Chemical Reactions Chapter Six As chemical reactions occur












- Slides: 18

Chemical Reactions Chapter Six


As chemical reactions occur, substances are changed into new substances.

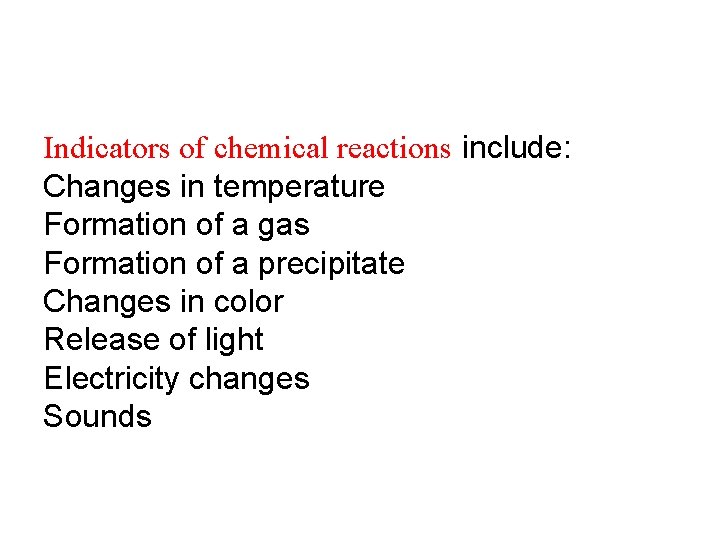
Indicators of chemical reactions include: Changes in temperature Formation of a gas Formation of a precipitate Changes in color Release of light Electricity changes Sounds

In chemical reactions, reactants are changed into products.

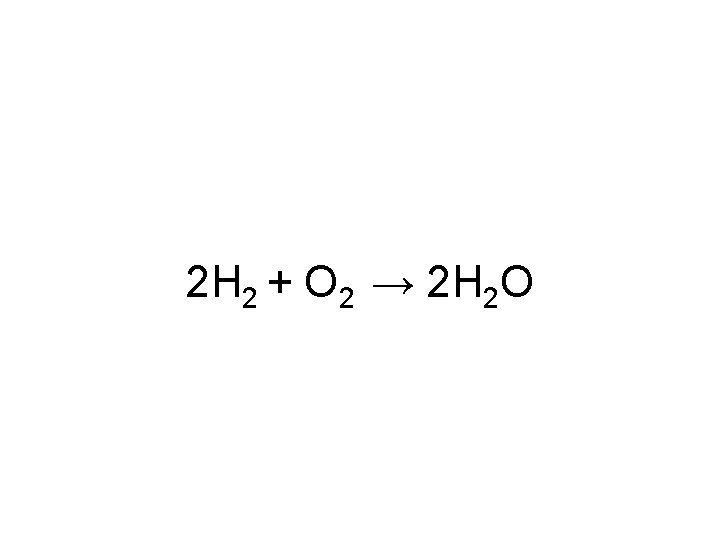
2 H 2 + O 2 → 2 H 2 O

As a chemical reaction happens, energy is involved. Energy is required to break chemical bonds. Energy is released as bonds form. The sum of these two actions determines if the reaction will cause the environment to heat up or get colder.

An exothermic reaction gives off heat.

An endothermic reaction absorbs heat.

Reaction Types • • • Synthesis Decomposition Single displacement Double displacement Combustion

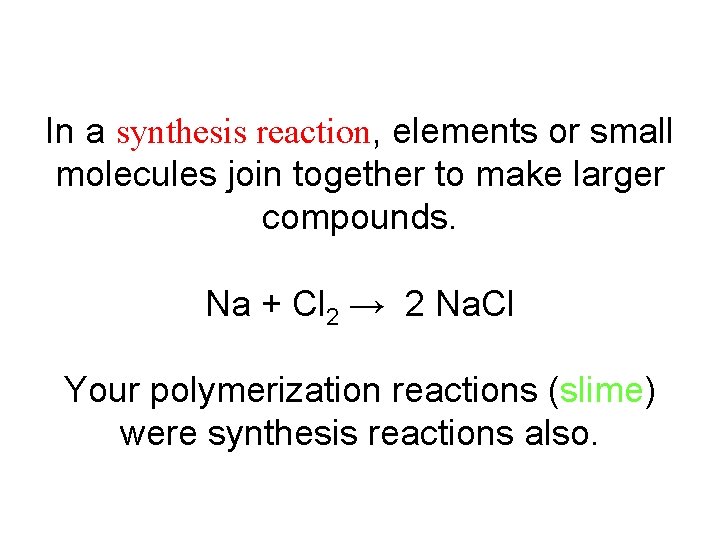
In a synthesis reaction, elements or small molecules join together to make larger compounds. Na + Cl 2 → 2 Na. Cl Your polymerization reactions (slime) were synthesis reactions also.

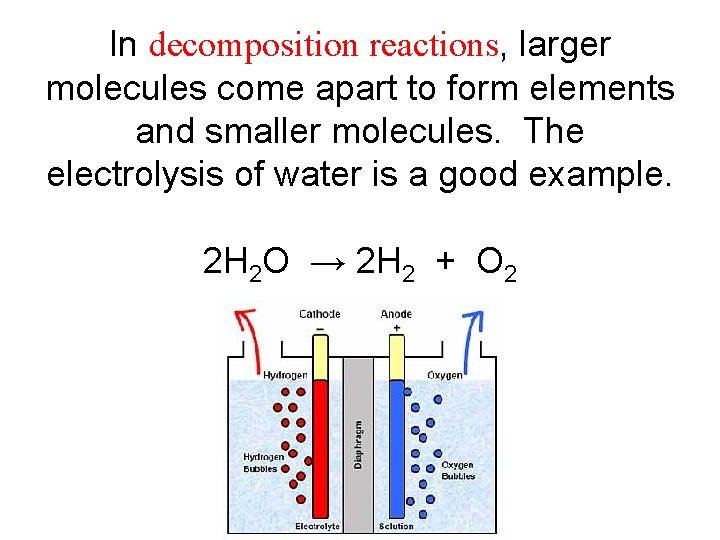
In decomposition reactions, larger molecules come apart to form elements and smaller molecules. The electrolysis of water is a good example. 2 H 2 O → 2 H 2 + O 2

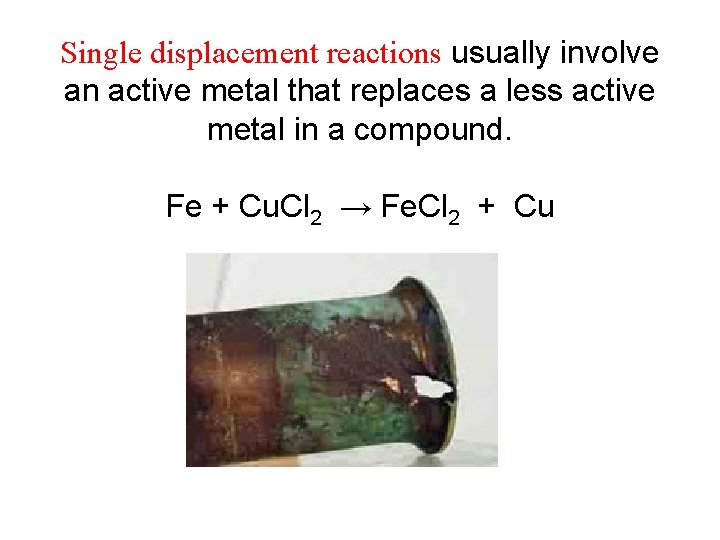
Single displacement reactions usually involve an active metal that replaces a less active metal in a compound. Fe + Cu. Cl 2 → Fe. Cl 2 + Cu

Double displacement reactions occur between two salt solutions that rearrange to form a colored precipitate. Ag. NO 3 + Na. Cl → Ag. Cl + Na. NO 3

Combustion reactions occur when hydrocarbons react with oxygen to give carbon dioxide and water. C 3 H 8 + 5 O 2 → 3 CO 2 + 4 H 2 O

Reaction rate can change. It can be controlled by changing the: • • • Temperature of the reactents Surface area of the reactents Concentration of the reactents Changing the pressure of gases Reacting smaller molecules Using catalysts (enzymes)

Some reactions are reversable.

 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of reactions
Types of reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Synthesis reaction
Synthesis reaction 5 types of chemical reactions
5 types of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Is photosynthesis endothermic
Is photosynthesis endothermic Reaction rates
Reaction rates