CHEMICAL REACTIONS n When chemical reactions occur OLD



![Equilibrium Expression n A ratio of [products] over [reactants] Each [ ] is raised Equilibrium Expression n A ratio of [products] over [reactants] Each [ ] is raised](https://slidetodoc.com/presentation_image_h/1b08e0b00d4241b511b184d055e4c443/image-18.jpg)
- Slides: 23

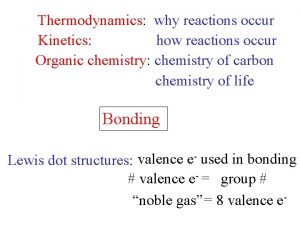
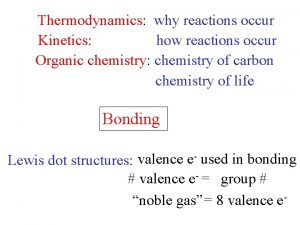
CHEMICAL REACTIONS n When chemical reactions occur OLD bonds (in the reactants) are broken and NEW bonds (in the products) are formed. n The energy needed to break old bonds and form new ones can be studied through THERMOCHEMISTRY.

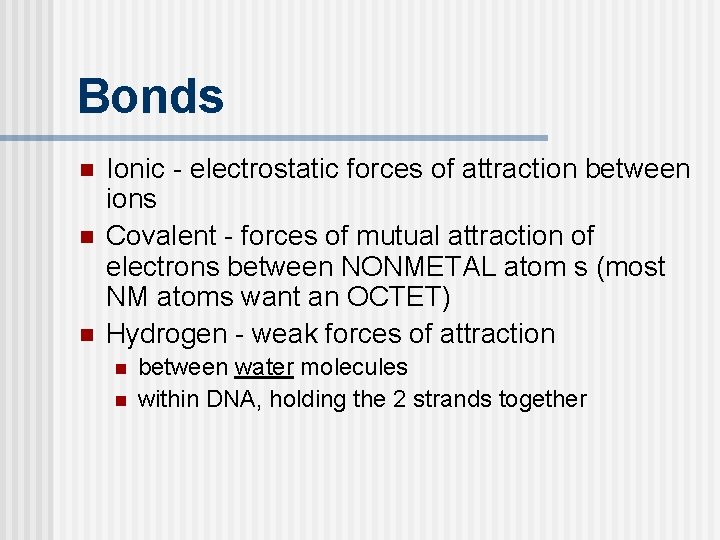
Bonds n n n Ionic - electrostatic forces of attraction between ions Covalent - forces of mutual attraction of electrons between NONMETAL atom s (most NM atoms want an OCTET) Hydrogen - weak forces of attraction n n between water molecules within DNA, holding the 2 strands together

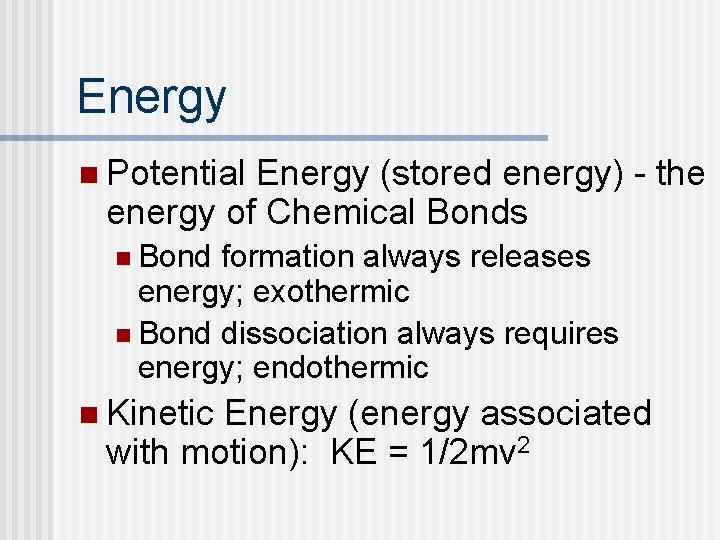
Energy n Potential Energy (stored energy) - the energy of Chemical Bonds n Bond formation always releases energy; exothermic n Bond dissociation always requires energy; endothermic n Kinetic Energy (energy associated with motion): KE = 1/2 mv 2

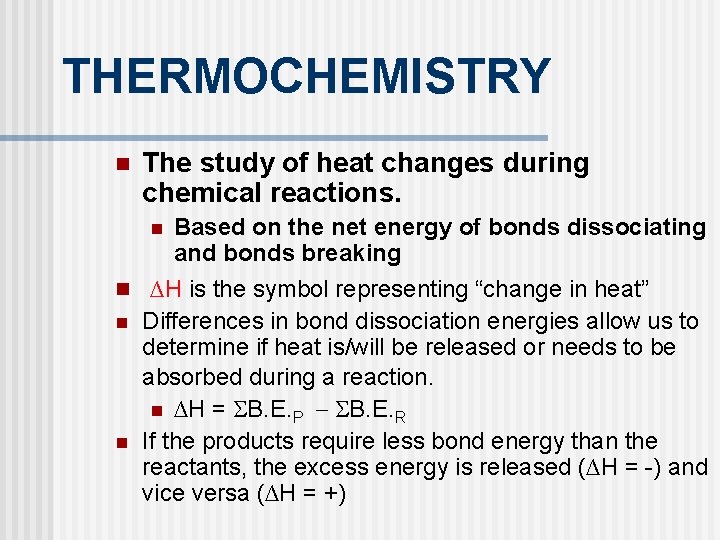
THERMOCHEMISTRY n The study of heat changes during chemical reactions. n Based on the net energy of bonds dissociating and bonds breaking n ∆H is the symbol representing “change in heat” n n Differences in bond dissociation energies allow us to determine if heat is/will be released or needs to be absorbed during a reaction. n ∆H = B. E. P B. E. R If the products require less bond energy than the reactants, the excess energy is released (∆H = -) and vice versa (∆H = +)

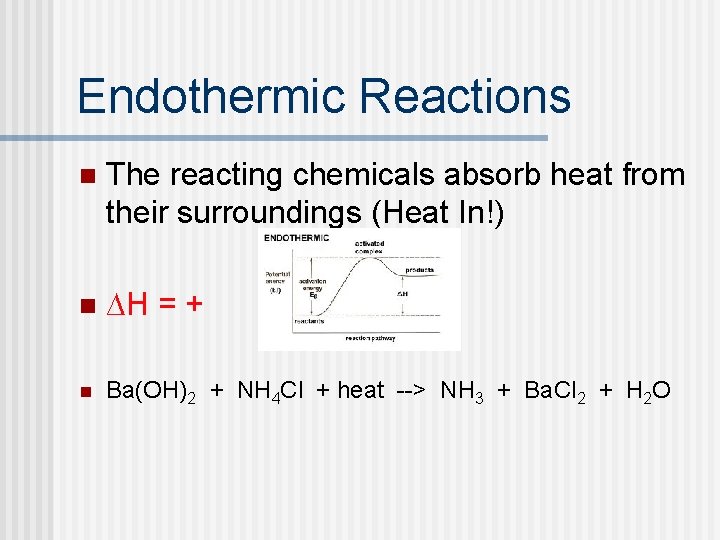
Endothermic Reactions n The reacting chemicals absorb heat from their surroundings (Heat In!) n ∆H = + n Ba(OH)2 + NH 4 Cl + heat --> NH 3 + Ba. Cl 2 + H 2 O

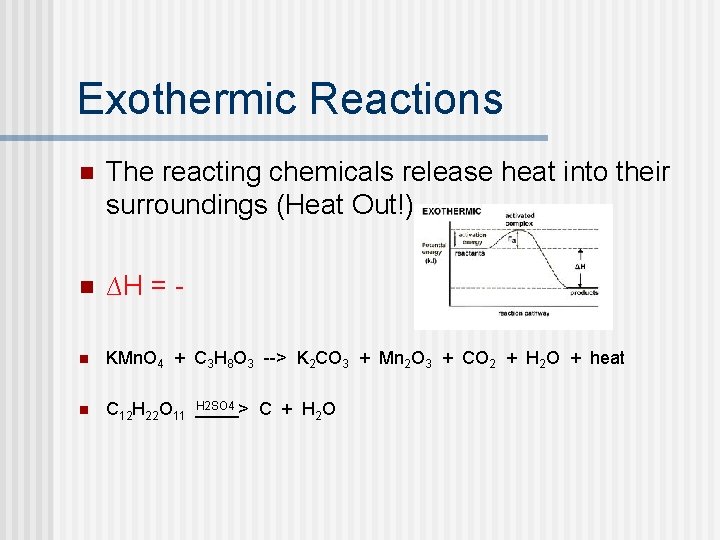
Exothermic Reactions n The reacting chemicals release heat into their surroundings (Heat Out!) n ∆H = - n KMn. O 4 + C 3 H 8 O 3 --> K 2 CO 3 + Mn 2 O 3 + CO 2 + H 2 O + heat n C 12 H 22 O 11 H 2 SO 4 > C + H 2 O

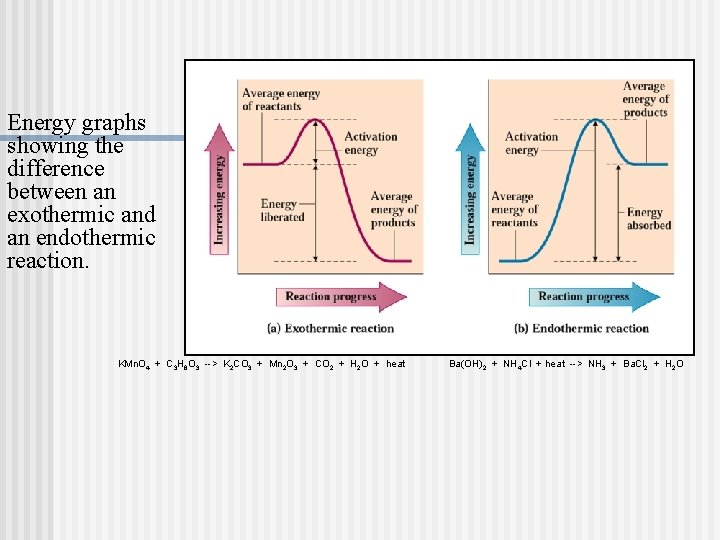
Energy graphs showing the difference between an exothermic and an endothermic reaction. KMn. O 4 + C 3 H 8 O 3 --> K 2 CO 3 + Mn 2 O 3 + CO 2 + H 2 O + heat Ba(OH)2 + NH 4 Cl + heat --> NH 3 + Ba. Cl 2 + H 2 O

Rate of Reaction Rate = Speed n The rate of a reaction depends on: n n n Temperature, Concentration of reactants, Catalysts Reactions require a specific amount of “activation” energy (Ea) in order for reactants to react effectively.

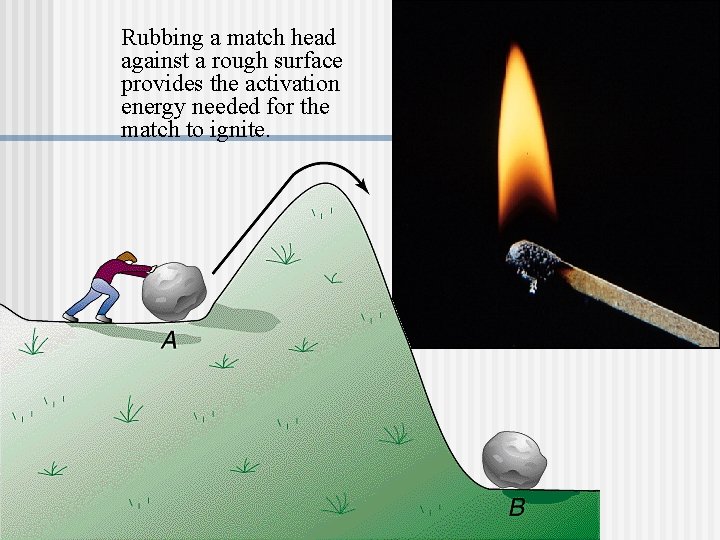
Rubbing a match head against a rough surface provides the activation energy needed for the match to ignite.

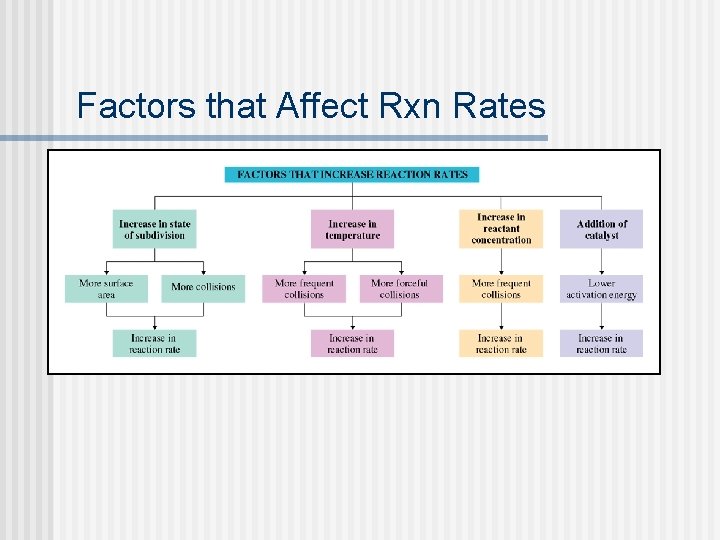
Factors that Affect Rxn Rates

Rxn Rates & Concentration Graphs showing how reaction rates and reactant concentration vary with time.

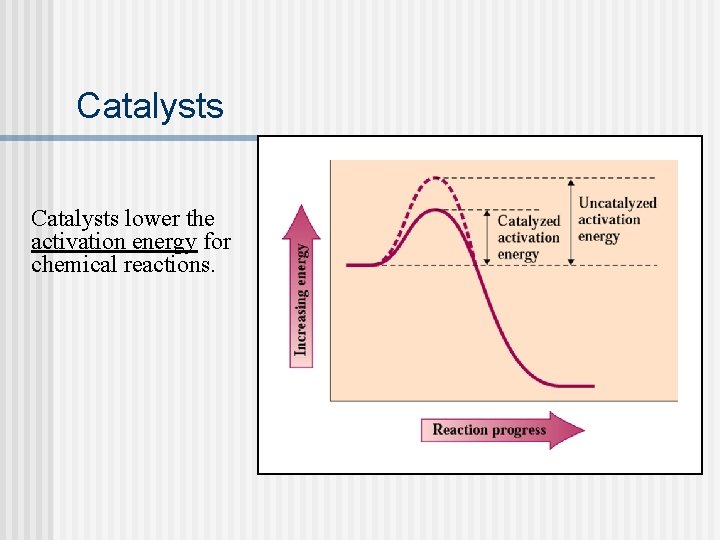
Catalysts lower the activation energy for chemical reactions.

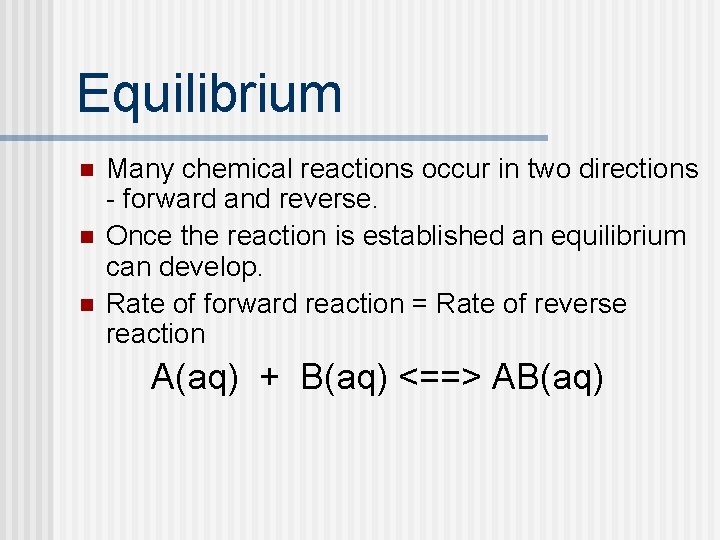
Equilibrium n n n Many chemical reactions occur in two directions - forward and reverse. Once the reaction is established an equilibrium can develop. Rate of forward reaction = Rate of reverse reaction A(aq) + B(aq) <==> AB(aq)

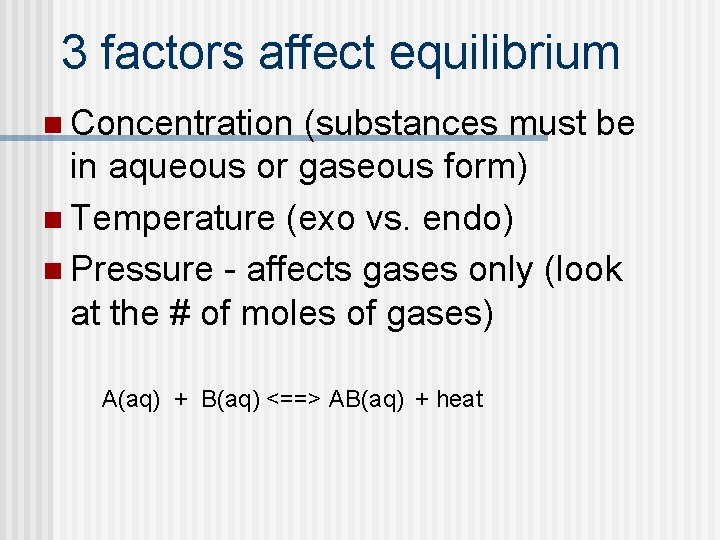
3 factors affect equilibrium n Concentration (substances must be in aqueous or gaseous form) n Temperature (exo vs. endo) n Pressure - affects gases only (look at the # of moles of gases) A(aq) + B(aq) <==> AB(aq) + heat

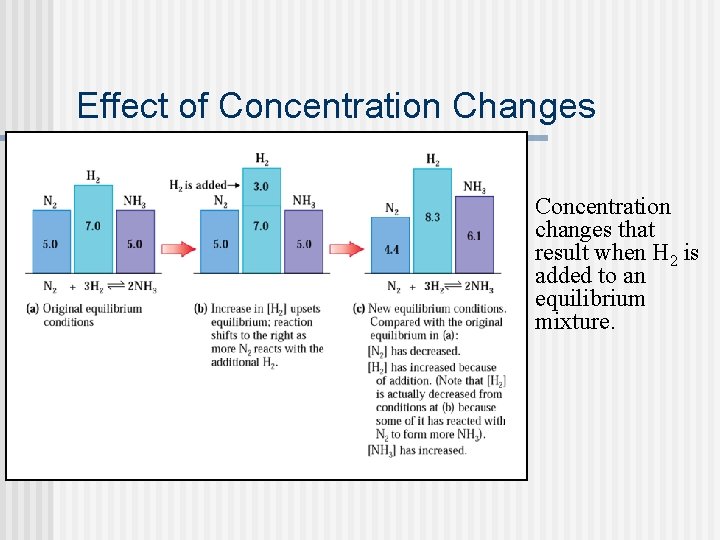
Effect of Concentration Changes Concentration changes that result when H 2 is added to an equilibrium mixture.

Effect of Temperature Equilibrium mixtures changing color with difference in temperatures.

Equilibrium Position n n A + 2 B <==> C + D This position is defined by the amounts of reactants and products If the equilibrium position shifts, equilibrium will have to be reestablished with different amounts of reactants and products. An equilibrium expression allows for a mathematical description of the position at equilibrium.
![Equilibrium Expression n A ratio of products over reactants Each is raised Equilibrium Expression n A ratio of [products] over [reactants] Each [ ] is raised](https://slidetodoc.com/presentation_image_h/1b08e0b00d4241b511b184d055e4c443/image-18.jpg)
Equilibrium Expression n A ratio of [products] over [reactants] Each [ ] is raised to the power equal to its coefficient in the balanced equation The ratio is set equal to a constant (Keq) A 2(aq) + 2 B(aq) <==> 2 AB(aq) Keq = Ex. : 2 NOCl(g) <==> 2 NO(g) + Cl 2(g) Ba. Cl 2(aq) + Na 2 SO 4(aq) <==> 2 Na. Cl(aq) + Ba. SO 4(s)

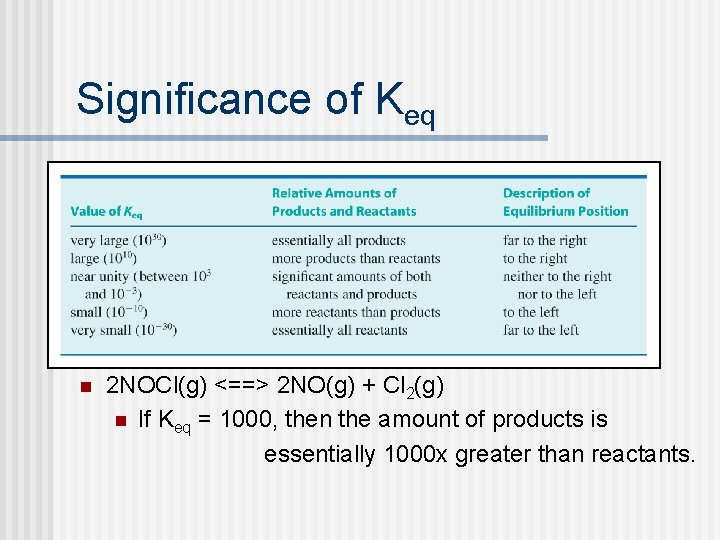
Significance of Keq n 2 NOCl(g) <==> 2 NO(g) + Cl 2(g) n If Keq = 1000, then the amount of products is essentially 1000 x greater than reactants.

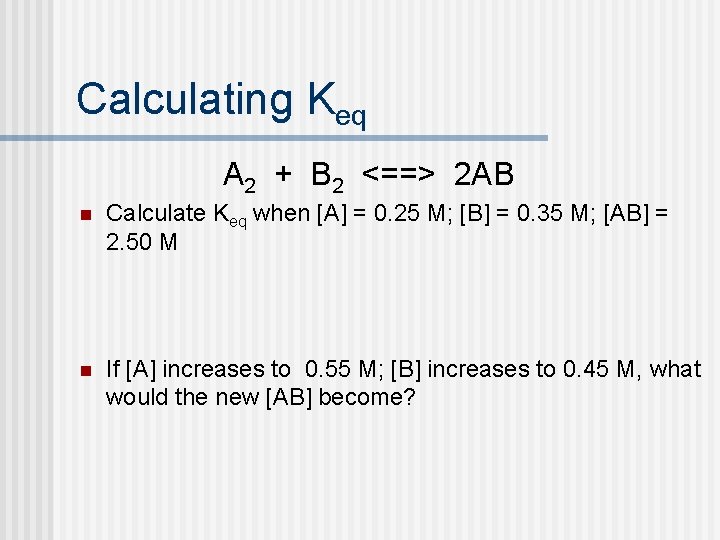
Calculating Keq A 2 + B 2 <==> 2 AB n Calculate Keq when [A] = 0. 25 M; [B] = 0. 35 M; [AB] = 2. 50 M n If [A] increases to 0. 55 M; [B] increases to 0. 45 M, what would the new [AB] become?

Chemical stress effects n n Le Chatelier’s Principle: A system in equilibrium which is stressed tries to return to equilibrium by shifting the reaction in a direction to relieve the stress So, if we increase the concentration of some participant in the equilibrium, the system will try to react away that substance. If we decrease the concentration of some participant in the equilibrium, the system will try to produce more of that substance. If we increase the temperature or pressure of the system, the system will try to reduce the temperature or pressure.

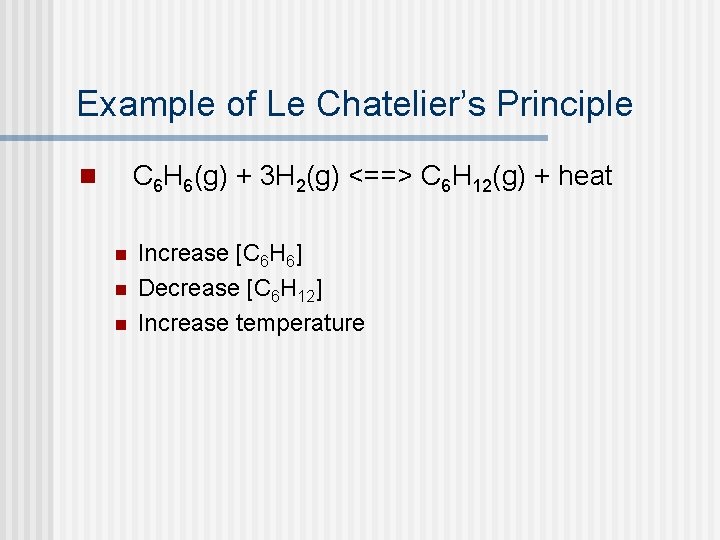
Example of Le Chatelier’s Principle C 6 H 6(g) + 3 H 2(g) <==> C 6 H 12(g) + heat n n Increase [C 6 H 6] Decrease [C 6 H 12] Increase temperature

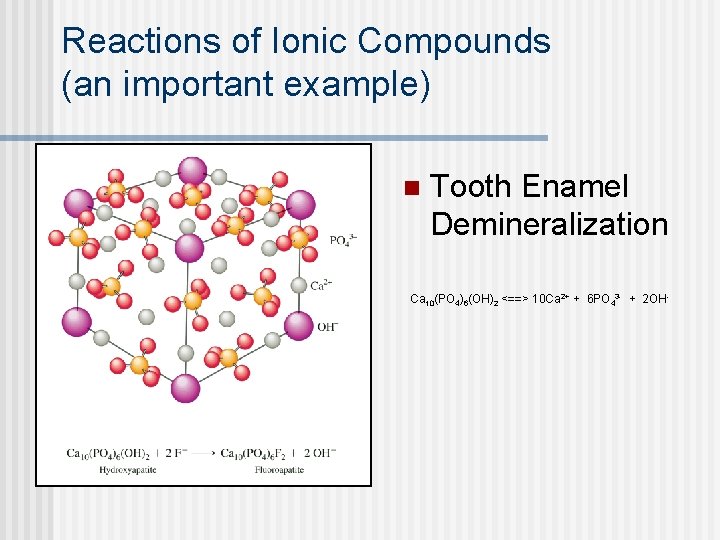
Reactions of Ionic Compounds (an important example) n Tooth Enamel Demineralization Ca 10(PO 4)6(OH)2 <==> 10 Ca 2+ + 6 PO 43 - + 2 OH-
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Photosynthesis endothermic or exothermic
Photosynthesis endothermic or exothermic Reaction rates
Reaction rates Examples of redox reaction
Examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Once upon a time there lived a boy
Once upon a time there lived a boy Once upon a time there lived an old man and an old woman
Once upon a time there lived an old man and an old woman Once upon a time there lived a poor woodcutter
Once upon a time there lived a poor woodcutter What is your
What is your Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Alkyne to alcohol
Alkyne to alcohol Type of chemical reactions
Type of chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Classify non aqueous solvents
Classify non aqueous solvents Describing chemical reactions
Describing chemical reactions Chemical reactions in welding
Chemical reactions in welding Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Chemical reaction rearrangement of atoms
Chemical reaction rearrangement of atoms Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Indications of chemical reactions
Indications of chemical reactions