Polyatomic Ions Polyatomic Ions Two or more different

![Concept Check: How many IONS are present in each of the following? A) [K]+[OH]- Concept Check: How many IONS are present in each of the following? A) [K]+[OH]-](https://slidetodoc.com/presentation_image/5007a043d3e4696067bd6be9354b8533/image-11.jpg)





- Slides: 18

Polyatomic Ions

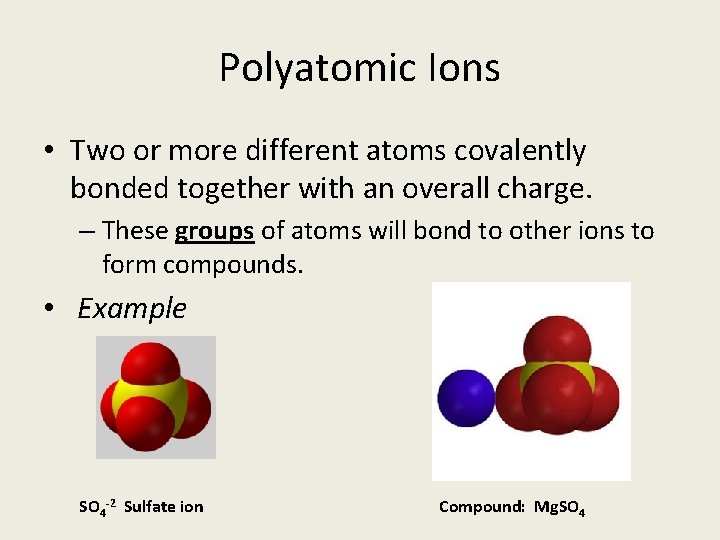
Polyatomic Ions • Two or more different atoms covalently bonded together with an overall charge. – These groups of atoms will bond to other ions to form compounds. • Example SO 4 -2 Sulfate ion Compound: Mg. SO 4

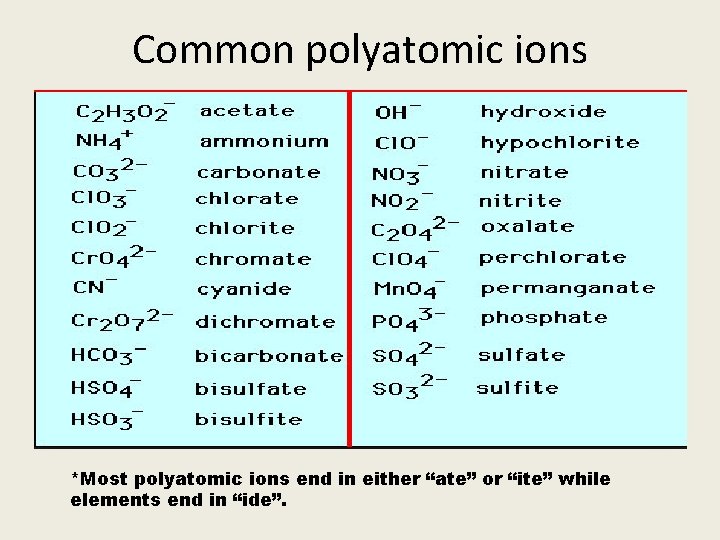
Common polyatomic ions *Most polyatomic ions end in either “ate” or “ite” while elements end in “ide”.

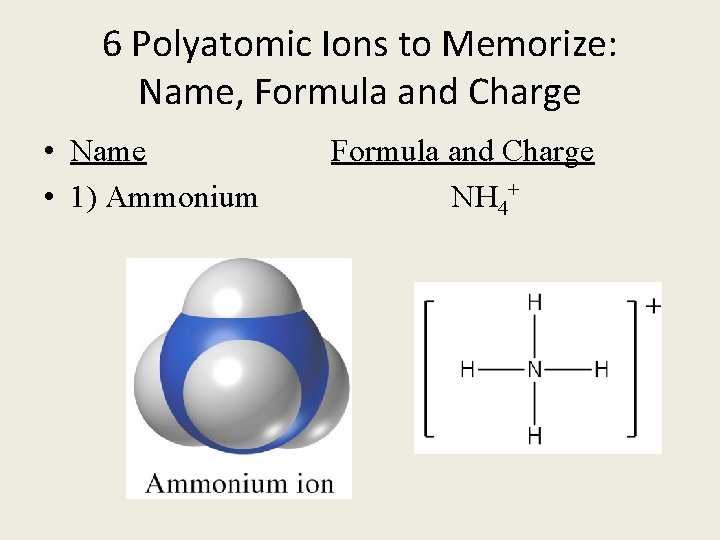
6 Polyatomic Ions to Memorize: Name, Formula and Charge • Name Formula and Charge • 1) Ammonium NH 4+

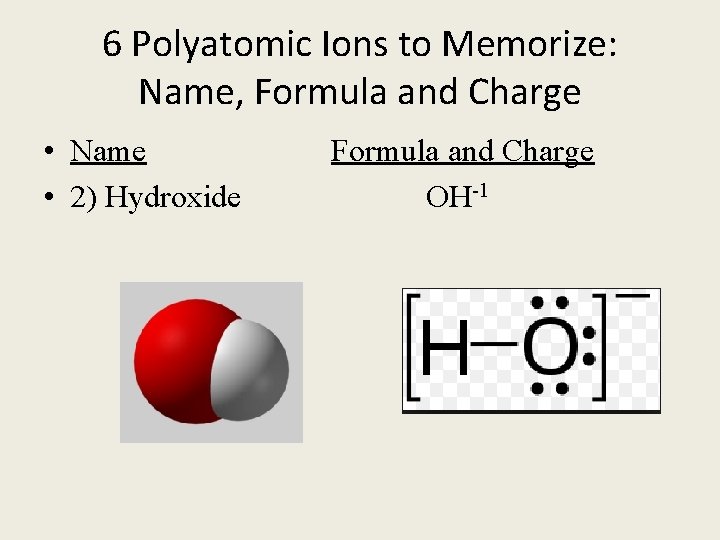
6 Polyatomic Ions to Memorize: Name, Formula and Charge • Name Formula and Charge • 2) Hydroxide OH-1

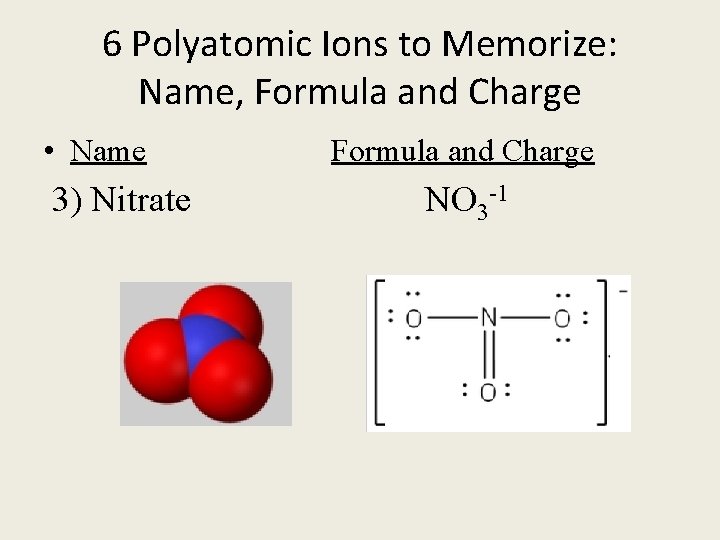
6 Polyatomic Ions to Memorize: Name, Formula and Charge • Name Formula and Charge 3) Nitrate NO 3 -1

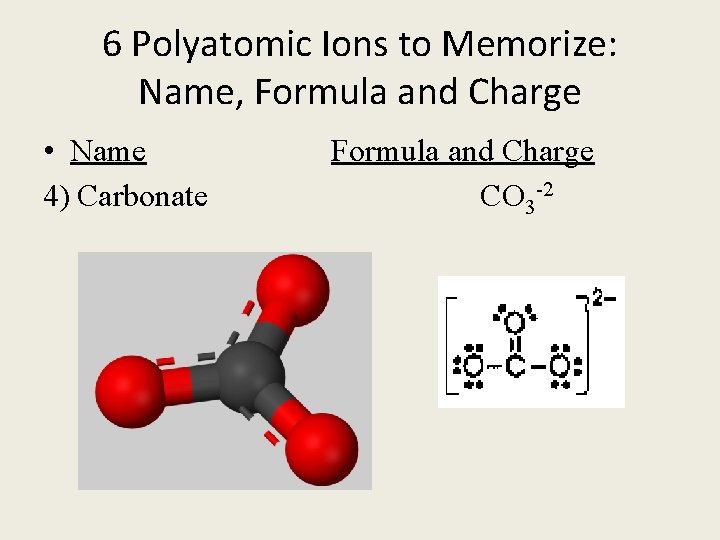
6 Polyatomic Ions to Memorize: Name, Formula and Charge • Name Formula and Charge 4) Carbonate CO 3 -2

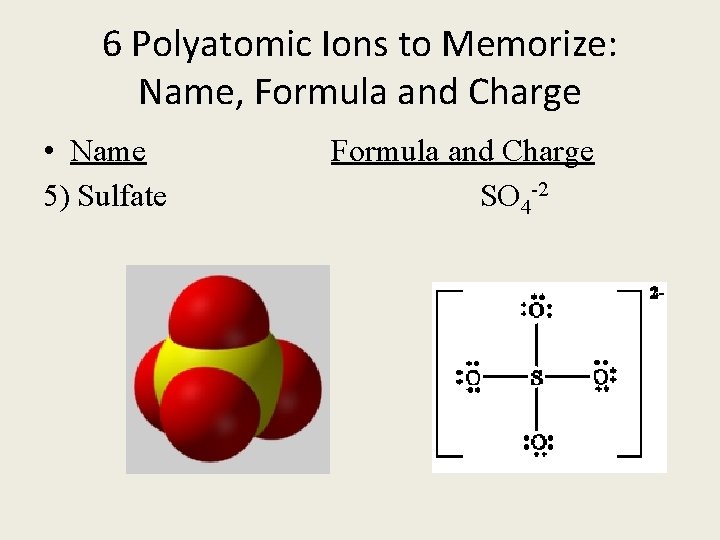
6 Polyatomic Ions to Memorize: Name, Formula and Charge • Name Formula and Charge 5) Sulfate SO 4 -2

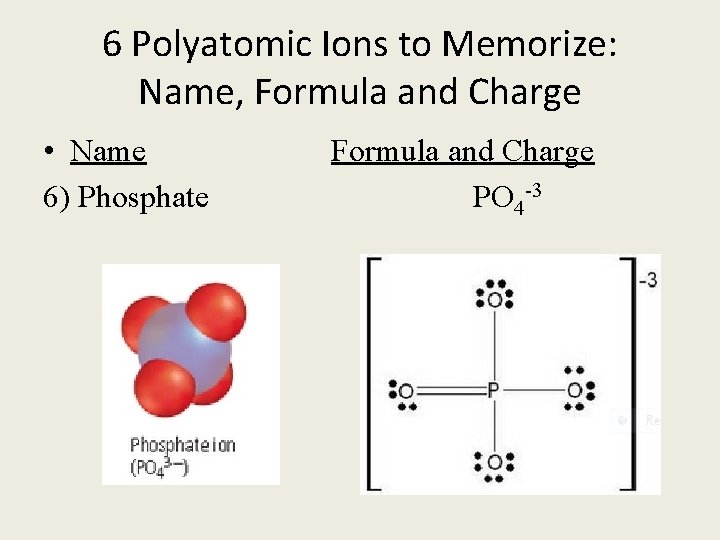
6 Polyatomic Ions to Memorize: Name, Formula and Charge • Name Formula and Charge 6) Phosphate PO 4 -3

Remember… • Many polyatomic ions include oxygen. – If the name ends in –ate, it will have more oxygen atoms than the other “form. ” – If the name ends in –ite, it will have fewer oxygen atoms than the other “form. ” – Example, sulfate SO 42 - vs sulfite SO 32 -
![Concept Check How many IONS are present in each of the following A KOH Concept Check: How many IONS are present in each of the following? A) [K]+[OH]-](https://slidetodoc.com/presentation_image/5007a043d3e4696067bd6be9354b8533/image-11.jpg)
Concept Check: How many IONS are present in each of the following? A) [K]+[OH]- B) [Na]+[CO 3 -2 ] [Na]+ Ans: 2 Ans: 3 Ans: 4 C) [Na]+ [PO 4]-3 [Na]+ [Na]+

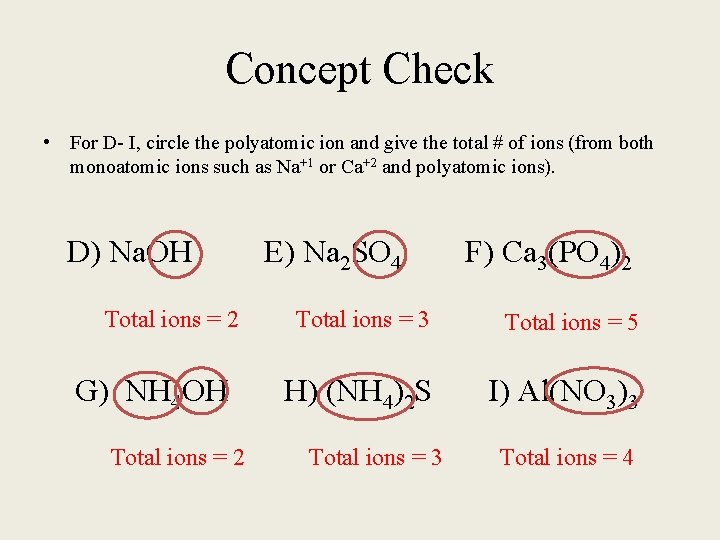
Concept Check • For D- I, circle the polyatomic ion and give the total # of ions (from both monoatomic ions such as Na+1 or Ca+2 and polyatomic ions). D) Na. OH E) Na 2 SO 4 F) Ca 3(PO 4)2 Total ions = 3 Total ions = 5 G) NH 4 OH H) (NH 4)2 S I) Al(NO 3)3 Total ions = 2 Total ions = 3 Total ions = 4

Naming Ternary Salts (compounds with a polyatomic ion) • Simple Naming Rule: Give each ion its name • Example - Name the following • Na. NO 3 sodium nitrate • Na 2 CO 3 sodium carbonate

Practice: Name the following: • Na. OH Ans: sodium hydroxide • Mg(C 2 H 3 O 2)2 Ans: magnesium acetate • NH 4 Cl • Ans: ammonium chloride

Writing Formulas • Find charges of monoatomic ions from periodic table • Look up or memorize charges of polyatomic ions • Sum of charges must total zero; use criss-cross if necessary • If more than one polyatomic ion use ( )

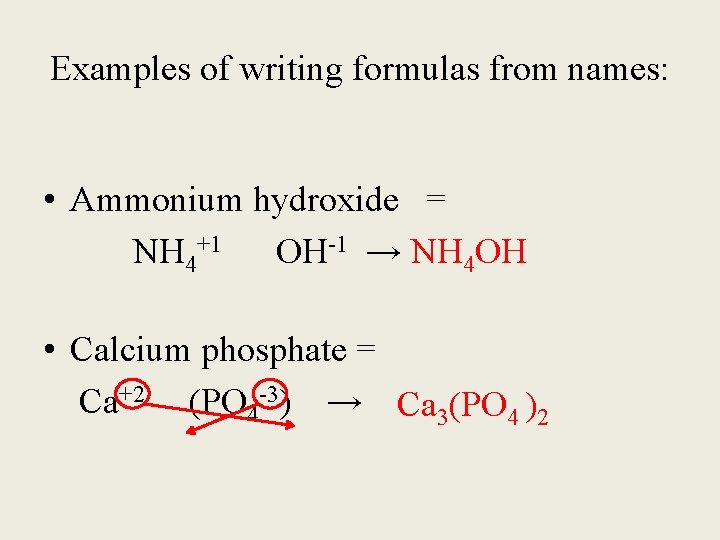
Examples of writing formulas from names: • Ammonium hydroxide = NH 4+1 OH-1 → NH 4 OH • Calcium phosphate = Ca+2 (PO 4 -3) → Ca 3(PO 4 )2

Practice Formulas • calcium nitrate Ans: Ca(NO 3)2 • Cesium hydroxide Ans: Cs. OH • Gallium carbonate • Ans: Ga 2(CO 3)3

Review Practice Formulas • calcium nitrate Ans: Ca(NO 3)2 • calcium nitride Ans: Ca 2 N 3 calcium nitrite Ans: Ca(NO 2)2