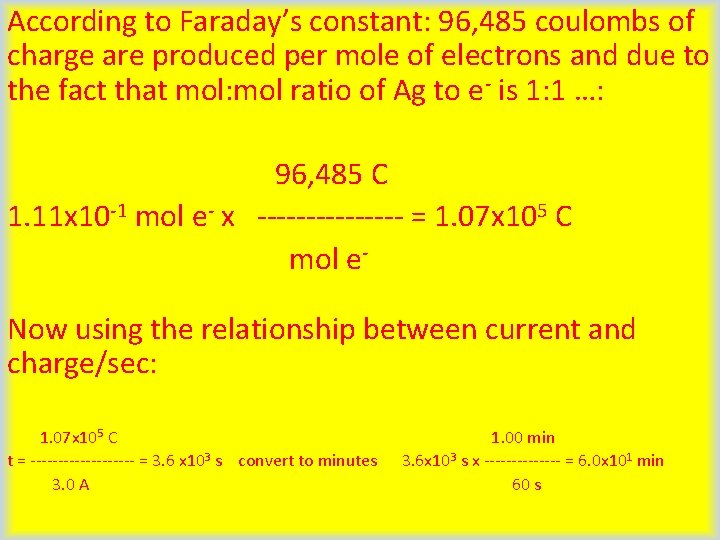
Let’s Try a Practice Problem! Silver can be plated out of a solution containing Ag+ according to the half-reaction: Ag+(aq) + e- Ag(s) How much time (in minutes) would it take to plate 12 g of silver using a current of 3. 0 A? According to the balanced reduction half-reaction above, it takes 1 mol e- to plate 1 mol Ag(s). Using the periodic table, we can see how may grams 1 mol Ag(s) is weighs. 107. 87 g 1 mol x ------ = 107. 87 g 1. 00 mol Now, we can mathematically solve for how may moles of silver are weigh 12 g: 1. 00 mol Ag(s) 12. 0 g Ag(s) x ---------- = 1. 11 x 10 -1 mol Ag(s) 107. 87 g Ag(s) (continued )

According to Faraday’s constant: 96, 485 coulombs of charge are produced per mole of electrons and due to the fact that mol: mol ratio of Ag to e- is 1: 1 …: 96, 485 C 1. 11 x 10 -1 mol e- x -------- = 1. 07 x 105 C mol e. Now using the relationship between current and charge/sec: 1. 07 x 105 C t = ---------- = 3. 6 x 103 s convert to minutes 3. 0 A 1. 00 min 3. 6 x 103 s x ------- = 6. 0 x 101 min 60 s

Predicting Products of Electrolysis (continued) • Aqueous solutions: – Predicting the species that oxidized and that which is reduced in aqueous solution is a little bit more challenging. In aqueous solution, water is present, so we must take into account that water can also either be oxidized or reduced. In order to make the prediction here, we must once again think N. I. O. and P. I. R. and make sure to take water into account. What would be oxidized and what would be reduced during the electrolysis of aqueous Na. I(aq)? (use the following electrode potentials for pure water at room temperature, because pure water at room temp. is not standard: [OH-]=[H+]=1. 0 x 10 -7 M. : Oxidation (anode) = 0. 82 V, and reduction (cathode) = -0. 41 V) The iodide ion is oxidized at the anode, and water would be reduced at the cathode. Which gas would be produced at the cathode? Hydrogen gas!

Let’s Try a Practice Problem! Predict the half-reactions occurring at the anode and cathode for the electrolysis of aqueous Na 2 SO 4? Let’s lay out our options: 2 Na+(aq) + SO 42 -(aq) Sodium can only be reduced and sulfate can only be oxidized. Now let’s compare each to water: Na+(aq) + e- Na(s) Eo = -2. 71 V 2 H 2 O(l) + 2 e- H 2(g) + 2 OH-(aq) E= -0. 41 V Water is reduced at the cathode. SO 42 -(aq) + 4 H+(aq) + 2 e- H 2 SO 4(aq) + H 2 O(l) Eo = 0. 20 V 2 H 2 O(l) O 2(g) + 4 H+(aq) + 4 e- E = 0. 82 V Sulfate is oxidized at the anode.

18. 8 pg. 907 #’s 90, 96 a, 98, & 100 Study for Quiz on Chapter 18
 Nuclear reaction equation
Nuclear reaction equation Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Are nonspontaneous processes impossible
Are nonspontaneous processes impossible Classify each process as spontaneous or nonspontaneous.
Classify each process as spontaneous or nonspontaneous. Redox reaction examples
Redox reaction examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet 5 chemical reactions
5 chemical reactions Solvent in chemical reactions
Solvent in chemical reactions