Thermodynamics why reactions occur Kinetics how reactions occur






- Slides: 14

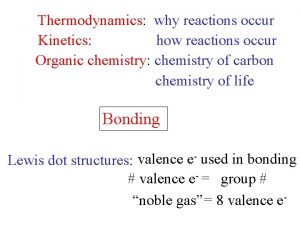
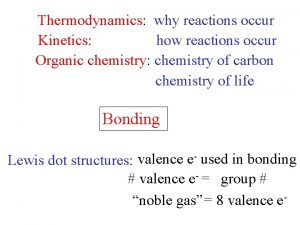
Thermodynamics: why reactions occur Kinetics: how reactions occur Organic chemistry: chemistry of carbon chemistry of life Bonding Lewis dot structures: valence e- used in bonding # valence e- = group # “noble gas” = 8 valence e-

Lewis structures CH 3 OH 1. Determine # valence eelement group number C IV H I O VI e 4 1 6 2. Place least electronegative element in center (never H)

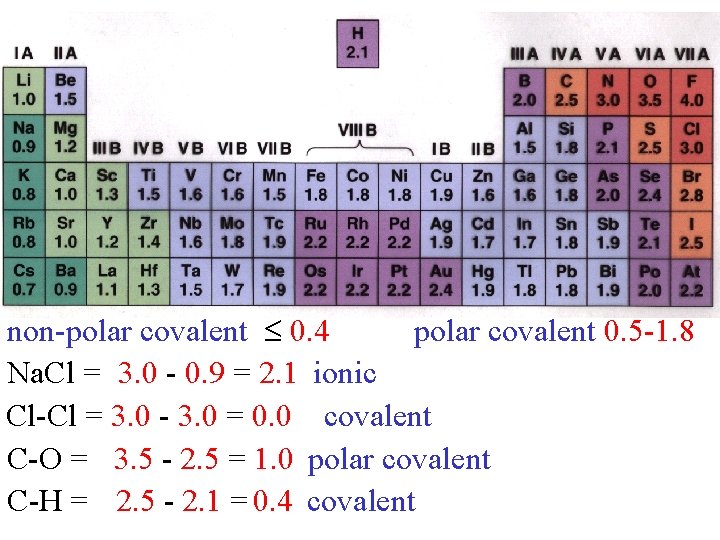
non-polar covalent 0. 4 polar covalent 0. 5 -1. 8 Na. Cl = 3. 0 - 0. 9 = 2. 1 ionic Cl-Cl = 3. 0 - 3. 0 = 0. 0 covalent C-O = 3. 5 - 2. 5 = 1. 0 polar covalent C-H = 2. 5 - 2. 1 = 0. 4 covalent

Lewis structures 1. Determine # valence e 2. Place least electronegative element in center (never H) C 3. Make single bond (2 e-) between each pair of atoms 4. Use remaining e- to satisfy octet rule 5. Use double or triple bonds to reduce # of unshared e-

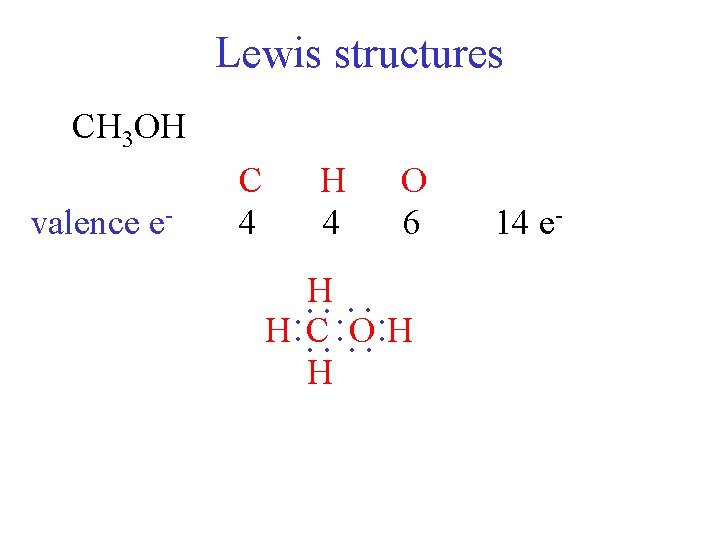
Lewis structures CH 3 OH H 4 O 6 : H H : C : O : H H : : : valence e- C 4 14 e-

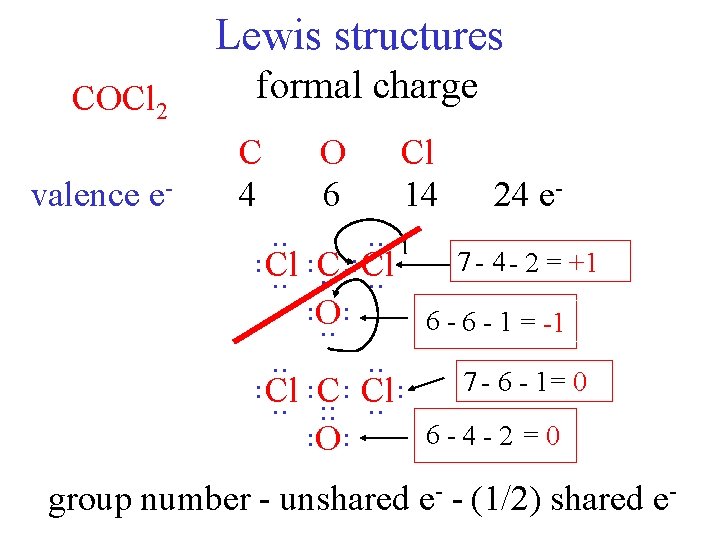
Lewis structures COCl 2 valence e- formal charge C 4 O 6 Cl 14 24 e- . . . . 7 4 2 = +1 Cl C Cl. . . O 6 - 1 = -1. . . 7 - 6 - 1= 0 Cl C Cl. . . . 6 -4 - 2 = 0 O. . group number - unshared e- - (1/2) shared e-

VSEPR Molecular geometries + H: O: H + : : H 2 O - - on O pairs of valence e tetrahedron 4 bonds angles 109. 5 o actually 108 o polar bonds dipole moment H-bonding . . H bound to O, N, F =H-bond donor. . . O, . . N, F. . = H-bond acceptor

VSEPR Molecular geometries H : C: H : O: : CH 2 O + 3 pairs of e- on C trigonal planar bonds angles 120 o polar bond dipole moment dipole-dipole

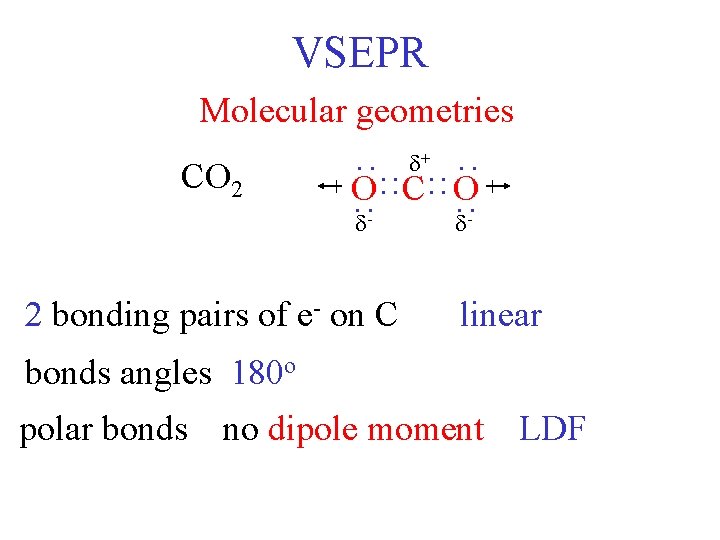
VSEPR Molecular geometries - 2 bonding pairs of e- on C : : : O : : C: : O : : : CO 2 + - linear bonds angles 180 o polar bonds no dipole moment LDF

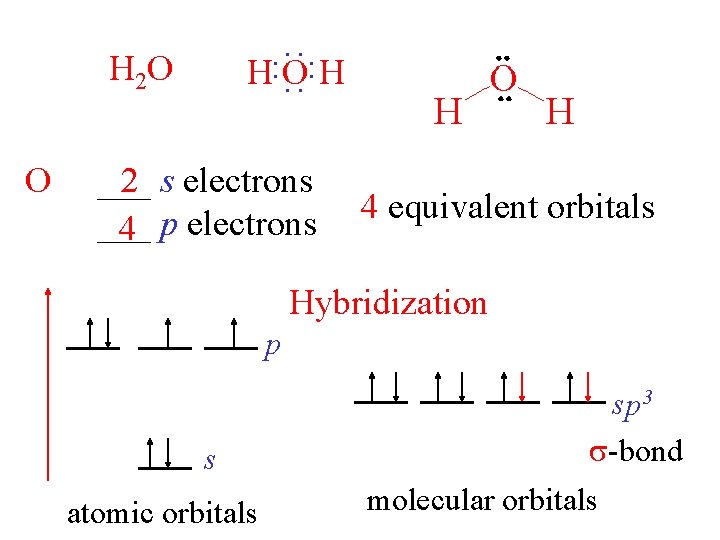
H: O: H O : : H 2 O ___ 2 s electrons ___ 4 p electrons 4 equivalent orbitals Hybridization p s atomic orbitals sp 3 -bond molecular orbitals

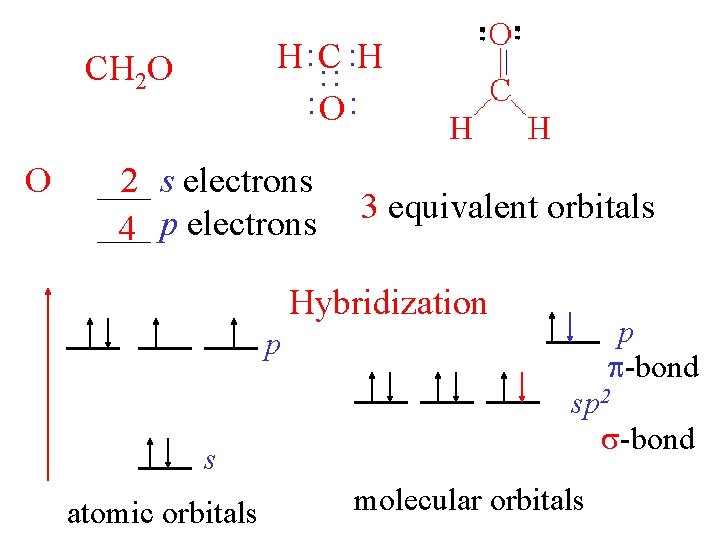
H : C : H : O : C : : CH 2 O ___ 2 s electrons ___ 2 p electrons 3 equivalent orbitals Hybridization p s atomic orbitals p -bond sp 2 -bond molecular orbitals

H : C : H : O : : CH 2 O ___ 2 s electrons ___ 4 p electrons 3 equivalent orbitals Hybridization p -bond p s atomic orbitals sp 2 -bond molecular orbitals

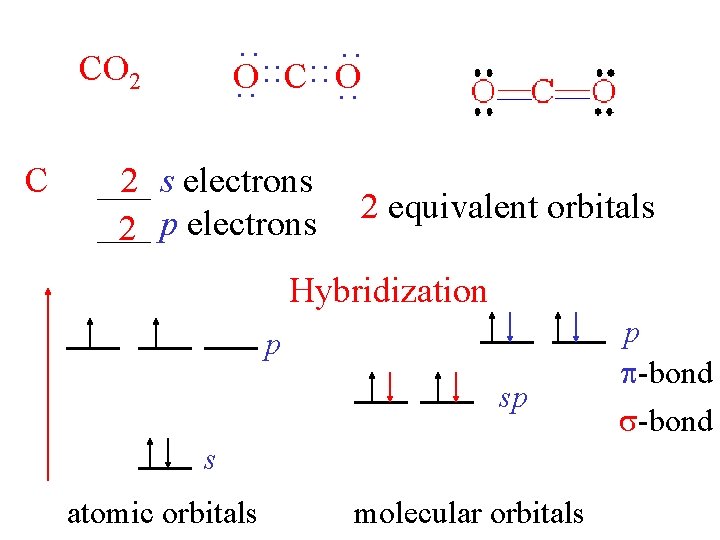
C : : CO 2 : : O : : C: : O ___ 2 s electrons ___ 2 p electrons 2 equivalent orbitals Hybridization p sp s atomic orbitals molecular orbitals p -bond

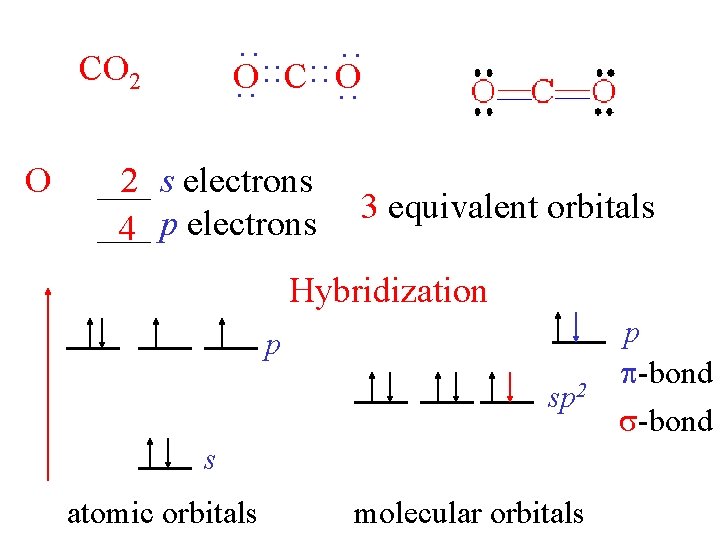
O : : CO 2 : : O : : C: : O ___ 2 s electrons ___ 4 p electrons 3 equivalent orbitals Hybridization p sp 2 s atomic orbitals molecular orbitals p -bond
 Pictures
Pictures Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Balancing redox reactions
Balancing redox reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Why study thermodynamics
Why study thermodynamics Dont ask
Dont ask Man is the
Man is the Why does hybridization occur
Why does hybridization occur Why hybridization occurs
Why hybridization occurs Why did the yazoo land fraud occur?
Why did the yazoo land fraud occur? Why do earthquakes occur
Why do earthquakes occur How do tides work
How do tides work Why does refraction occur brainpop
Why does refraction occur brainpop