Solution Stoichiometry Solution Stoichiometry Solution stoichiometry follows the








- Slides: 10

Solution Stoichiometry

Solution Stoichiometry Solution stoichiometry follows the same general steps as mass to mass stoichiometry… the only difference is that you will be starting with a volume and concentration of a solution or asked to solve for a volume or concentration of a product. In order to solve solution stoich’ problems, you need to be confident with mass to mass stoich and molarity calcs. Go back and review those concepts if necessary!

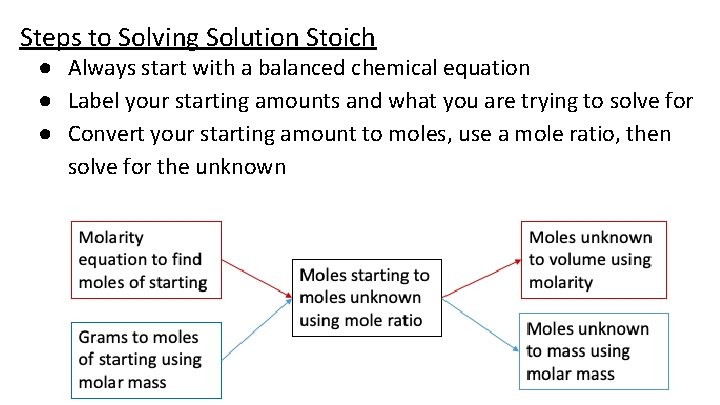
Steps to Solving Solution Stoich ● Always start with a balanced chemical equation ● Label your starting amounts and what you are trying to solve for ● Convert your starting amount to moles, use a mole ratio, then solve for the unknown

Using Molarity as a Conversion Factor Instead of doing a separate step using molarity and volume to solve for moles, you can use molarity directly in your conversion. Remember that the units of molarity are moles/L, so you can plug in a molarity as a number of moles over 1 liter. Short video explanation of using molarity as a conversion factor to convert to moles or liters

Solution Stoichiometry Example 1: Volume to Volume Mg(OH)2 (aq) + 2 HCl (aq) → Mg. Cl 2 (aq) + 2 H 2 O (l) How many m. L of 2. 5 M HCl are needed to completely react with 275 m. L of 1. 5 M Mg(OH)2 according to the reaction? Steps to solve: 1. The equation is already given & balanced. Identify what you have (275 m. L of 1. 5 M Mg(OH)2) and what you are solving for (volume of 2. 5 M HCl) 2. Convert volume of Mg(OH)2 to moles 3. Do a mole to mole ratio from Mg(OH)2 to HCl 4. Convert moles of HCl to volume (m. L) using molarity Answer = 330 m. L HCl Video Explanation HERE!

Solution Stoichiometry Example 2: Volume to Mass Ag. NO 3 (aq) + Cu (s) → Cu(NO 3)2 (aq) + Ag(s) 55 m. L of 2. 5 M silver nitrate reacts with copper wire according to the reaction above. Calculate the mass of silver produced. Steps to solve: 1. The equation is already given & balanced. Identify what you have (55 m. L of 2. 5 M Ag. NO 3) and what you are solving for (g of Ag) 2. Convert volume of Ag. NO 3 to moles 3. Mole to mole ratio of Ag. NO 3 to Ag Answer = 15 g Ag 4. Moles of Ag to grams (using molar mass) Video Explanation HERE! (Start at 8: 28)

Solution Stoichiometry Example 3: Mass to Volume Mg(OH)2 (aq) + 2 HCl (aq) → Mg. Cl 2 (aq) + 2 H 2 O (l) If 35 g of Mg. Cl 2 is produced in the chemical reaction above, then how many m. L of 0. 75 M HCl will be consumed? Steps to solve: 1. The equation is already given & balanced. Identify what you have (35 g of Mg. Cl 2) and what you are solving for (m. L of 0. 75 M HCl). 2. Convert grams Mg. Cl 2 to moles (using molar mass) 3. Mole to mole ratio Mg. Cl 2 to HCl 4. Convert moles HCl to m. L using the molarity Answer = 980 m. L Video Explanation HERE! (Start at 12: 55)

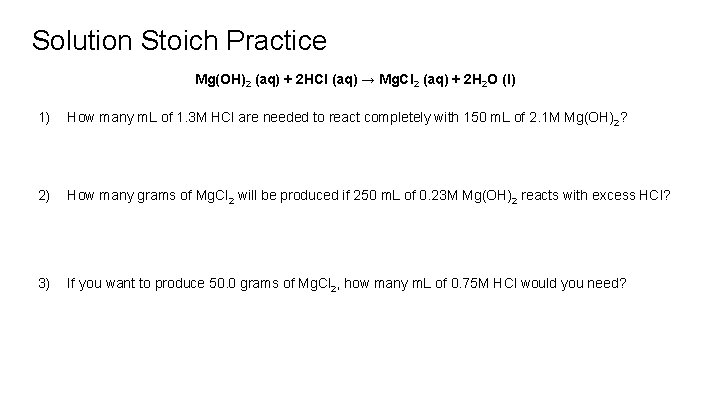
Solution Stoich Practice Mg(OH)2 (aq) + 2 HCl (aq) → Mg. Cl 2 (aq) + 2 H 2 O (l) 1) How many m. L of 1. 3 M HCl are needed to react completely with 150 m. L of 2. 1 M Mg(OH)2? 2) How many grams of Mg. Cl 2 will be produced if 250 m. L of 0. 23 M Mg(OH)2 reacts with excess HCl? 3) If you want to produce 50. 0 grams of Mg. Cl 2, how many m. L of 0. 75 M HCl would you need?

Solution Stoich Practice Answers 1. = 484. 6 m. L or 480 m. L (2 sf) 2. = 5. 48 g Mg. Cl 2 or 5. 5 g (2 sf) 3. = 1400 m. L HCl Need help? Video explanation HERE

At Home Experiment - Make your own ice cream Half & Half + Vanilla + Sugar → Ice Cream 1. 2. 3. 4. Add ½ cup of half and half to a sandwich or quart sized ziplock bag Add ¼ tsp vanilla Determine the total volume inside the bag using the conversions provided. Consider the sugar to be the solute. If the molarity of the “ice cream solution” equals 0. 3080 M, calculate how many grams of sugar should go into the solution. 5. If you don’t have a kitchen scale, use the conversion table to add that amount of sugar to the bag 6. Squeeze the air out of the bag and seal. Place it inside a gallon size ziplock bag half full of ice + at least 6 Tablespoons of salt (rock salt works best) 7. Shake the big bag until the ice cream freezes. Send a selfie of your homemade ice cream to your teacher : ) Helpful Conversions 1 cup = 0. 2366 L 1 tsp = 4. 929 m. L Sugar = C 12 H 22 O 11 1 tsp = 4. 20 grams