Principles of Immunochemical Techniques Introduction Immunochemical reactions form
























- Slides: 79

Principles of Immunochemical Techniques

Introduction • Immunochemical reactions form the basis of a diverse range of sensitive and specific clinical assays. • identification and quantitation of specific substances • an antibody is used as a reagent to detect an antigen of interest. • The exquisite specificity and the high affinity of antibodies for specific antigens, coupled with the unique ability of antibodies to cross link antigens,

BASIC CONCEPTS • ANTIBODIES – Immunoglobulins consist of five general classes designated as Ig. G, Ig. A, Ig. M, Ig. D, and Ig. E.

Schematic diagram of Ig. G antibody molecule showing carbohydrate (Cbh). Disulfide bonds (-S-S-). and major fragments produced by proteolytic enzyme treatment (F[ab′]2. Fc, Fab, Fd).

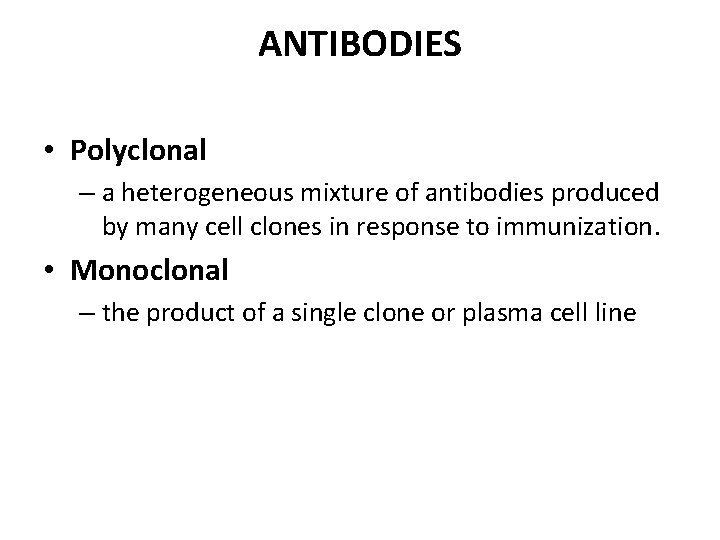
ANTIBODIES • Polyclonal – a heterogeneous mixture of antibodies produced by many cell clones in response to immunization. • Monoclonal – the product of a single clone or plasma cell line

Production of antibodies • Polyclonal antiserum – raised in a normal animal host in response to immunogen administration. • The usual method of production of monoclonal antibodies – Fusing sensitized lymphocytes from the spleens or lymph nodes of mice that have been immunized, with a murine myeloma cell line – Phage display

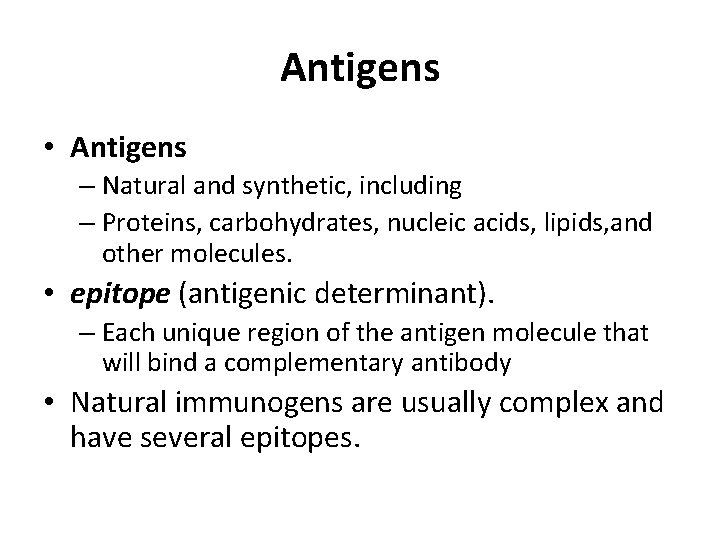
Antigens • Antigens – Natural and synthetic, including – Proteins, carbohydrates, nucleic acids, lipids, and other molecules. • epitope (antigenic determinant). – Each unique region of the antigen molecule that will bind a complementary antibody • Natural immunogens are usually complex and have several epitopes.

• Hapten – a chemically defined determinant that when conjugated to an immunogenic carrier stimulates the synthesis of antibody specific for the hapten

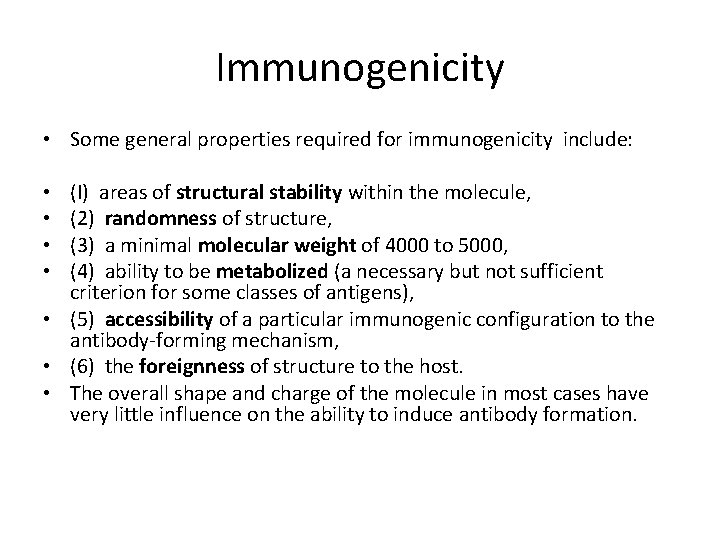
Immunogenicity • Some general properties required for immunogenicity include: (I) areas of structural stability within the molecule, (2) randomness of structure, (3) a minimal molecular weight of 4000 to 5000, (4) ability to be metabolized (a necessary but not sufficient criterion for some classes of antigens), • (5) accessibility of a particular immunogenic configuration to the antibody-forming mechanism, • (6) the foreignness of structure to the host. • The overall shape and charge of the molecule in most cases have very little influence on the ability to induce antibody formation. • •

• The strength or energy of interaction between the antibody and antigen is described by two terms • Affinity – thermodynamic quantity defining the energy of interaction of a single antibody-combining site and its corresponding epitope on the antigen. • Avidity – the overall strength of binding of an antibody and an antigen

ANTIGEN ANTIBODY BINDING • BINDING FORCES – van der Waals-London dipole-dipole interaction, hydrophobic interaction, and ionic coulombic bonding • Distance, Temperature (van der Waals) • aqueous or other polar solutions (hydrophobic) • dielectric constant (aqueous solutions / added salt) – layer of charged particles tend to shield • p. H, temperature, and ionic strength of the reaction medium should influence the binding of antigen and antibody.

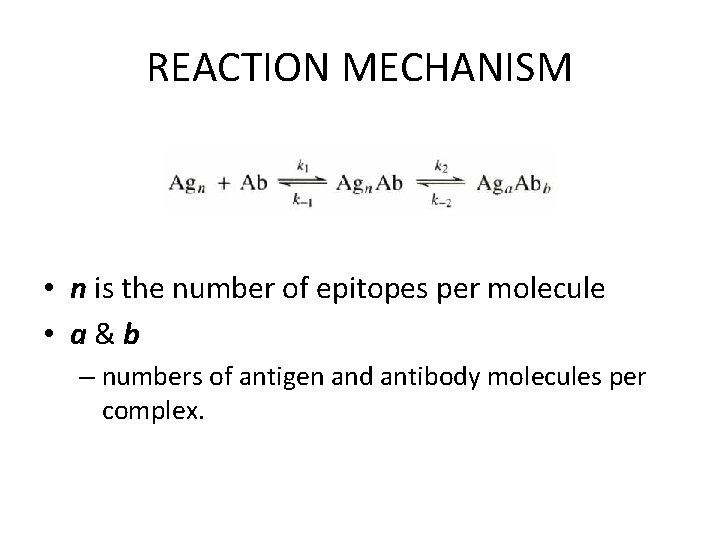
REACTION MECHANISM • n is the number of epitopes per molecule • a&b – numbers of antigen and antibody molecules per complex.

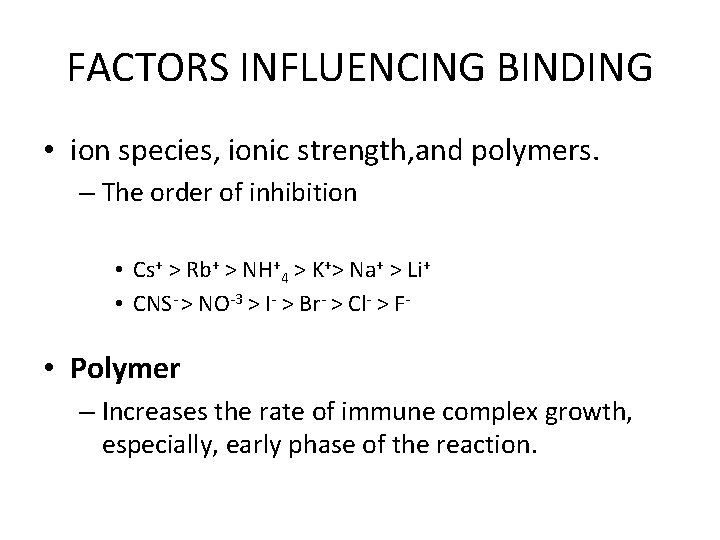
FACTORS INFLUENCING BINDING • ion species, ionic strength, and polymers. – The order of inhibition • Cs+ > Rb+ > NH+4 > K+> Na+ > Li+ • CNS- > NO-3 > I- > Br- > Cl- > F- • Polymer – Increases the rate of immune complex growth, especially, early phase of the reaction.

FACTORS INFLUENCING BINDING

TYPES OF REACTIONS • The precipitin reaction • at a solid-liquid interface.

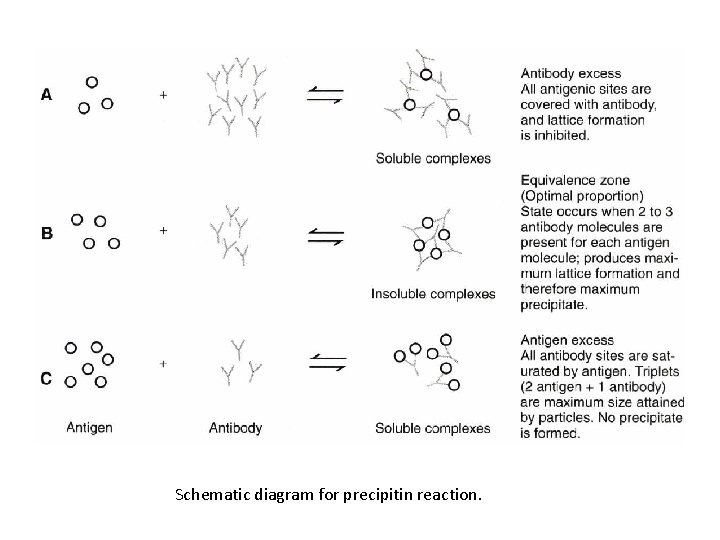
Schematic diagram for precipitin reaction.

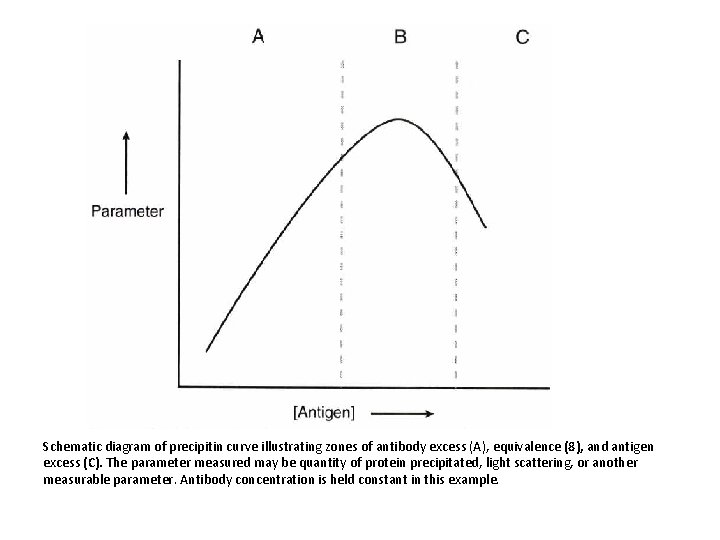
Schematic diagram of precipitin curve illustrating zones of antibody excess (A), equivalence (8), and antigen excess (C). The parameter measured may be quantity of protein precipitated, light scattering, or another measurable parameter. Antibody concentration is held constant in this example.

Reactions at a Solid-Liquid Interface • the antigen or antibody of interest is bound to a solid phase, – such as a cell membrane, or to a synthetic particle (polystyrene or cellulose) • provide lower detection limits – additional low-avidity antibody binding occurs

QUALITATIVE METHODS • Passive gel diffusion, • Immunoelectrophoresis (IEP), • Western and dot blotting. • Antigen antibody ratio, salt concentration, and polymer enhancement have the same influence on the antigen antibody reaction in gels as they have on reactions in solution.

• Passive diffusion – simple diffusion – double diffusion. • a concentration gradient is established for both reactants (antigen and antibody). • Ouchterlony method.

Double immunodiffusion in two dimensions by the Ouchterlony technique. A, Reaction of identity: B, reaction of non identity; C, reaction of partial identity; D, scheme for spur formation. Ag, Antigen; Ab, antibody.

• the position of the precipitin band is in part a function molecular masses of both antigen and antibody. • a negative reaction does not necessarily imply absence of antibody or antigen. – too small for the detection limit – antibody may be nonprecipitating.

Immunoelectrophoresis (IEP) • for the study of antigen mixtures, • the evaluation of the specificity of antiserum, • the evaluation of human gammopathies. – applied to the evaluation of human myeloma proteins. • Electrophoretic separation • Formation of precipitin arcs – shape and position are characteristic of the individual separated proteins in the specimen – By comparison with a known control separated on the same plate,

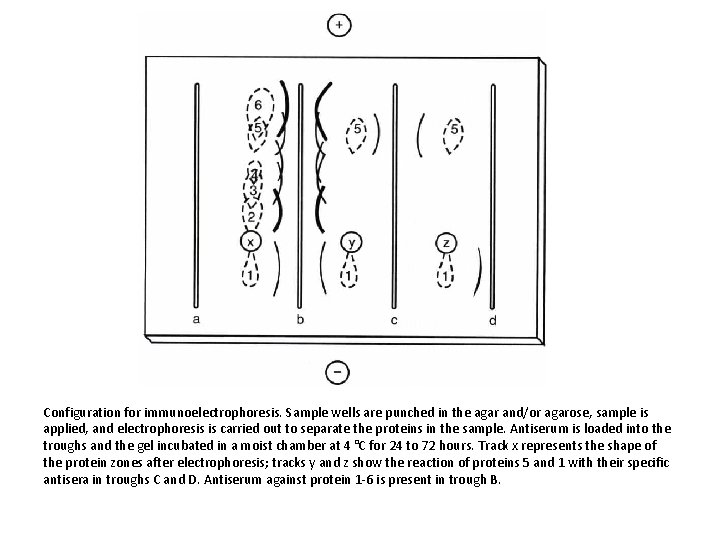
Configuration for immunoelectrophoresis. Sample wells are punched in the agar and/or agarose, sample is applied, and electrophoresis is carried out to separate the proteins in the sample. Antiserum is loaded into the troughs and the gel incubated in a moist chamber at 4 °C for 24 to 72 hours. Track x represents the shape of the protein zones after electrophoresis; tracks y and z show the reaction of proteins 5 and 1 with their specific antisera in troughs C and D. Antiserum against protein 1 -6 is present in trough B.

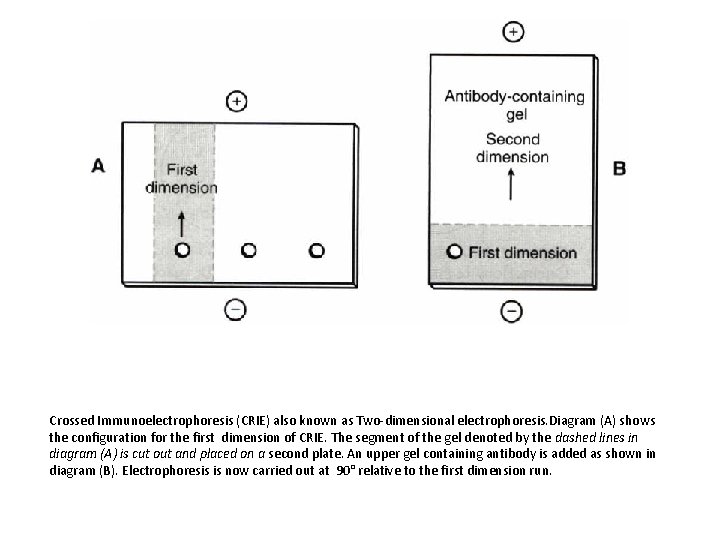
Crossed Immunoelectrophoresis (CRIE) also known as Two-dimensional electrophoresis. Diagram (A) shows the configuration for the first dimension of CRIE. The segment of the gel denoted by the dashed lines in diagram (A) is cut out and placed on a second plate. An upper gel containing antibody is added as shown in diagram (B). Electrophoresis is now carried out at 90° relative to the first dimension run.

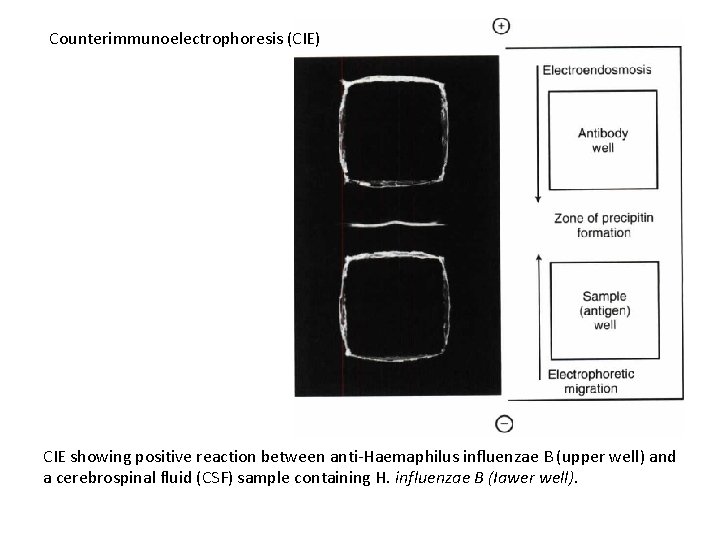
Counterimmunoelectrophoresis (CIE) CIE showing positive reaction between anti-Haemaphilus influenzae B (upper well) and a cerebrospinal fluid (CSF) sample containing H. influenzae B (Iawer well).

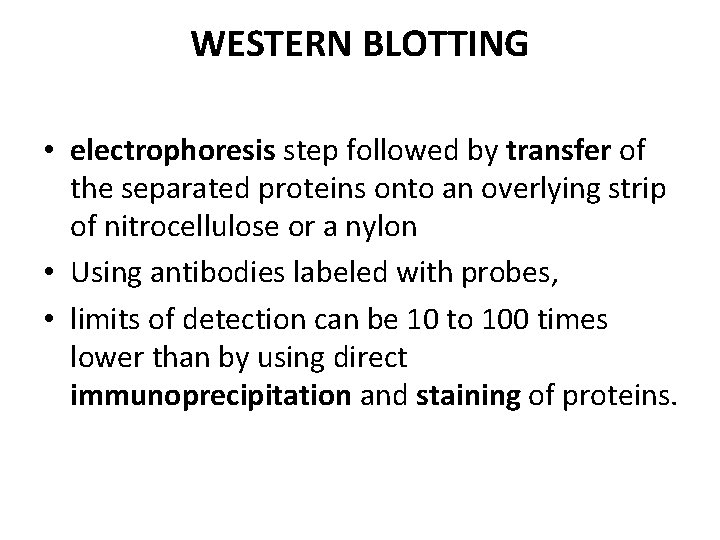
WESTERN BLOTTING • electrophoresis step followed by transfer of the separated proteins onto an overlying strip of nitrocellulose or a nylon • Using antibodies labeled with probes, • limits of detection can be 10 to 100 times lower than by using direct immunoprecipitation and staining of proteins.

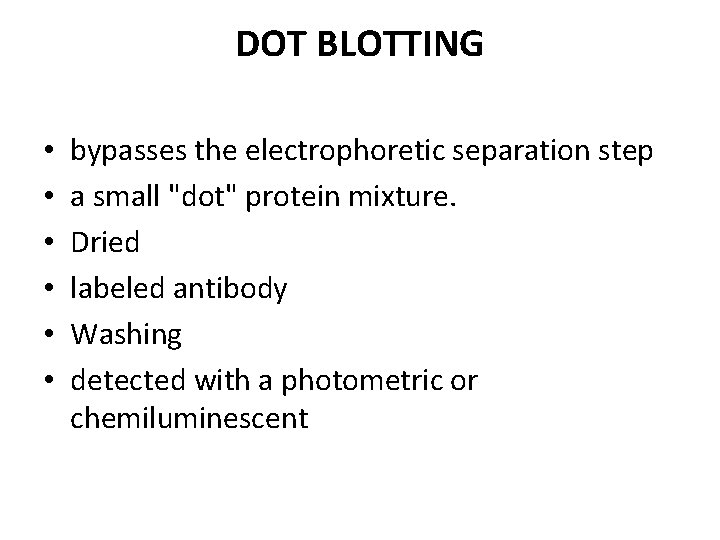
DOT BLOTTING • • • bypasses the electrophoretic separation step a small "dot" protein mixture. Dried labeled antibody Washing detected with a photometric or chemiluminescent

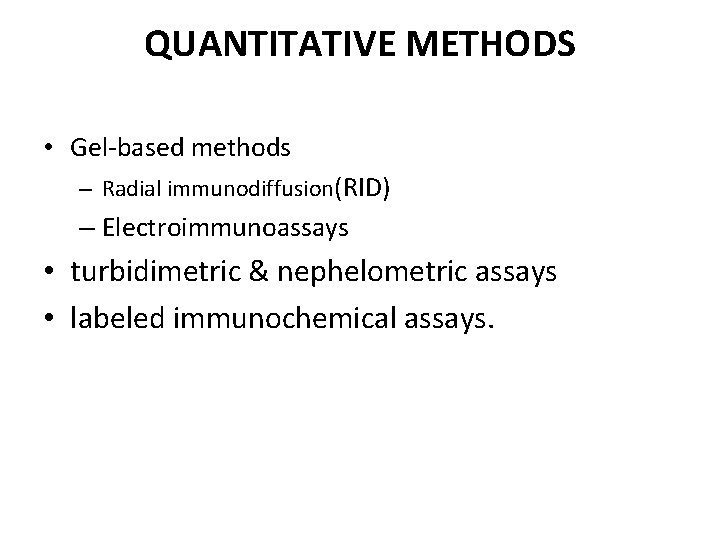
QUANTITATIVE METHODS • Gel-based methods – Radial immunodiffusion(RID) – Electroimmunoassays • turbidimetric & nephelometric assays • labeled immunochemical assays.

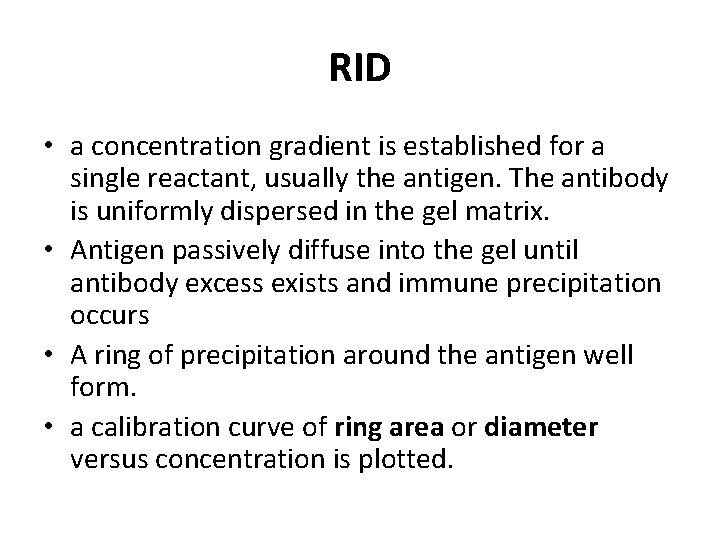
RID • a concentration gradient is established for a single reactant, usually the antigen. The antibody is uniformly dispersed in the gel matrix. • Antigen passively diffuse into the gel until antibody excess exists and immune precipitation occurs • A ring of precipitation around the antigen well form. • a calibration curve of ring area or diameter versus concentration is plotted.

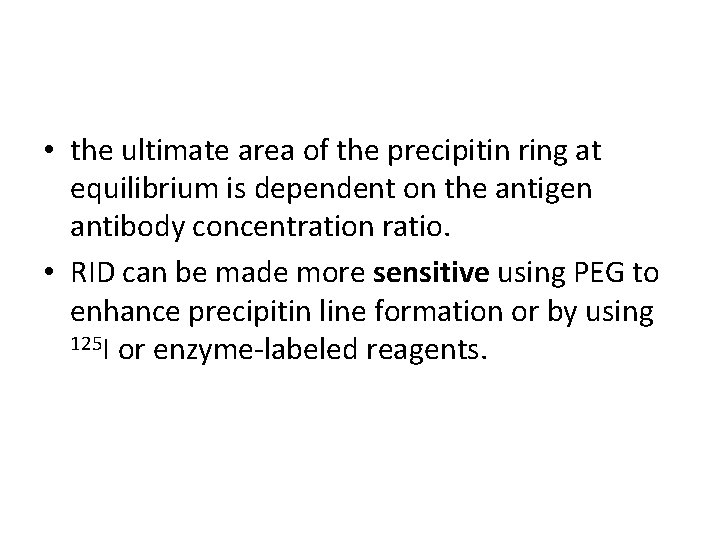
• the ultimate area of the precipitin ring at equilibrium is dependent on the antigen antibody concentration ratio. • RID can be made more sensitive using PEG to enhance precipitin line formation or by using 125 I or enzyme-labeled reagents.

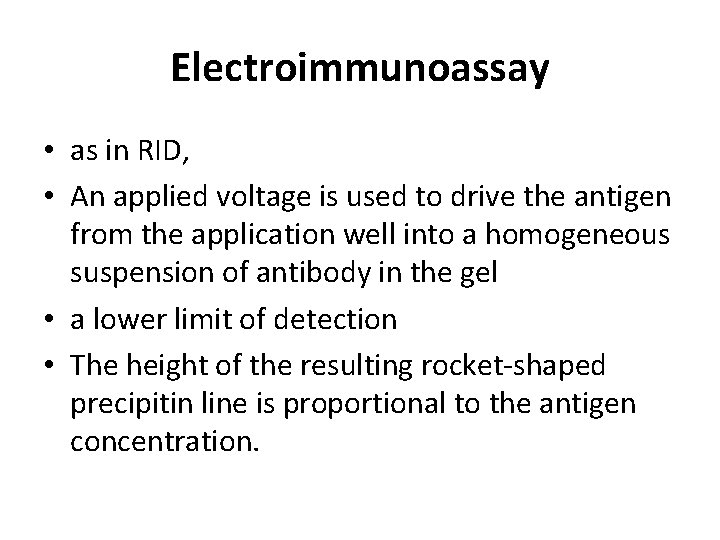
Electroimmunoassay • as in RID, • An applied voltage is used to drive the antigen from the application well into a homogeneous suspension of antibody in the gel • a lower limit of detection • The height of the resulting rocket-shaped precipitin line is proportional to the antigen concentration.

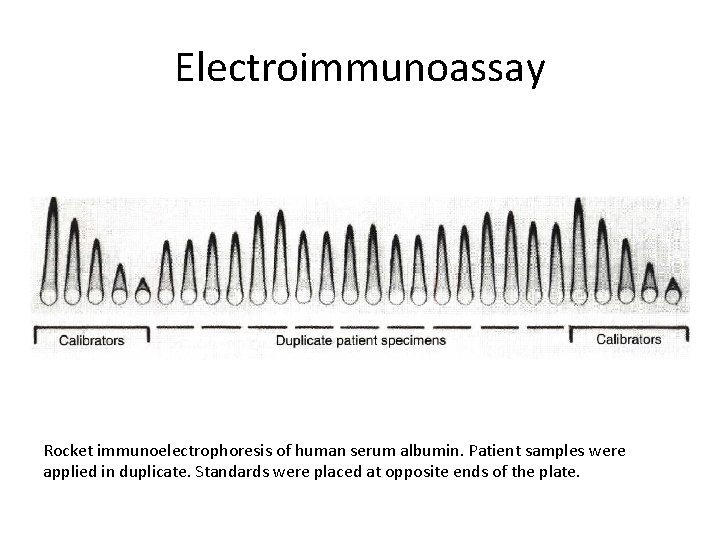
Electroimmunoassay Rocket immunoelectrophoresis of human serum albumin. Patient samples were applied in duplicate. Standards were placed at opposite ends of the plate.

Electroimmunoassay • Electroimmunoassay methods produce the best results with antigens having a strong anodic mobility and intermediate to low molecular weight. • p. H 8. 6 (the most common p. H used for the method)

TURBIDIMETRIC & NEPHELOMETRIC ASSAYS • In many clinical laboratories, gel-based methods are restricted to qualitative studies. • Quantitative data are more commonly obtained by turbidimetric and nephelometric methods, radioimmunoassays, enzyme immunoassays (EIAs), and fluorometric immunoassays.

TURBIDIMETRIC & NEPHELOMETRIC ASSAYS • the rate of formation of immune complexes are measured • the reaction between antigen and antibody begins within milliseconds and continues for hours. • Rate assays (Kinetic) – Change (dls/dt) in intensity of scattered light (Is) within the first few minutes of the reaction

TURBIDIMETRIC & NEPHELOMETRIC ASSAYS • Equilibrium(pseudo equilibrium) assays – wait 30 to 60 minutes • Improvement (Turbidimetric & Nephelometric assays) – addition of water-soluble linear polymers • increasing the reaction rate – This allows the use of much lower reactant concentrations – results in a more stable immune complex suspension. – Using a latex-enhanced procedure • for low molecular weight proteins (e. g. , myoglobin, MW 17, 800), assay detection limits can be lowered, based on antibody-coated latex beads.

LABELED IMMUNOCHEMICAL ASSAYS • The previously discussed methods rely on evaluating the immune complex formation as an index of antigen antibody reaction. • the initial binding of the antibody and antigen has been used with labeled antigens and antibodies

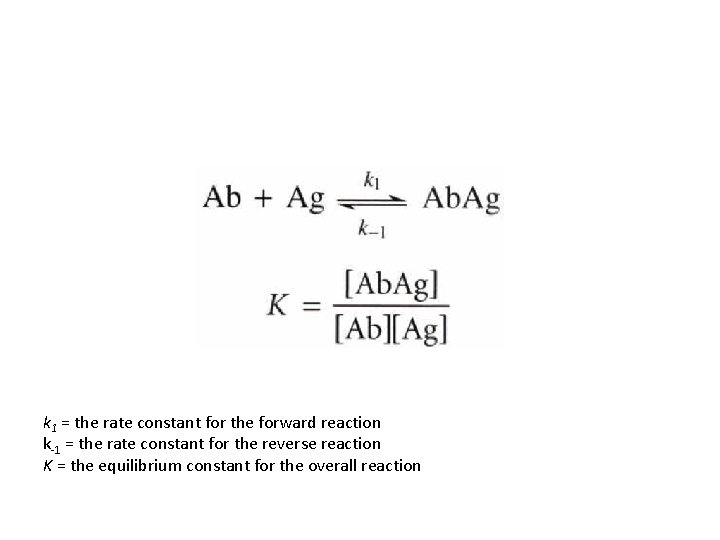
k 1 = the rate constant for the forward reaction k-1 = the rate constant for the reverse reaction K = the equilibrium constant for the overall reaction

LABELED IMMUNOCHEMICAL ASSAYS • the concentration of Ab, Ag, and Ab. Ag will be dependent on the magnitude of k 1 and k-1 • For polyclonal antiserum, the average avidity of the antibody populations will determine K (typically 108 to 1010 L/mol) • the magnitude of k 1 in comparison with k-1 will determine the ultimate limit of detection attainable with a given antibody population.

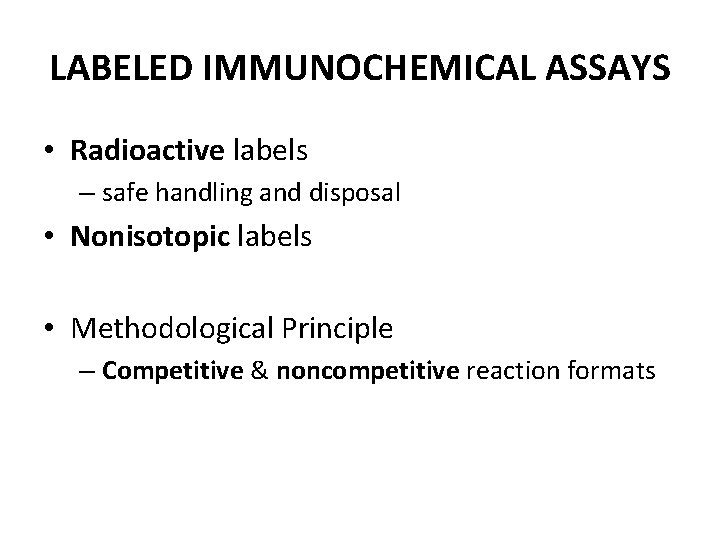
LABELED IMMUNOCHEMICAL ASSAYS • Radioactive labels – safe handling and disposal • Nonisotopic labels • Methodological Principle – Competitive & noncompetitive reaction formats


Immunoassay designs. L, Label.

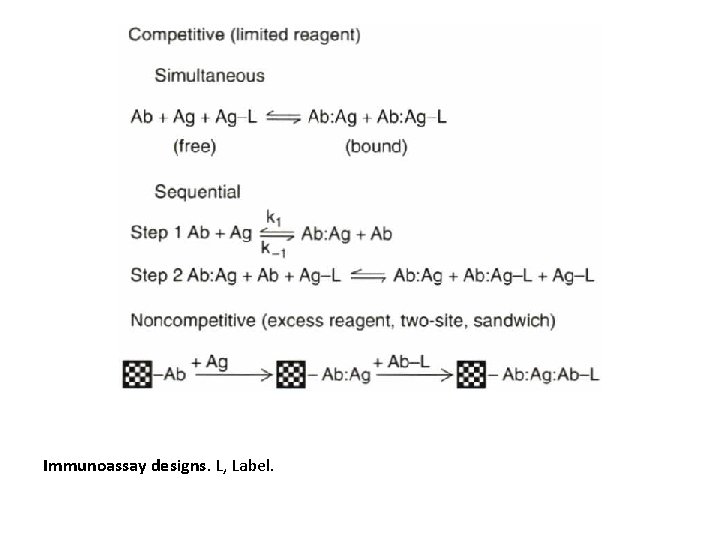
A schematic diagram of the dose-response curve for a typical immunoassay. The analytically useful portion of the curve is bracketed by points a and b.

• sequential immunoassay has lower detection limit compared with that of a simultaneous assay, provided k 1 >> k-1


• In noncompetitive assays, the capture and labeled antibody can be either polyclonal or monoclonal. • If monoclonal antibodies having specificity for distinct epitopes are used, it is possible to incubate the sample and conjugate simultaneously with the capture antibody, thus simplifying the assay protocol.

• Noncompetitive immunoassays can be performed in either a simultaneous or sequential mode. • Hook effect (simultaneous mode) – a situation can occur in which a high concentration of analyte can saturate both the capture and labeled antibodies. • the assay response drops – reduces the number of complexes formed and produces a falsely low result.

• Prone to Hook effect – Assays for analytes for which the normal pathological concentration range is very wide (e. g. , h. CG, AFP) • Dilutions • Sequential assay format

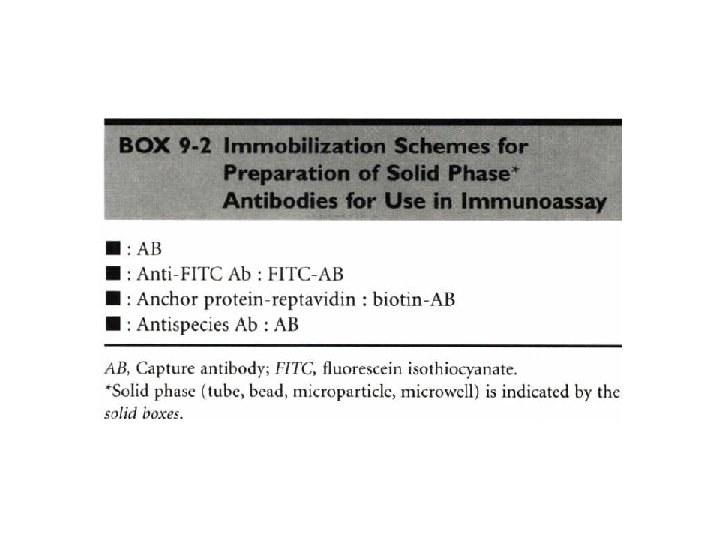
Heterogeneous Vs Homogeneous lmmunochemical Assays • Immunochemical assays that require a separation of the free from the bound label are termed heterogeneous • The most widely used of these techniques are precipitation and liquid phase and solid phase adsorption.


Homogeneous Assays • In this type of assay, the activity of the label attached to the antigen is directly modulated by antibody binding, with the magnitude of the modulation being proportional to the concentration of the antigen or antibody being measured. • technically easier and faster

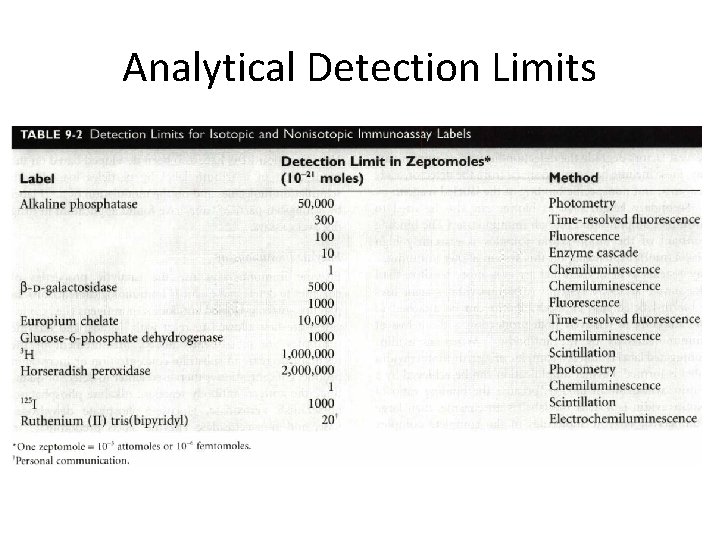
Analytical Detection Limits

has low specific activity compared with enzyme labels and chemiluminescent and fluorescent labels • Enzyme labels provide an amplification • 125 I – can be improved by • a chemiluminescent or bioluminescent reaction. • Fluorescent labels – background signal from the detector, assay reagents, and nonspecific binding of the labeled reagent.

• strategies to lower the analytical detection limits of immunoassays – biotin-avidin system • Ag: Ab-biotin: avidin label – use of streptavidin-thyroglobulin conjugates and macromolecular complexes of multiplelabeled thyroglobulin and streptavidin-thyroglobulin

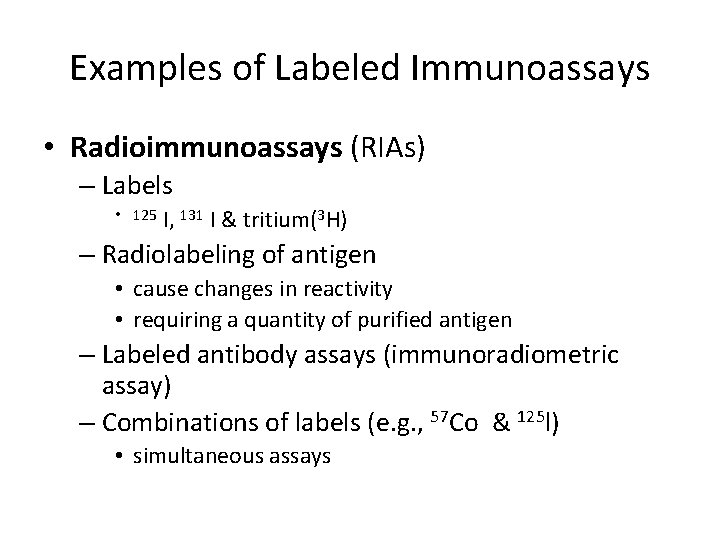
Examples of Labeled Immunoassays • Radioimmunoassays (RIAs) – Labels • 125 I, 131 I & tritium(3 H) – Radiolabeling of antigen • cause changes in reactivity • requiring a quantity of purified antigen – Labeled antibody assays (immunoradiometric assay) – Combinations of labels (e. g. , 57 Co & 125 l) • simultaneous assays

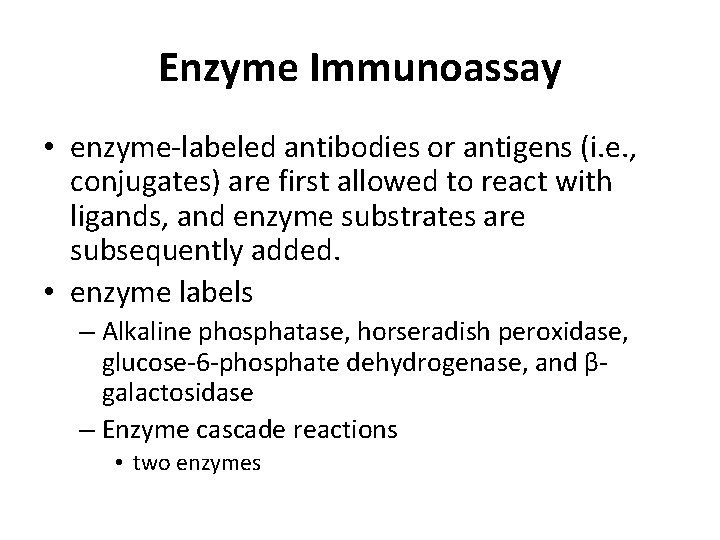
Enzyme Immunoassay • enzyme-labeled antibodies or antigens (i. e. , conjugates) are first allowed to react with ligands, and enzyme substrates are subsequently added. • enzyme labels – Alkaline phosphatase, horseradish peroxidase, glucose-6 -phosphate dehydrogenase, and βgalactosidase – Enzyme cascade reactions • two enzymes

Enzyme Immunoassay • Detection systems – Photometry • versatile, reliable, simple to operate, and relatively inexpensive. – fluorescent & chemiluminescent measurement • High sensitivity

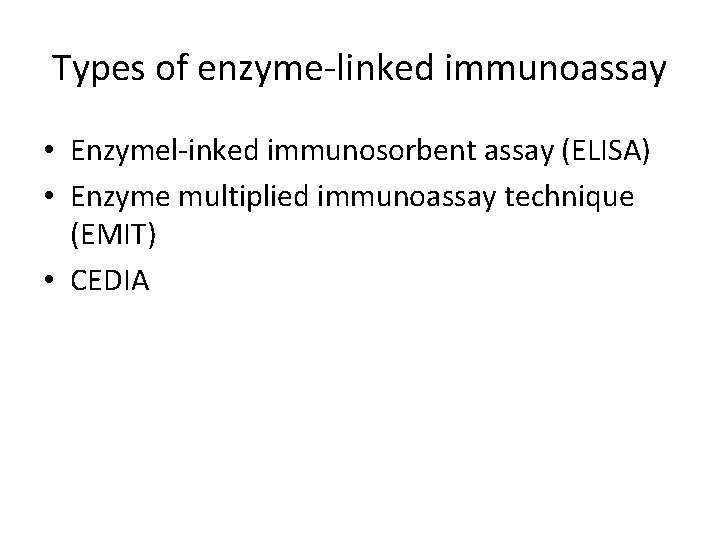
Types of enzyme-linked immunoassay • Enzymel-inked immunosorbent assay (ELISA) • Enzyme multiplied immunoassay technique (EMIT) • CEDIA

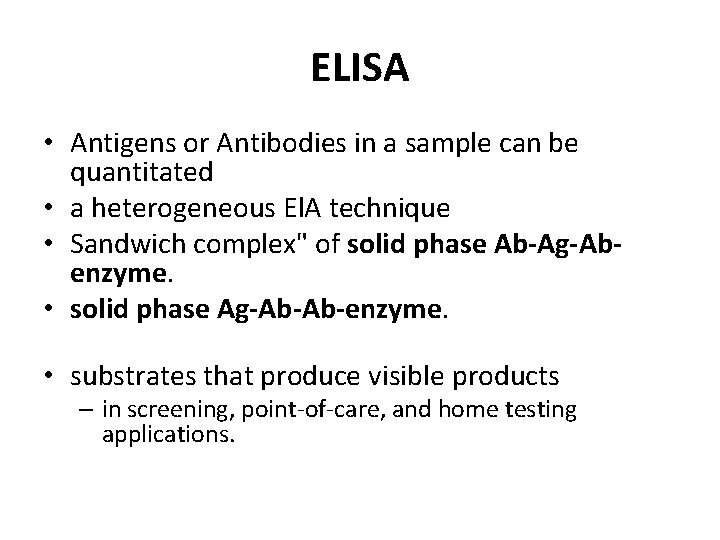
ELISA • Antigens or Antibodies in a sample can be quantitated • a heterogeneous El. A technique • Sandwich complex" of solid phase Ab-Ag-Abenzyme. • solid phase Ag-Ab-Ab-enzyme. • substrates that produce visible products – in screening, point-of-care, and home testing applications.

Enzyme Multiplied Immunoassay Technique. • A homogeneous EIA (does not require a separation step) • Simple to perform, easily automated – used to develop a wide variety of drug, hormone, and metabolite assays

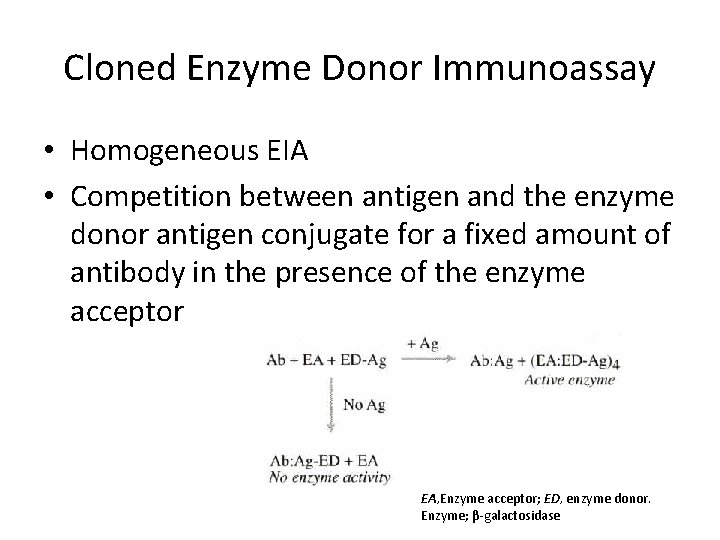
Cloned Enzyme Donor Immunoassay • Homogeneous EIA • Competition between antigen and the enzyme donor antigen conjugate for a fixed amount of antibody in the presence of the enzyme acceptor EA, Enzyme acceptor; ED, enzyme donor. Enzyme; β-galactosidase

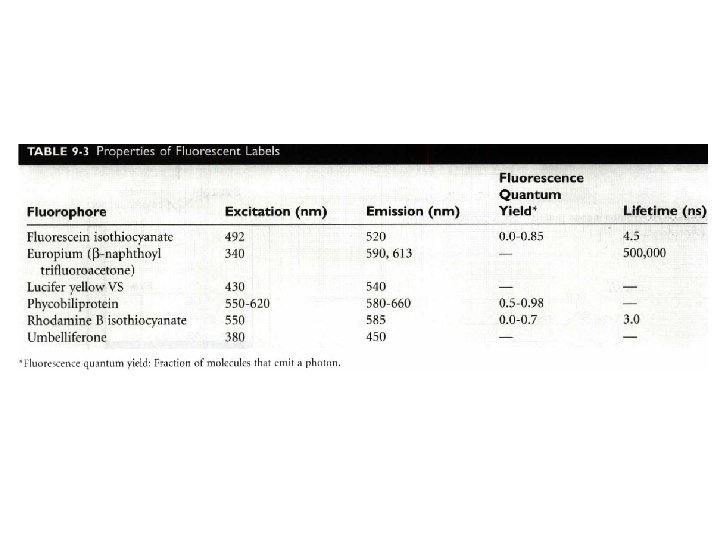
Fluoroimmunoassay • Limitations of this technique – background fluorescence – From drugs, drug metabolites, and protein-bound substances, such as bilirubin – Resolution • use of rare earth (lanthanide) chelates and background rejection (time-resolved) • A europium chelate label is excited by a pulse of excitation light (0. 5 µs), and the long-lived fluorescence emission from the label is measured after a delay (400 to 800µs). By this time, any short-lived background signal has decayed.


Chemiluminescence Immunoassay • Light emission produced during a chemical reaction • Chemiluminescent labels – Isoluminol and acridinium esters • Oxidation of isoluminol by hydrogen peroxide produces a relatively long-lived light emission at 425 nm • Oxidation of an acridinium ester by alkaline hydrogen peroxide produces a rapid flash of light at 429 nm.

Simplified Immunoassays • for use in physicians' offices or the home • Early efforts – based on agglutination and inhibition of agglutination using labeled red blood cells or latex particles in a slide format. • Sandwich immunoassays

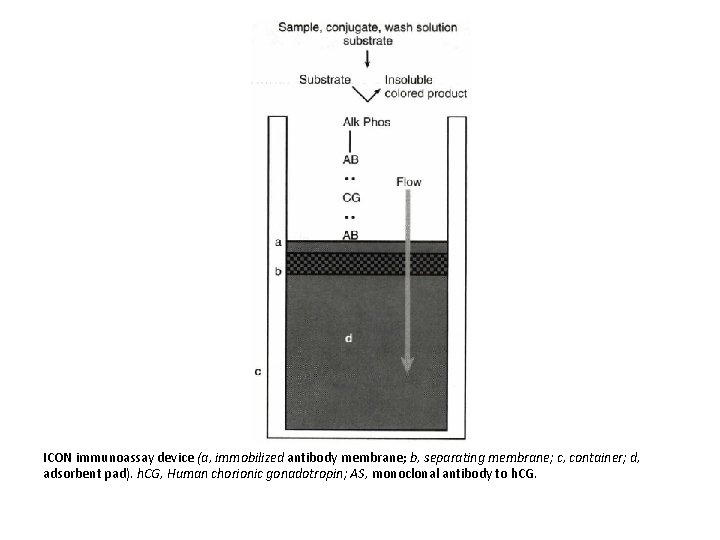
ICON immunoassay device (a, immobilized antibody membrane; b, separating membrane; c, container; d, adsorbent pad). h. CG, Human chorionic gonadotropin; AS, monoclonal antibody to h. CG.

Simplified Immunoassays • Simultaneous multianalyte immunoassays – more analytes are detected in a single assay – based on either • Discrete reaction zones or • combinations of different labels. • Discrete reaction zones – Each test zone consists of antibodies for a specific (drug) immobilized on the membrane surface.

Simplified Immunoassays • Combinations of distinguishable labels – Europium (613 nm, emission lifetime 730 μs) and samarium (643 nm, emission lifetime 50 μs) chelates

• Protein Microarrays – Arrays of hundreds or thousands of micrometersized dots of antigens or antibodies immobilized on the surface of a glass or plastic chip • Facilitates simultaneous multianalyte immunoassays

INTERFERENCES IN IMMUNOASSAYS • Falsely low results – Hook effect, at high antigen concentrations • False negative or false positive results – sample contains antianimal antibodies. – To minimize this problem • Nonimmune serum or Ig. G from the species used to raise the antibodies has been a popular choice for this purpose.

OTHER IMMUNOCHEMICAL TECHNIQUES • Cytochemical • agglutination assays

Immunocytochemistry • labeled antibody reagents as specific probes for protein and peptide antigens – evaluate single cells for synthetic capability – for identification of various cell lines.

AGGLUTINATION ASSAYS • qualitative & quantitative measurement of antigens and antibodies. • the visible clumping of particulates, such as cells and latex particles, is used as an indicator • Ig. M antibodies are more likely to produce complete agglutination – Size & valence • When Ig. G – Chemical enhancement or an antiglobulin agglutination method.

AGGLUTINATION ASSAYS • the ratio of antigen and antibody is critical. – Extremes result in inhibition of aggregation. • incomplete agglutination – a weak antigen antibody reaction – only Ig. G is involved • enhancement – Lowering the ionic strength – Introducing polymeric molecules, such as • polymerized albumin (5% to 30%), dextran, polybrene, polyvinylpyrrolidone, or PEG.

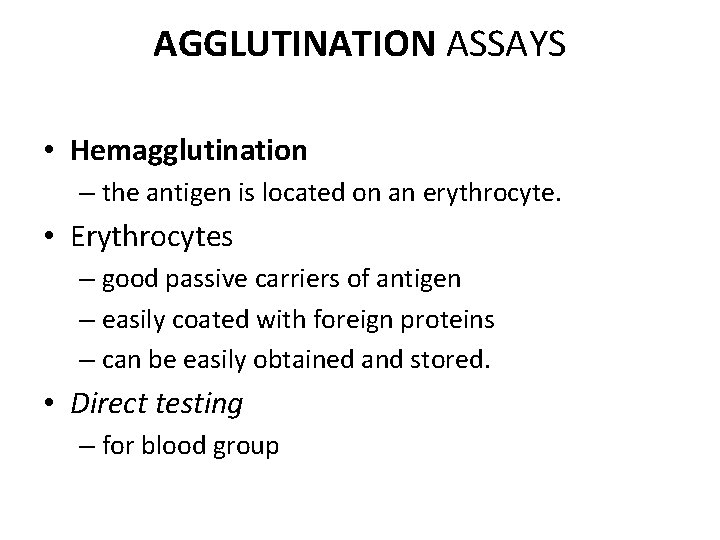
AGGLUTINATION ASSAYS • Hemagglutination – the antigen is located on an erythrocyte. • Erythrocytes – good passive carriers of antigen – easily coated with foreign proteins – can be easily obtained and stored. • Direct testing – for blood group

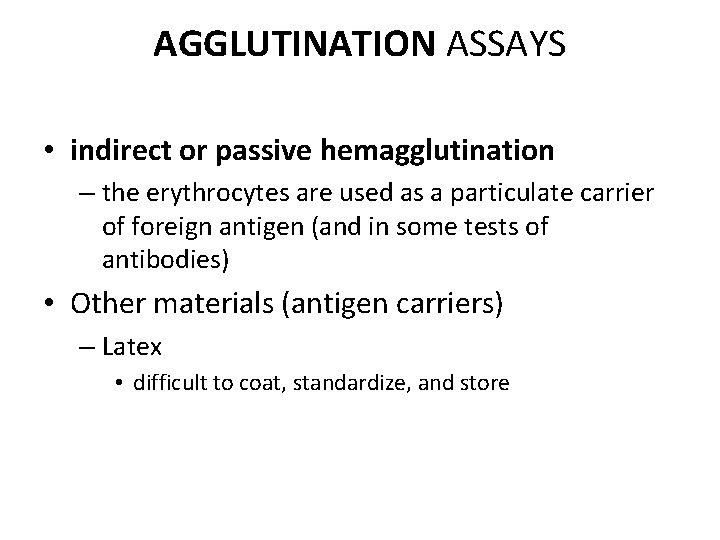
AGGLUTINATION ASSAYS • indirect or passive hemagglutination – the erythrocytes are used as a particulate carrier of foreign antigen (and in some tests of antibodies) • Other materials (antigen carriers) – Latex • difficult to coat, standardize, and store

AGGLUTINATION ASSAYS • Hemagglutination inhibition – the ability of antigens, haptens, or other substances to specifically inhibit hemagglutination of sensitized (coated) cells by antibody is determined.

• In general, – the agglutination methods are quite sensitive but are not as quantitative as other immunochemical methods • Nonisotopic immunoassays, – as convenient as agglutination, replacing agglutination methods
 Principles of immunochemical reaction
Principles of immunochemical reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Redox reaction example
Redox reaction example Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Are ionic compounds metals or nonmetals
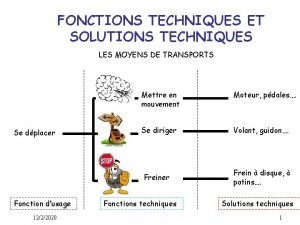
Are ionic compounds metals or nonmetals Fonctions techniques
Fonctions techniques Present continuous tense interrogative negative sentences
Present continuous tense interrogative negative sentences Desuggestopedia principles
Desuggestopedia principles Principles and techniques of fundraising
Principles and techniques of fundraising Principles and techniques of fundraising
Principles and techniques of fundraising Compilers: principles, techniques, and tools
Compilers: principles, techniques, and tools Principles & techniques of electrofishing
Principles & techniques of electrofishing Purpose of community bag
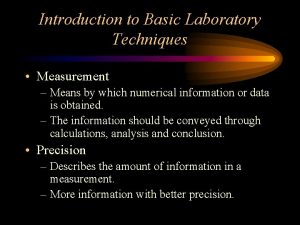
Purpose of community bag Basic laboratory techniques introduction
Basic laboratory techniques introduction Introduction to sql programming techniques
Introduction to sql programming techniques Introduction to sql programming techniques
Introduction to sql programming techniques Introduction of principles of marketing
Introduction of principles of marketing Henri fayol 14 principles of management introduction
Henri fayol 14 principles of management introduction What is the cost principle
What is the cost principle Haccp meaning
Haccp meaning Introduction to machinery principles
Introduction to machinery principles Homeostasis animals examples
Homeostasis animals examples Basic principles of animal form and function
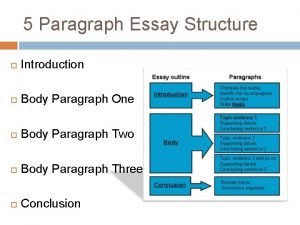
Basic principles of animal form and function Body paragraph structure
Body paragraph structure Fast reactions
Fast reactions Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Unit 5 chemical reactions
Unit 5 chemical reactions Redox rules
Redox rules Types of reactions
Types of reactions 5 reaction types
5 reaction types Single replacement reaction examples
Single replacement reaction examples Types of reactions chemistry
Types of reactions chemistry Chemical reaction types
Chemical reaction types Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Inputs of light reactions in photosynthesis
Inputs of light reactions in photosynthesis Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions Antiperiplanar
Antiperiplanar Elimination reaction of alkyl halides
Elimination reaction of alkyl halides Concurrent reactions in trusses
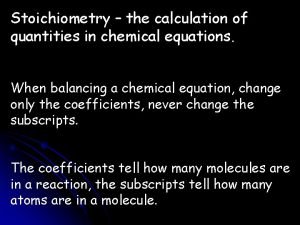
Concurrent reactions in trusses The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Single replacement reaction stoichiometry
Single replacement reaction stoichiometry Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Reversible and irreversible reactions
Reversible and irreversible reactions Oxidation half equations
Oxidation half equations Reactions in aqueous solutions
Reactions in aqueous solutions Balancing aqueous solutions
Balancing aqueous solutions Homogeneous reaction definition
Homogeneous reaction definition Predicting products of chemical reactions
Predicting products of chemical reactions Chemistry predicting products
Chemistry predicting products Combination reaction equation
Combination reaction equation Light reactions photosynthesis
Light reactions photosynthesis Unit 11 chemical reactions
Unit 11 chemical reactions Redox reaction chart
Redox reaction chart Dissolving metal reduction
Dissolving metal reduction Alpha beta gamma neutron
Alpha beta gamma neutron Natural transmutation
Natural transmutation Balancing nuclear reactions
Balancing nuclear reactions Gamma decay nuclear equation
Gamma decay nuclear equation Nuclear bombardment reactions
Nuclear bombardment reactions Lesson 90 solid evidence precipitation reactions
Lesson 90 solid evidence precipitation reactions Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Light independent reaction
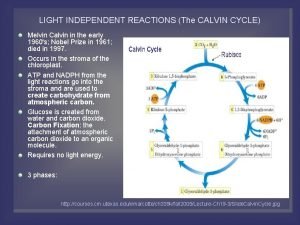
Light independent reaction Urea cycle notes
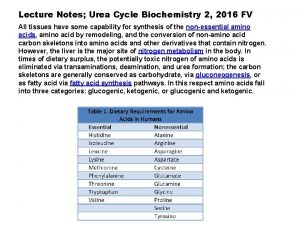
Urea cycle notes Respiration reaction
Respiration reaction Jean piaget 1896-1980
Jean piaget 1896-1980 Four types of chemical reactions
Four types of chemical reactions Biochemistry
Biochemistry Gluconeogenesis irreversible steps
Gluconeogenesis irreversible steps Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Www.biology-roots.com
Www.biology-roots.com Enzymes affect the reactions in living cells by
Enzymes affect the reactions in living cells by Rnx chemical
Rnx chemical Define concentration cells
Define concentration cells Examples of redox reaction
Examples of redox reaction Decomposition reactions examples
Decomposition reactions examples Describing chemical reactions
Describing chemical reactions