UNIT 7 CHEMICAL REACTIONS INTRO TO REACTIONS CHEMICAL








- Slides: 14

UNIT 7 -CHEMICAL REACTIONS: INTRO TO REACTIONS

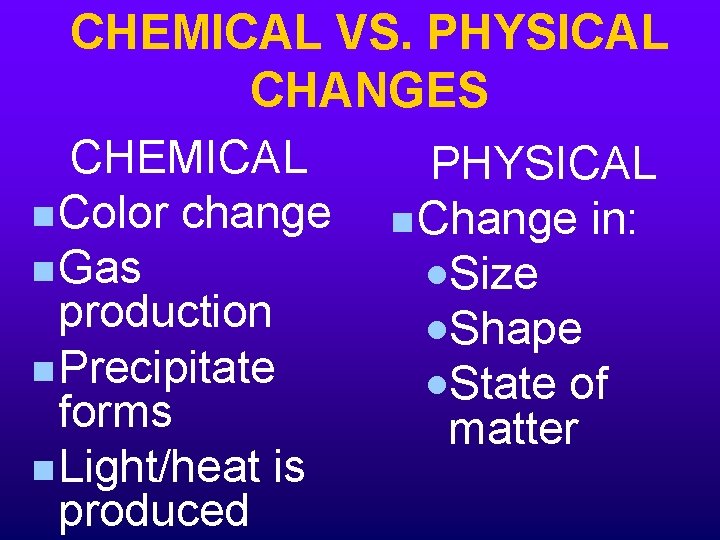
CHEMICAL VS. PHYSICAL CHANGES CHEMICAL PHYSICAL n Color change n Change in: n Gas ·Size production ·Shape n Precipitate ·State of forms matter n Light/heat is produced

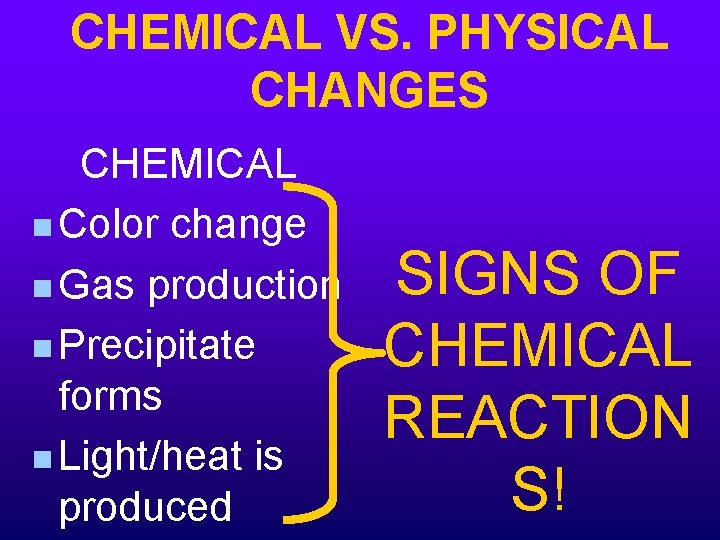
CHEMICAL VS. PHYSICAL CHANGES CHEMICAL n Color change n Gas production n Precipitate forms n Light/heat is produced SIGNS OF CHEMICAL REACTION S!

VOCABULARY n. EXERGONIC: reaction that releases energy n. ENDERGONI C: reaction that absorbs energy

VOCABULARY n EXOTHERMIC: reaction that releases energy as heat n ENDOTHERMIC : reaction that absorbs energy as heat

Coefficient n. A small whole number placed in front of a compound in a chemical reaction that multiplies its quantity. 2 H 2 O = two water molecules

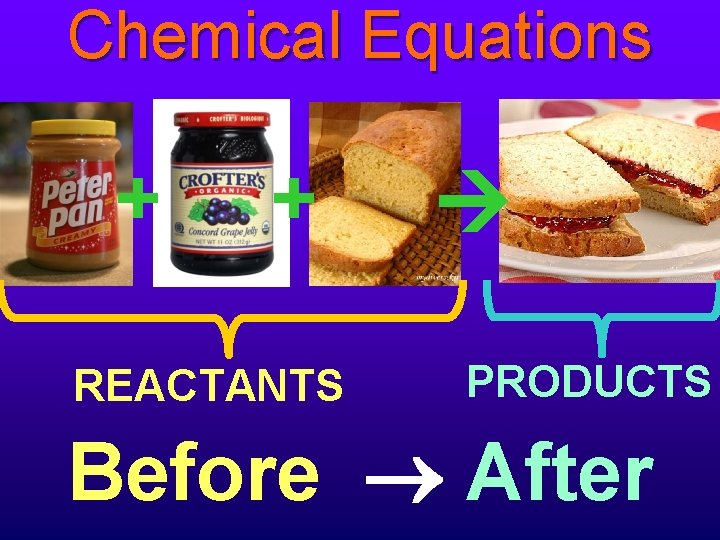
Chemical Equations + + REACTANTS PRODUCTS Before After

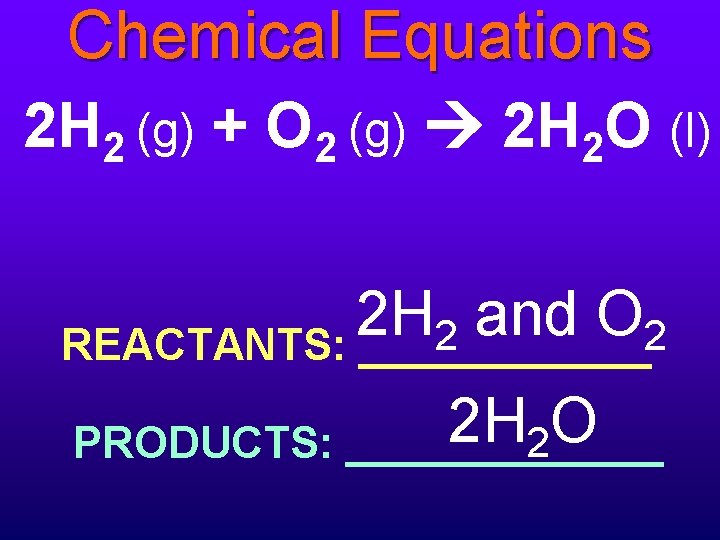
Chemical Equations 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 2 H and O 2 2 REACTANTS: ______ 2 H O 2 PRODUCTS: _______

SYMBOLS in Chemical Equations + Plus, and Produces, forms, yields (s) solid (l) liquid (g) gas (aq) Aqueous (solid in water)

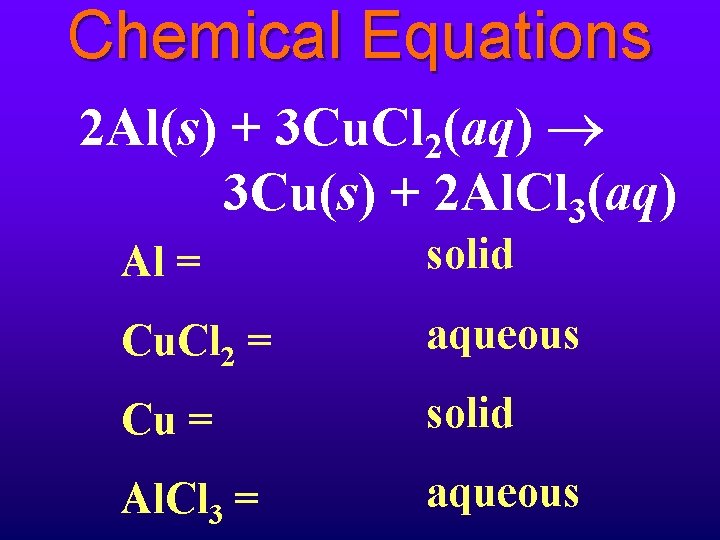
Chemical Equations 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq) Al = solid Cu. Cl 2 = aqueous Cu = solid Al. Cl 3 = aqueous

Writing Equations 2 H 2(g) + O 2(g) 2 H 2 O(g) n Identify the substances involved. n Use symbols to show: ·How many? - coefficient ·Of what? - chemical formula ·In what state? - physical state n Remember the diatomic elements.

Writing Equations Two atoms of solid aluminum react with three units of aqueous copper(II) chloride to produce three atoms of solid copper and two units of aqueous • How many? aluminum chloride. • Of what? • In what state? 2 Al(s)+ 3 Cu. Cl 2 (aq) 3 Cu (s) + 2 Al. Cl 3 (aq)

Describing Equations Describing Coefficients: ·individual atom = “atom” ·covalent substance = “molecule” ·ionic substance = “unit” 3 CO 2 3 molecules of carbon dioxide 2 Mg 2 atoms of magnesium 4 Mg. O 4 units of magnesium oxide

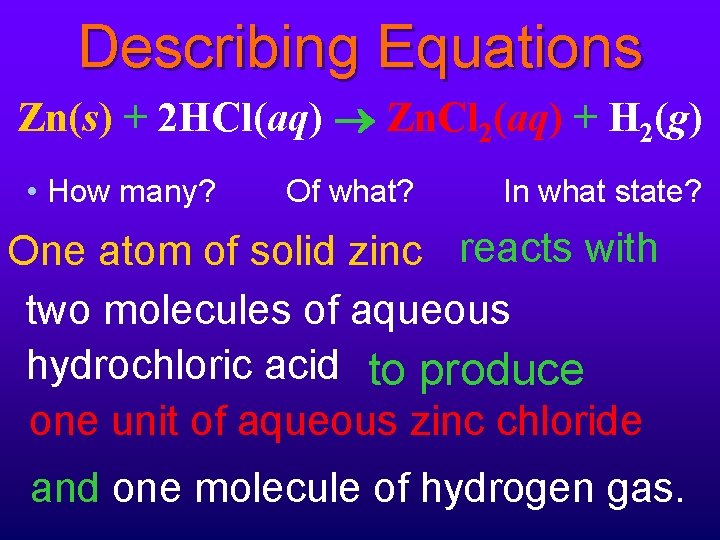
Describing Equations Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? Of what? In what state? One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas.
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 11 chemical reactions
Unit 11 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Redox reactions half reactions
Redox reactions half reactions What is bivariate data
What is bivariate data Ap government unit 1 study guide
Ap government unit 1 study guide Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Stoichiometry island diagram
Stoichiometry island diagram Redox rules
Redox rules



























