Solution Stoichiometry Solution Stoichiometry Solution Concentration Molarity What
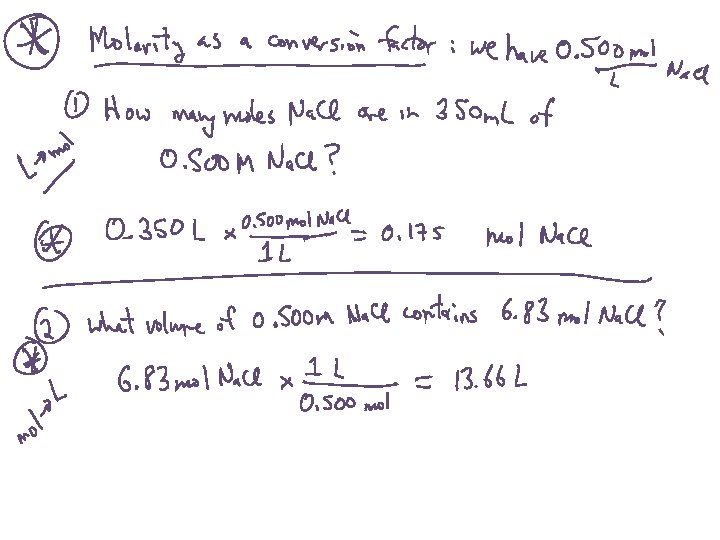









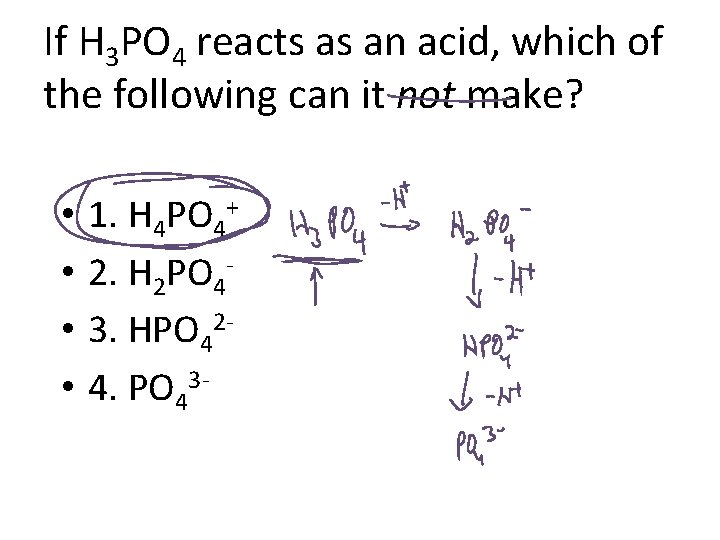
![Finding [H 3 O+] from p. H [H 3 O+] = 10 -p. H Finding [H 3 O+] from p. H [H 3 O+] = 10 -p. H](https://slidetodoc.com/presentation_image_h2/94e8fc5201c84b2288abfa3b0c08a6ae/image-47.jpg)








- Slides: 92


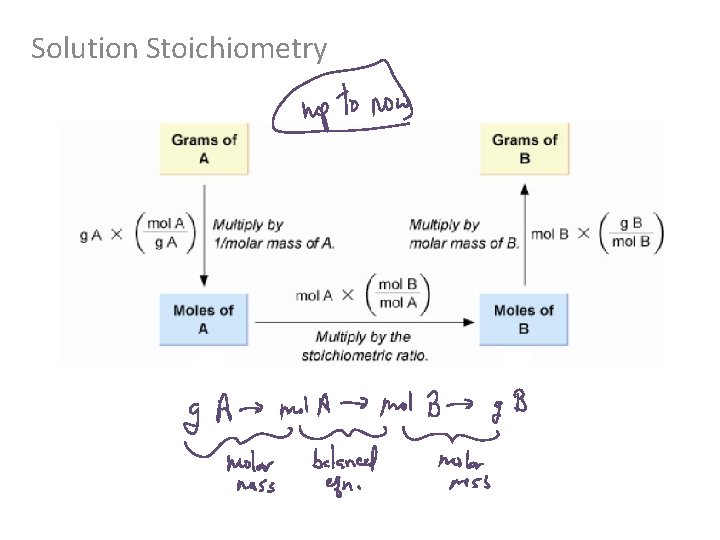
Solution Stoichiometry

Solution Stoichiometry
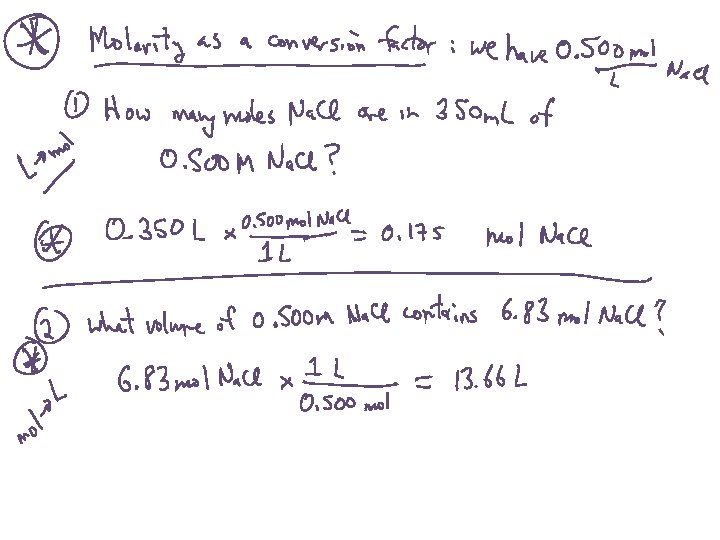

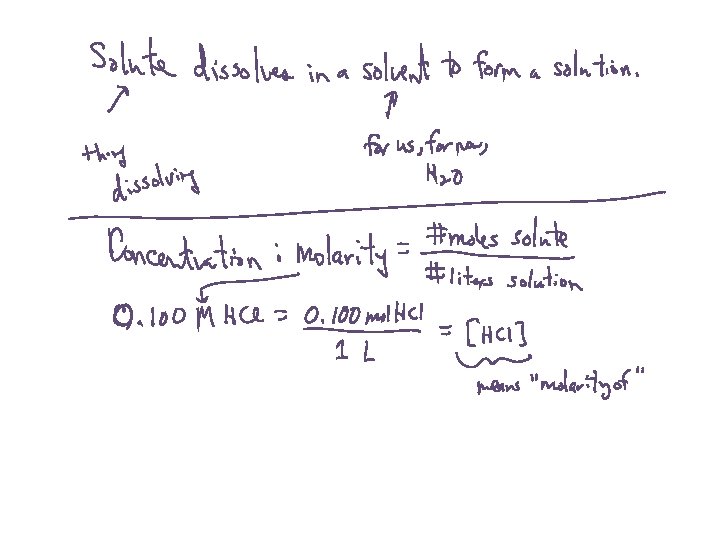
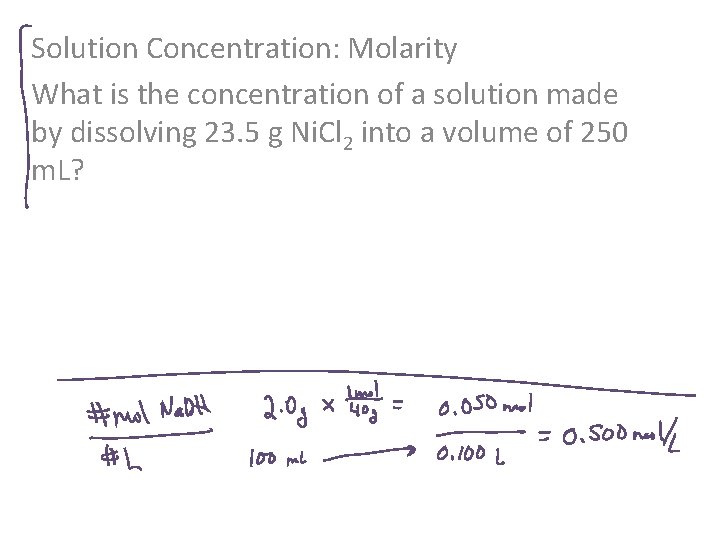
Solution Concentration: Molarity What is the concentration of a solution made by dissolving 23. 5 g Ni. Cl 2 into a volume of 250 m. L?

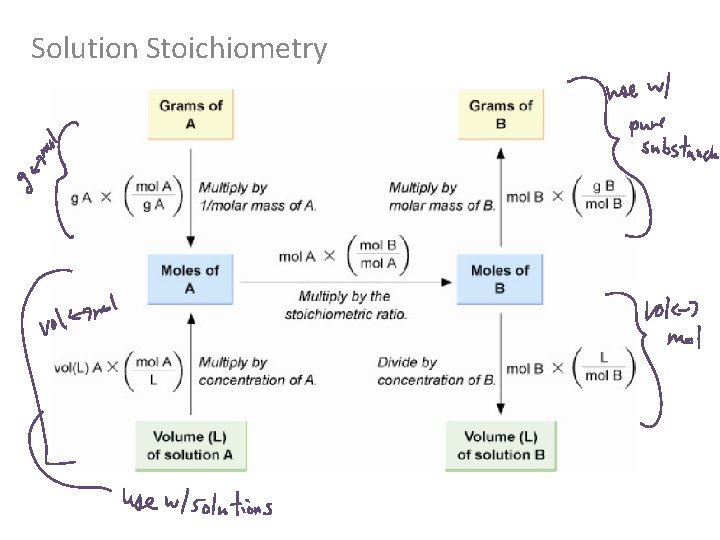
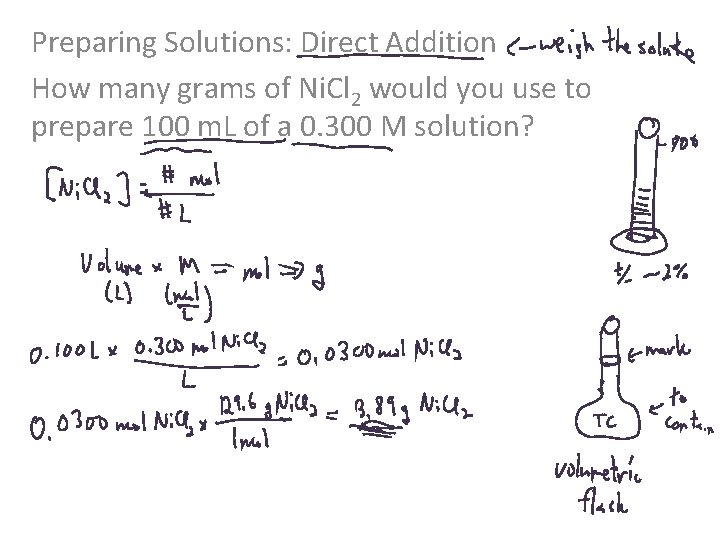
Preparing Solutions: Direct Addition How many grams of Ni. Cl 2 would you use to prepare 100 m. L of a 0. 300 M solution?

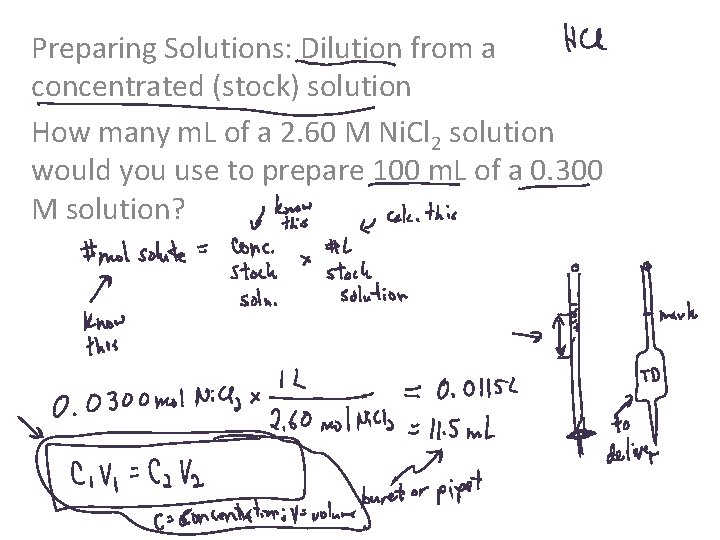
Preparing Solutions: Dilution from a concentrated (stock) solution How many m. L of a 2. 60 M Ni. Cl 2 solution would you use to prepare 100 m. L of a 0. 300 M solution?

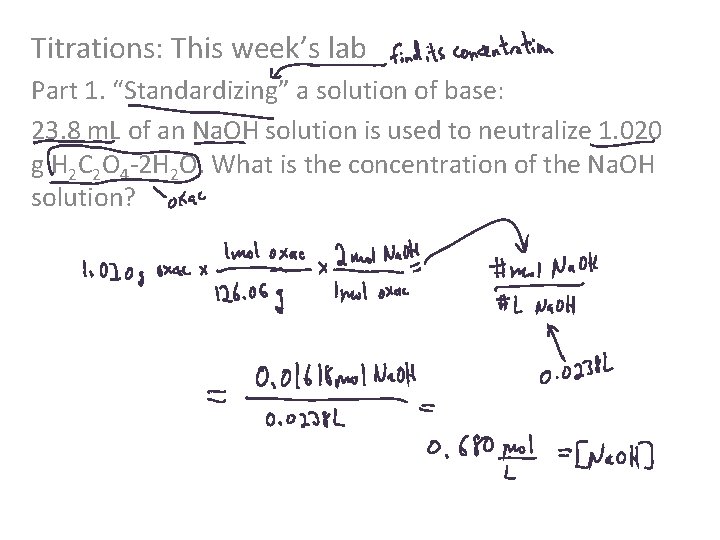
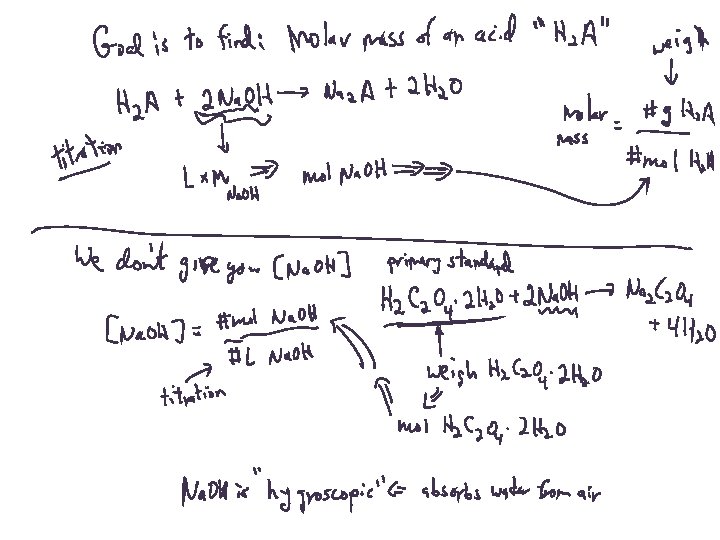
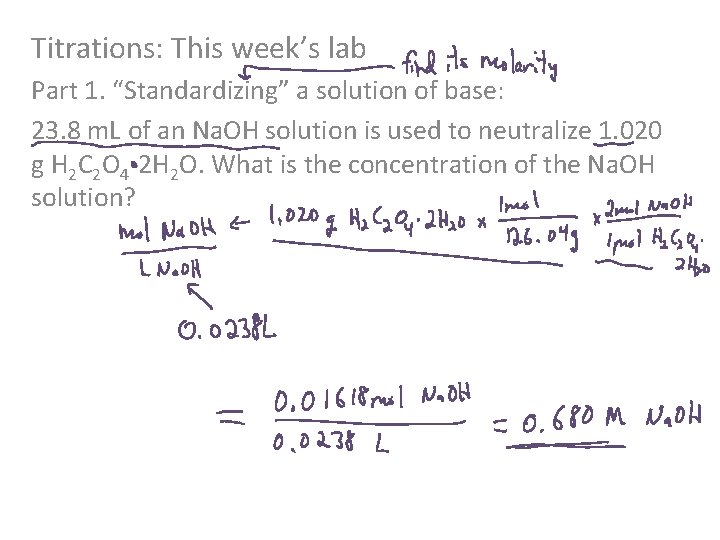
Titrations: This week’s lab Part 1. “Standardizing” a solution of base: 23. 8 m. L of an Na. OH solution is used to neutralize 1. 020 g H 2 C 2 O 4 -2 H 2 O. What is the concentration of the Na. OH solution?

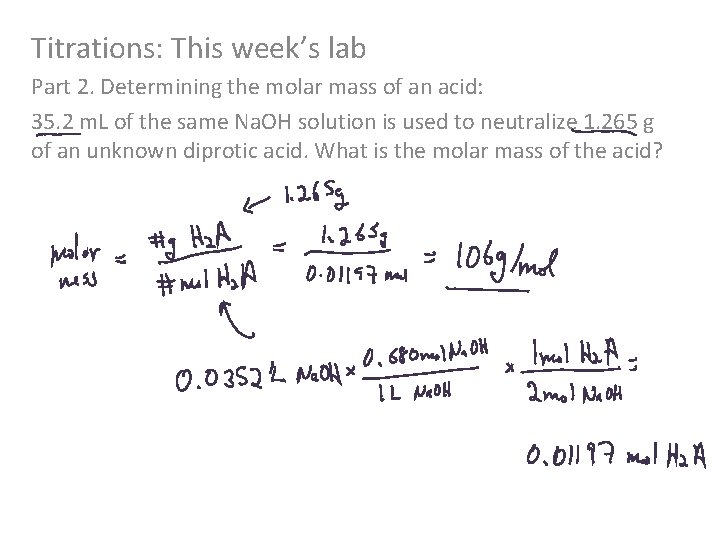
Titrations: This week’s lab Part 2. Determining the molar mass of an acid: 35. 2 m. L of the same Na. OH solution is used to neutralize 1. 265 g of an unknown diprotic acid. What is the molar mass of the acid?



Preparing Solutions: Dilution from a concentrated (stock) solution How many m. L of a 2. 60 M Ni. Cl 2 solution would you use to prepare 100 m. L of a 0. 300 M solution?

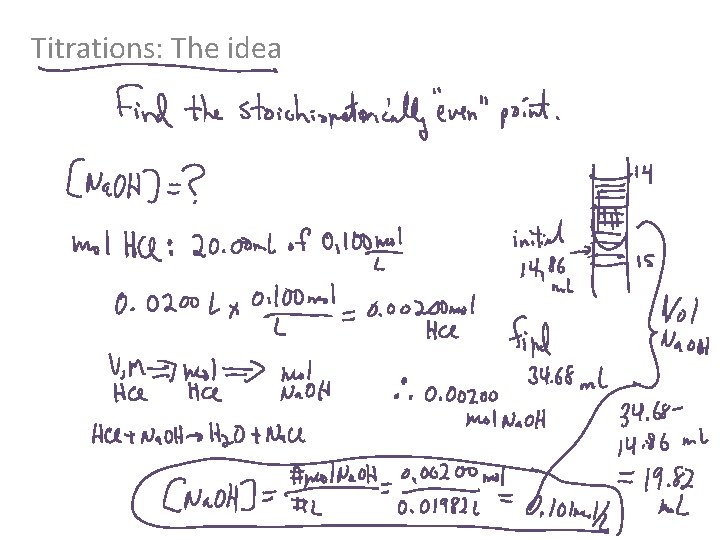
Titrations: The idea



Titrations: This week’s lab Part 1. “Standardizing” a solution of base: 23. 8 m. L of an Na. OH solution is used to neutralize 1. 020 g H 2 C 2 O 4 -2 H 2 O. What is the concentration of the Na. OH solution?

Titrations: This week’s lab Part 2. Determining the molar mass of an acid: 35. 2 m. L of the same Na. OH solution is used to neutralize 1. 265 g of an unknown diprotic acid. What is the molar mass of the acid?


Labs this week…

Chapter 4: Types of Chemical Reactions Goals: • To be able to predict chemical reactivity. • To know how to synthesize specific compounds.

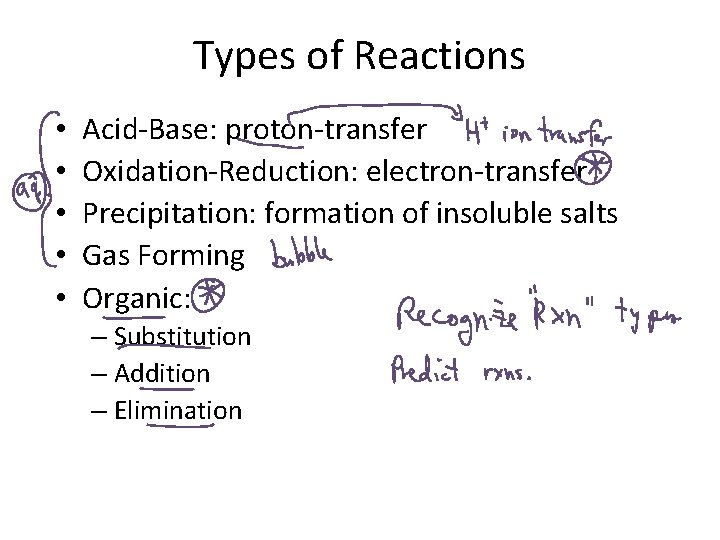
Types of Reactions • • • Acid-Base: proton-transfer Oxidation-Reduction: electron-transfer Precipitation: formation of insoluble salts Gas Forming Organic: – Substitution – Addition – Elimination

Reactions in Aqueous Solution Unless mentioned, all reactions studied this and next week occur in aqueous solution.

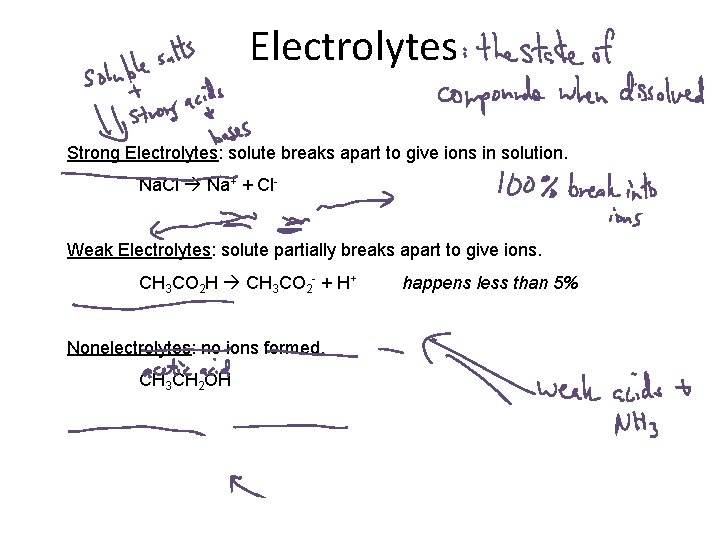
Electrolytes Strong Electrolytes: solute breaks apart to give ions in solution. Na. Cl Na+ + Cl. Weak Electrolytes: solute partially breaks apart to give ions. CH 3 CO 2 H CH 3 CO 2 - + H+ Nonelectrolytes: no ions formed. CH 3 CH 2 OH happens less than 5%

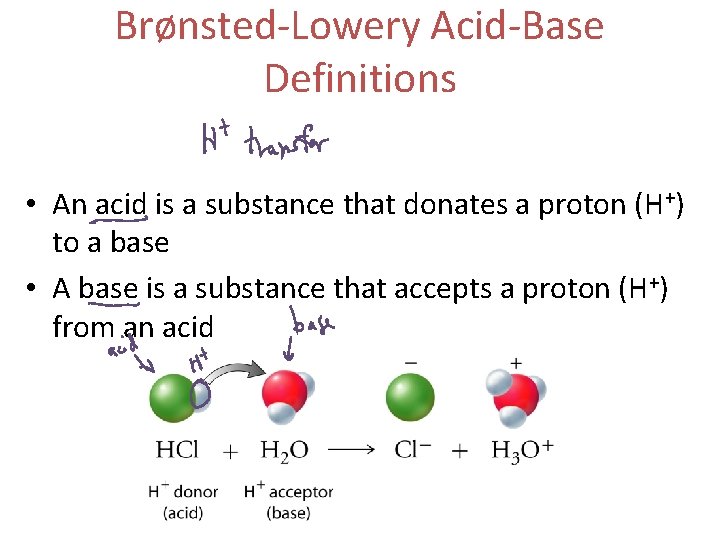
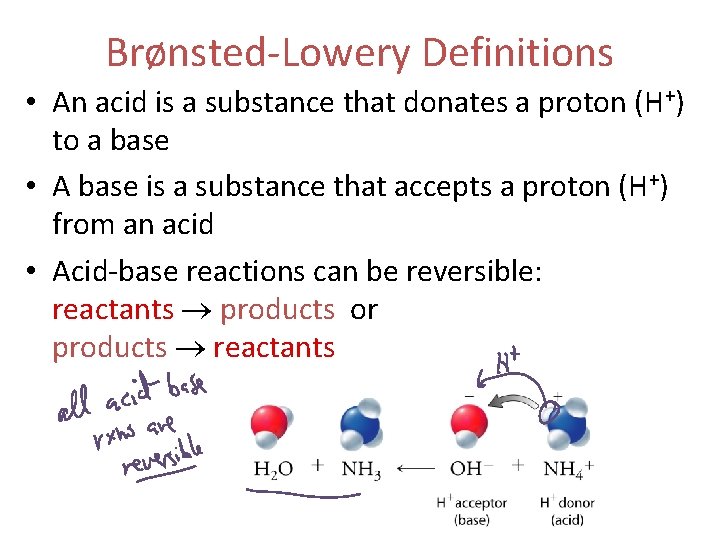
Brønsted-Lowery Acid-Base Definitions • An acid is a substance that donates a proton (H+) to a base • A base is a substance that accepts a proton (H+) from an acid

Brønsted-Lowery Definitions • acid: donates a proton (H+) to a base • base: accepts a proton (H+) from an acid • Acid-base reactions can be reversible: reactants products or products reactants

Brønsted-Lowery Definitions • An acid is a substance that donates a proton (H+) to a base • A base is a substance that accepts a proton (H+) from an acid • Acid-base reactions can be reversible: reactants products or products reactants

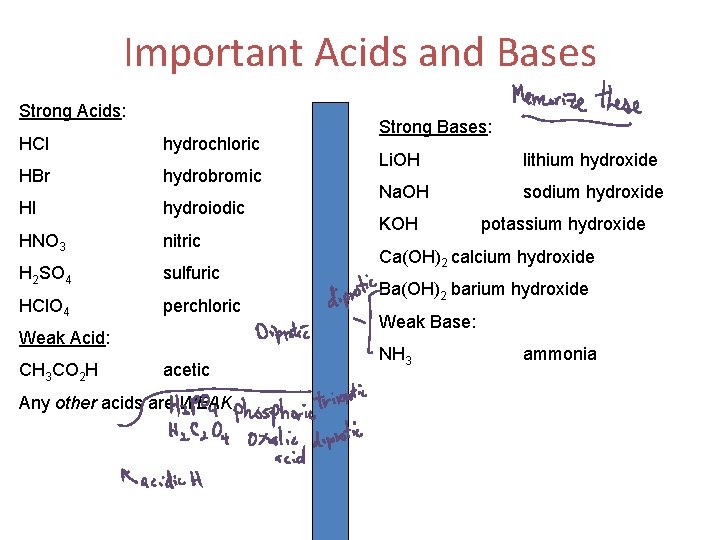
Important Acids and Bases Strong Acids: HCl hydrochloric HBr hydrobromic HI hydroiodic HNO 3 nitric H 2 SO 4 sulfuric HCl. O 4 perchloric Weak Acid: CH 3 CO 2 H acetic Any other acids are WEAK Strong Bases: Li. OH lithium hydroxide Na. OH sodium hydroxide KOH potassium hydroxide Ca(OH)2 calcium hydroxide Ba(OH)2 barium hydroxide Weak Base: NH 3 ammonia

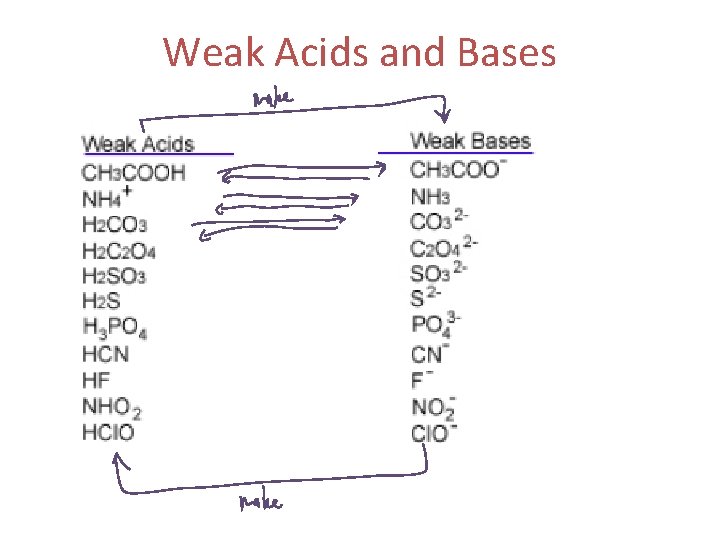
Weak Acids and Bases

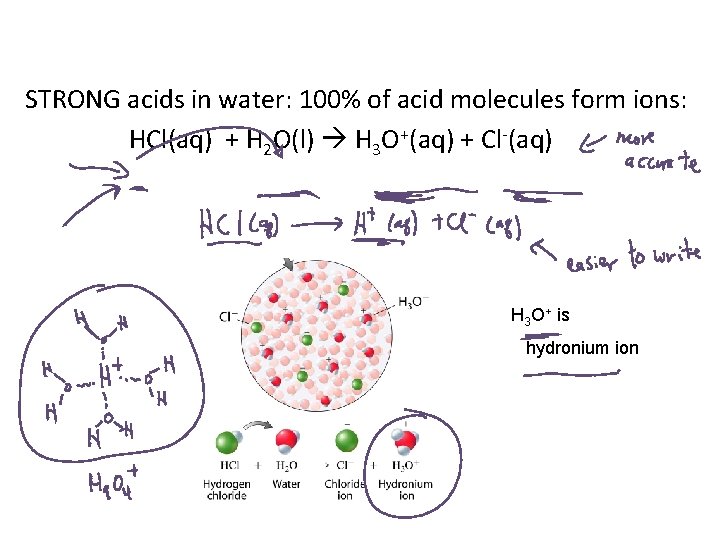
STRONG acids in water: 100% of acid molecules form ions: HCl(aq) + H 2 O(l) H 3 O+(aq) + Cl-(aq) H 3 O+ is hydronium ion

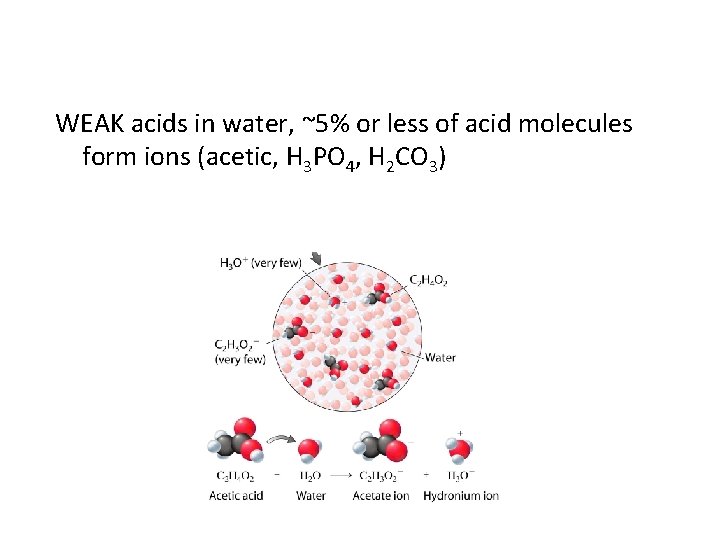
WEAK acids in water, ~5% or less of acid molecules form ions (acetic, H 3 PO 4, H 2 CO 3)

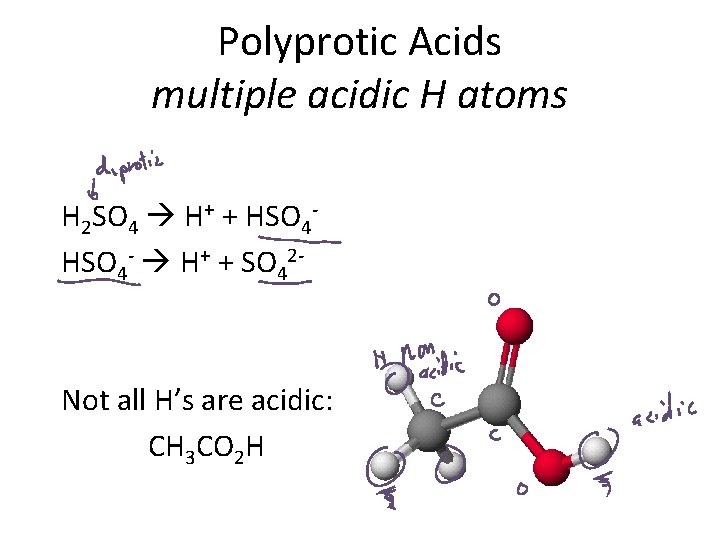
Polyprotic Acids multiple acidic H atoms H 2 SO 4 H+ + HSO 4 - H+ + SO 42 - Not all H’s are acidic: CH 3 CO 2 H
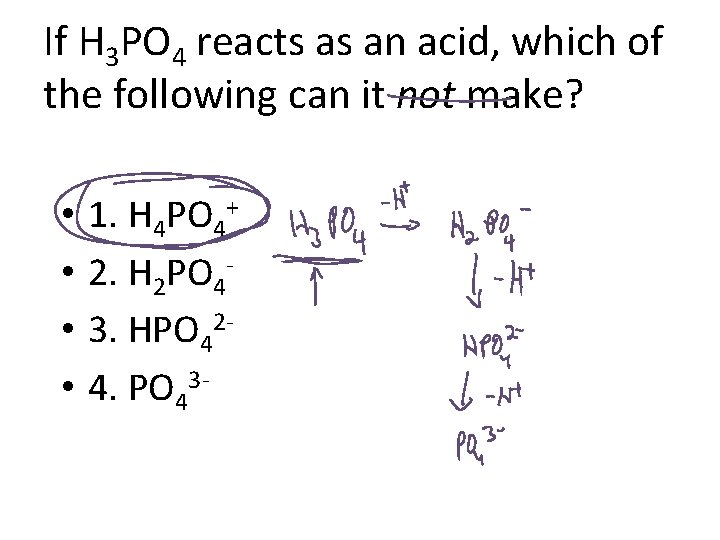
If H 3 PO 4 reacts as an acid, which of the following can it not make? • • 1. H 4 PO 4+ 2. H 2 PO 43. HPO 424. PO 43 -

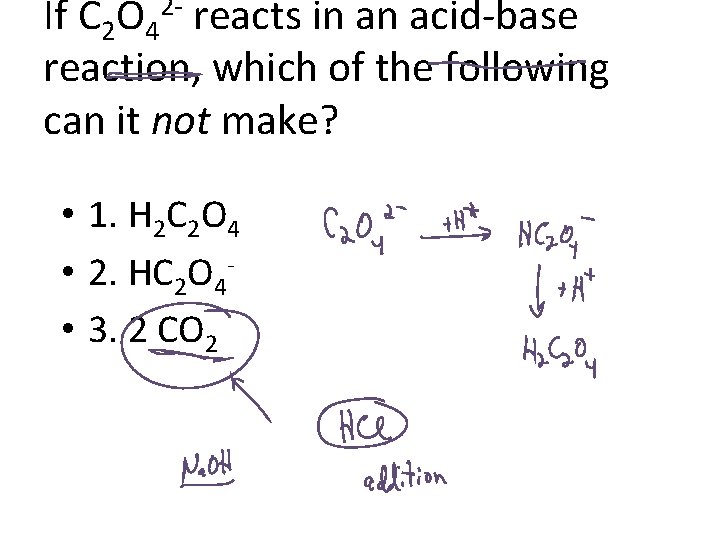
If C 2 O 4 reacts in an acid-base reaction, which of the following can it not make? 2 - • 1. H 2 C 2 O 4 • 2. HC 2 O 4 • 3. 2 CO 2

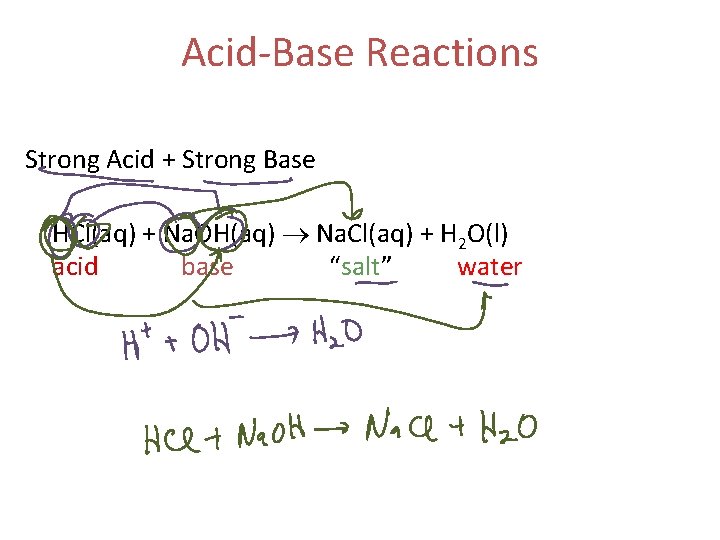
Acid-Base Reactions Strong Acid + Strong Base HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) acid base “salt” water

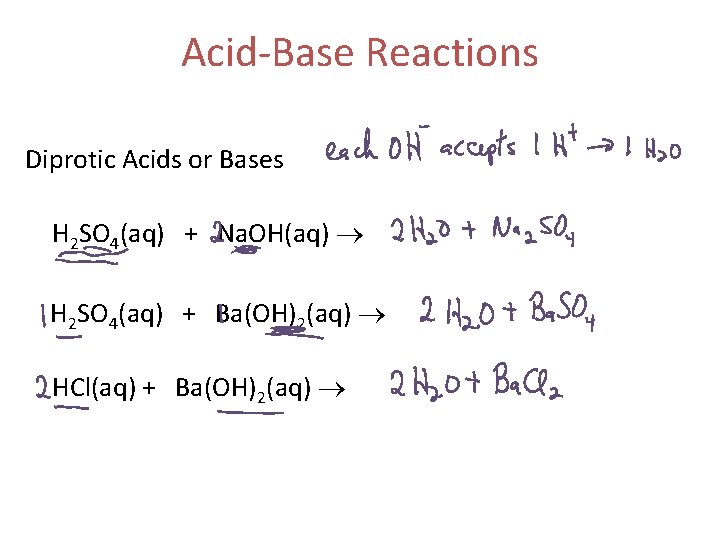
Acid-Base Reactions Diprotic Acids or Bases H 2 SO 4(aq) + Na. OH(aq) H 2 SO 4(aq) + Ba(OH)2(aq) HCl(aq) + Ba(OH)2(aq)

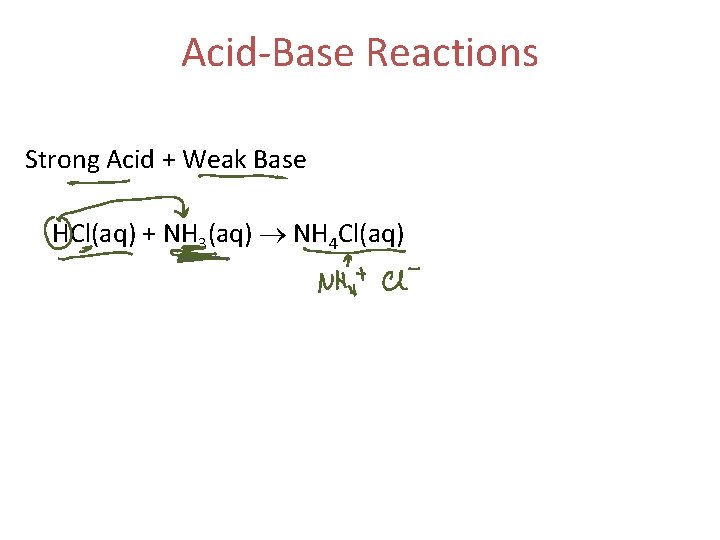
Acid-Base Reactions Strong Acid + Weak Base HCl(aq) + NH 3(aq) NH 4 Cl(aq)

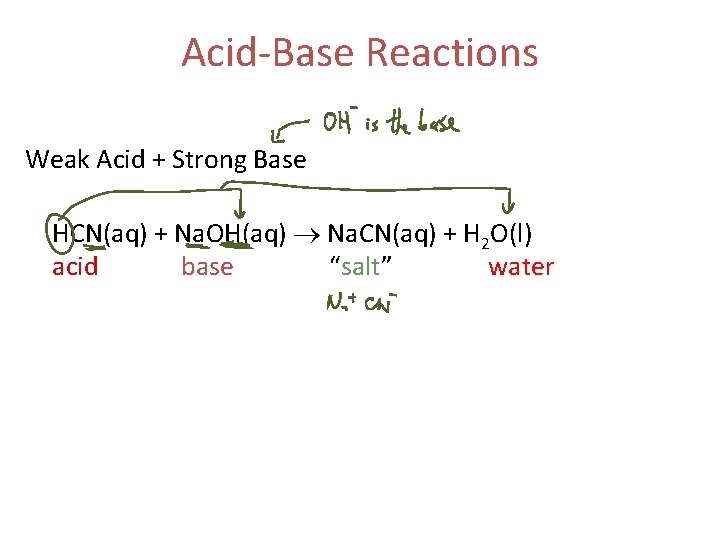
Acid-Base Reactions Weak Acid + Strong Base HCN(aq) + Na. OH(aq) Na. CN(aq) + H 2 O(l) acid base “salt” water

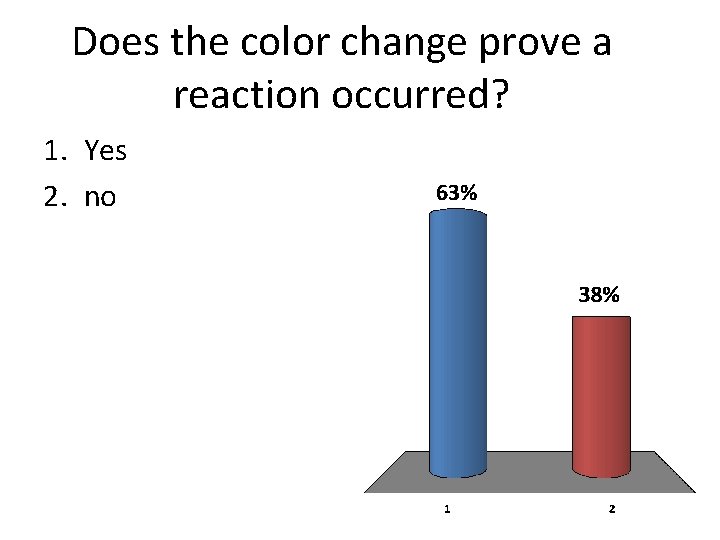
Does the color change prove a reaction occurred? 1. Yes 2. no

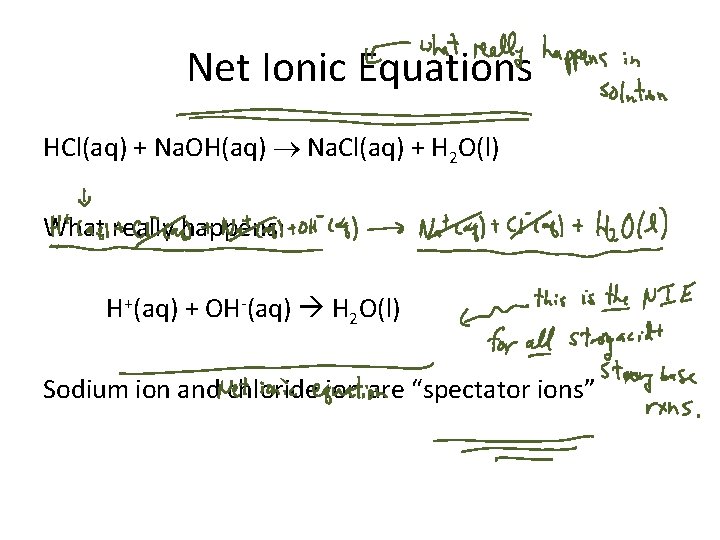
Net Ionic Equations HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) What really happens: H+(aq) + OH-(aq) H 2 O(l) Sodium ion and chloride ion are “spectator ions”

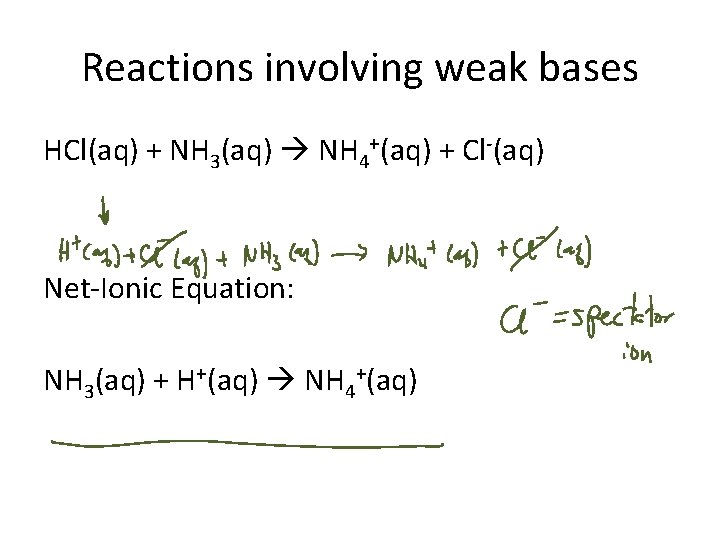
Reactions involving weak bases HCl(aq) + NH 3(aq) NH 4+(aq) + Cl-(aq) Net-Ionic Equation: NH 3(aq) + H+(aq) NH 4+(aq)

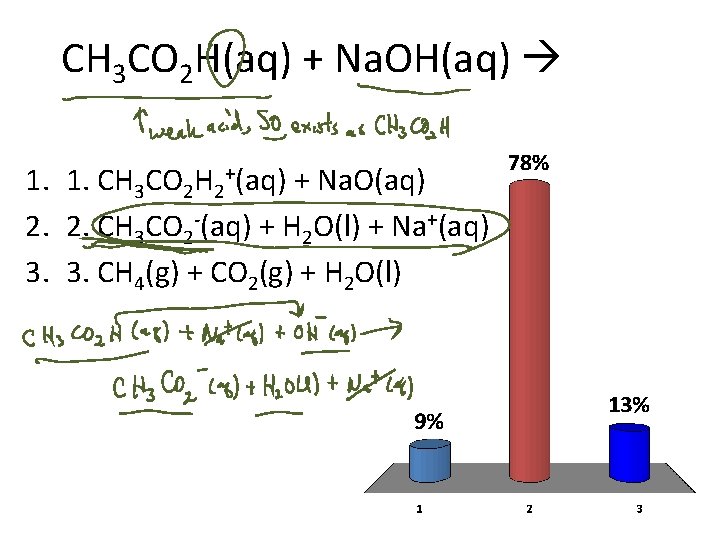
CH 3 CO 2 H(aq) + Na. OH(aq) 1. 1. CH 3 CO 2 H 2+(aq) + Na. O(aq) 2. 2. CH 3 CO 2 -(aq) + H 2 O(l) + Na+(aq) 3. 3. CH 4(g) + CO 2(g) + H 2 O(l)

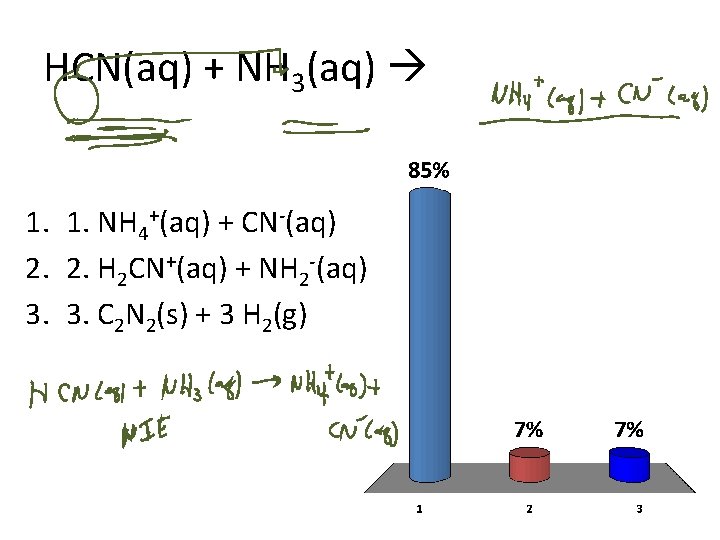
HCN(aq) + NH 3(aq) 1. 1. NH 4+(aq) + CN-(aq) 2. 2. H 2 CN+(aq) + NH 2 -(aq) 3. 3. C 2 N 2(s) + 3 H 2(g)

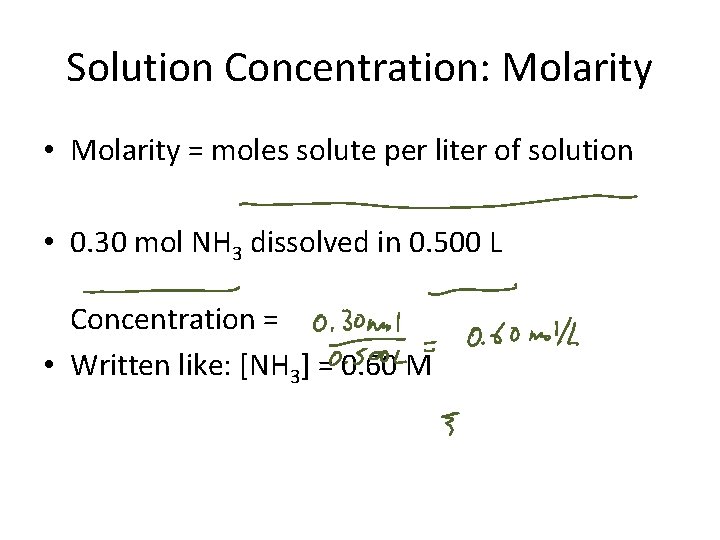
Solution Concentration: Molarity • Molarity = moles solute per liter of solution • 0. 30 mol NH 3 dissolved in 0. 500 L Concentration = • Written like: [NH 3] = 0. 60 M

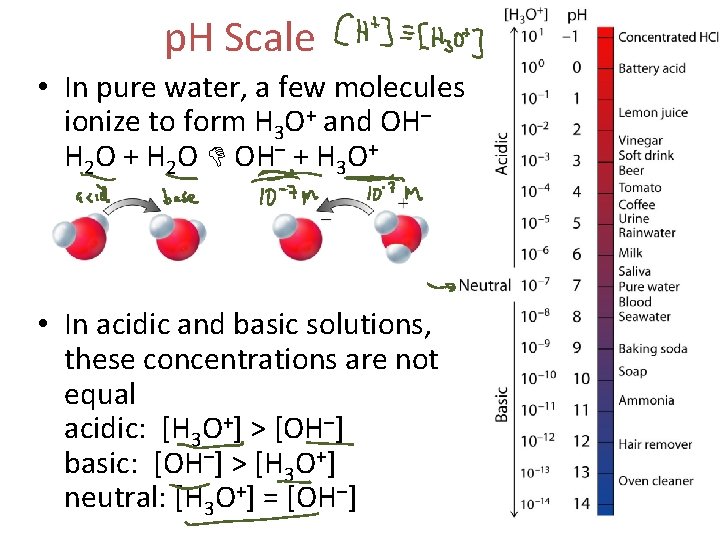
p. H Scale • In pure water, a few molecules ionize to form H 3 O+ and OH– H 2 O + H 2 O OH– + H 3 O+ • In acidic and basic solutions, these concentrations are not equal acidic: [H 3 O+] > [OH–] basic: [OH–] > [H 3 O+] neutral: [H 3 O+] = [OH–]

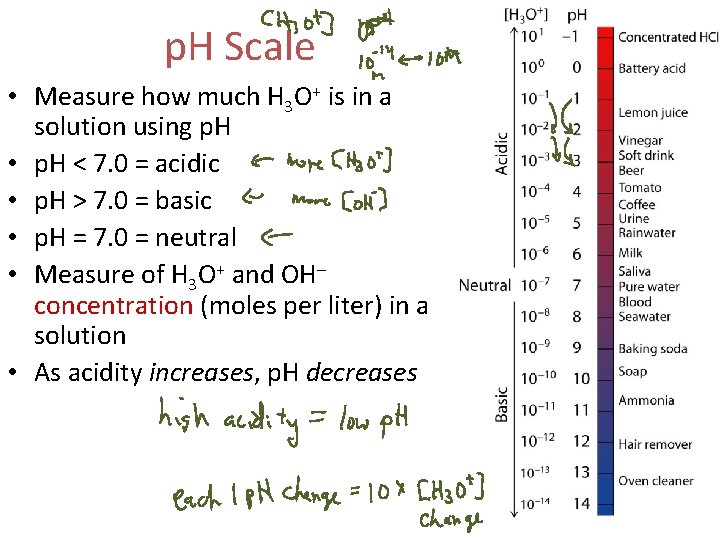
p. H Scale • Measure how much H 3 O+ is in a solution using p. H • p. H < 7. 0 = acidic • p. H > 7. 0 = basic • p. H = 7. 0 = neutral • Measure of H 3 O+ and OH– concentration (moles per liter) in a solution • As acidity increases, p. H decreases

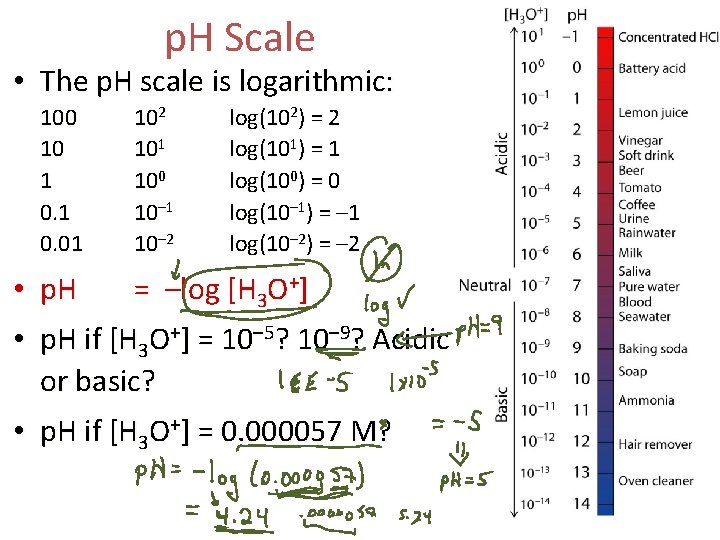
p. H Scale • The p. H scale is logarithmic: 100 10 1 0. 01 102 101 100 10– 1 10– 2 log(102) = 2 log(101) = 1 log(100) = 0 log(10– 1) = – 1 log(10– 2) = – 2 • p. H = –log [H 3 O+] • p. H if [H 3 O+] = 10– 5? 10– 9? Acidic or basic? • p. H if [H 3 O+] = 0. 000057 M?
![Finding H 3 O from p H H 3 O 10 p H Finding [H 3 O+] from p. H [H 3 O+] = 10 -p. H](https://slidetodoc.com/presentation_image_h2/94e8fc5201c84b2288abfa3b0c08a6ae/image-47.jpg)
Finding [H 3 O+] from p. H [H 3 O+] = 10 -p. H What is [H 3 O+] if p. H = 8. 9?

p. H: Quantitative Measure of Acidity • Acidity is related to concentration of H+ (or H 3 O +) • p. H = -log[H 3 O+]
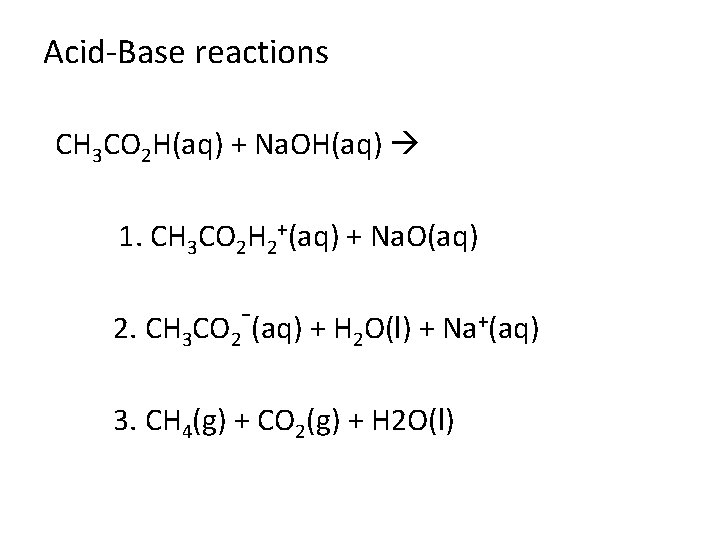
Acid-Base reactions CH 3 CO 2 H(aq) + Na. OH(aq) 1. CH 3 CO 2 H 2+(aq) + Na. O(aq) 2. CH 3 CO 2 (aq) + H 2 O(l) + Na+(aq) 3. CH 4(g) + CO 2(g) + H 2 O(l)
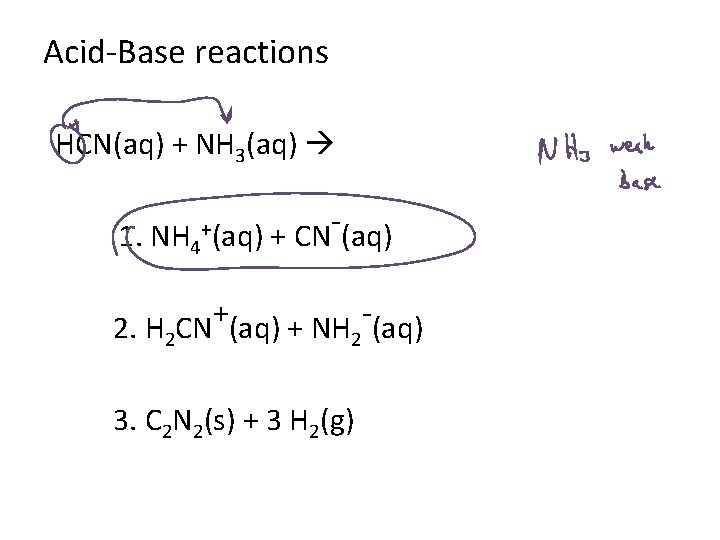
Acid-Base reactions HCN(aq) + NH 3(aq) 1. NH 4 +(aq) + CN (aq) + 2. H 2 CN (aq) + NH 2 (aq) 3. C 2 N 2(s) + 3 H 2(g)

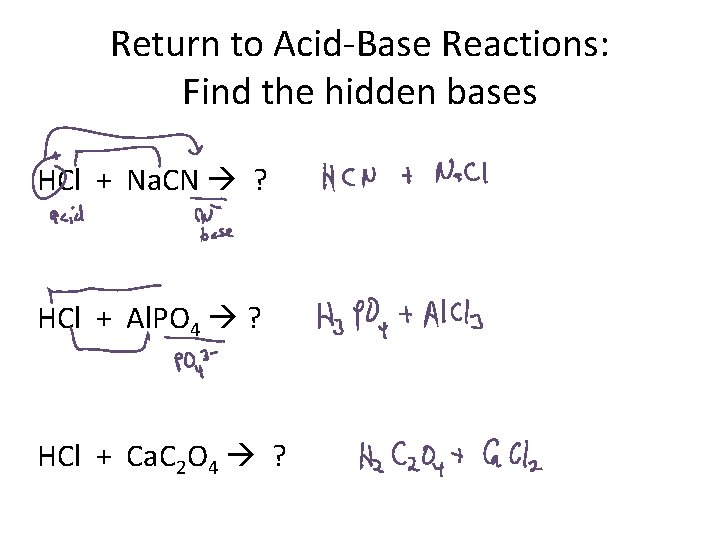
Acid-Base Reactions: Find the hidden bases Thing to know: Anions of weak acids are bases. Example: CH 3 COOH = weak acid CH 3 COO- therefore = weak base HCN = weak acid CN- = weak base H 3 PO 4 = weak acid PO 43 - = weak base

Return to Acid-Base Reactions: Find the hidden bases HCl + Na. CN ? HCl + Al. PO 4 ? HCl + Ca. C 2 O 4 ?


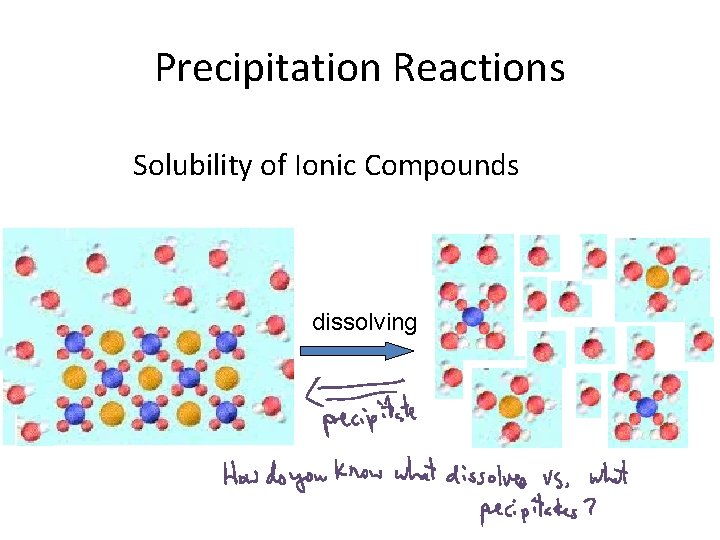
Precipitation Reactions Solubility of Ionic Compounds dissolving

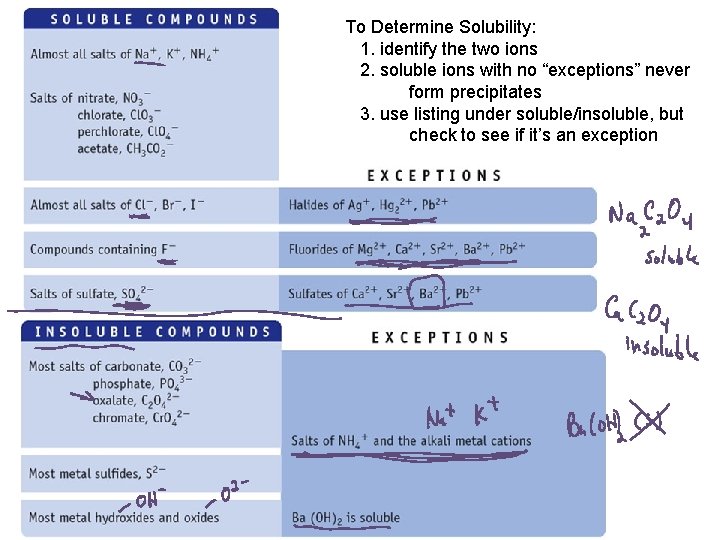
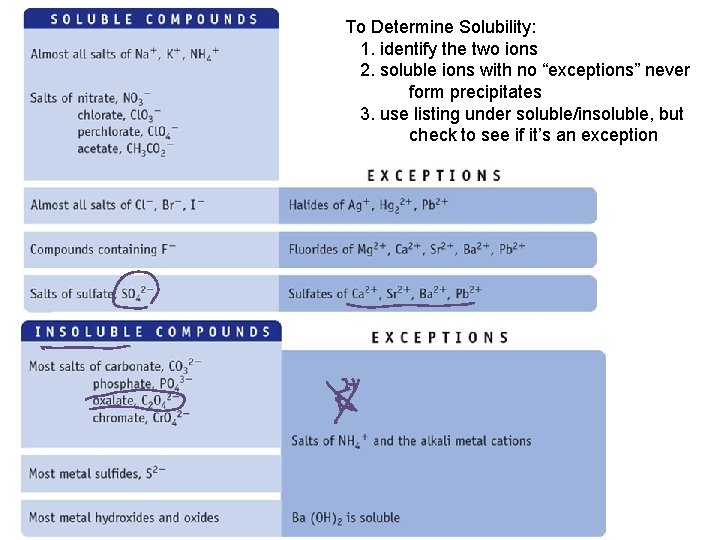
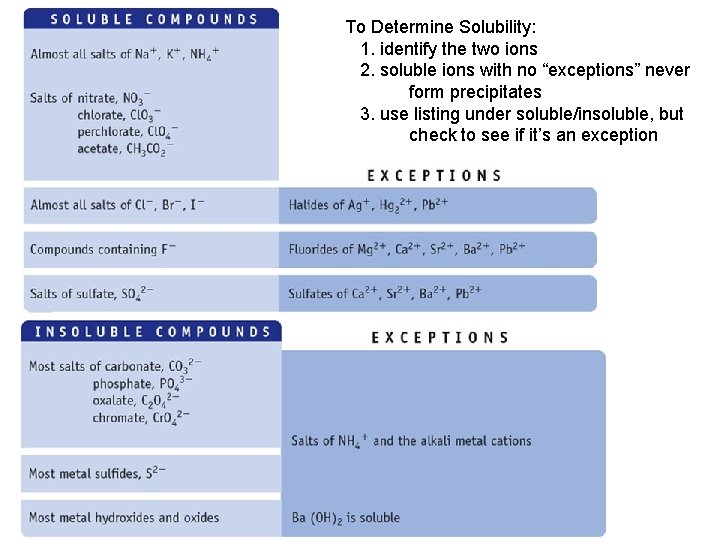
To Determine Solubility: 1. identify the two ions 2. soluble ions with no “exceptions” never form precipitates 3. use listing under soluble/insoluble, but check to see if it’s an exception

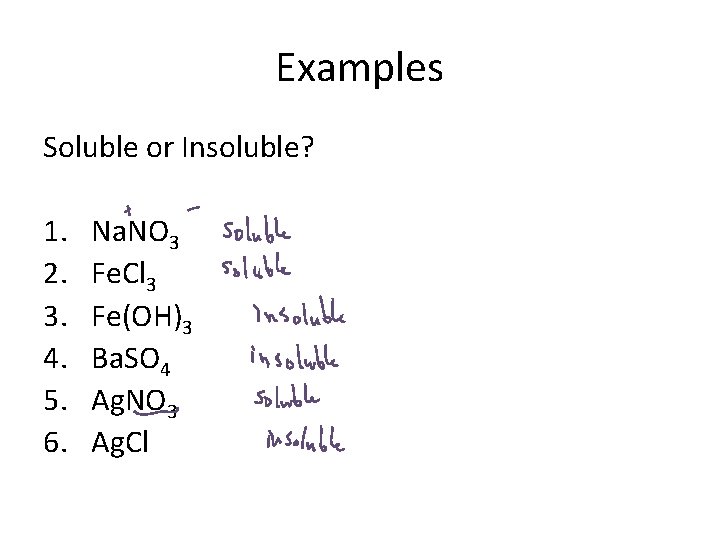
Examples Soluble or Insoluble? 1. 2. 3. 4. 5. 6. Na. NO 3 Fe. Cl 3 Fe(OH)3 Ba. SO 4 Ag. NO 3 Ag. Cl

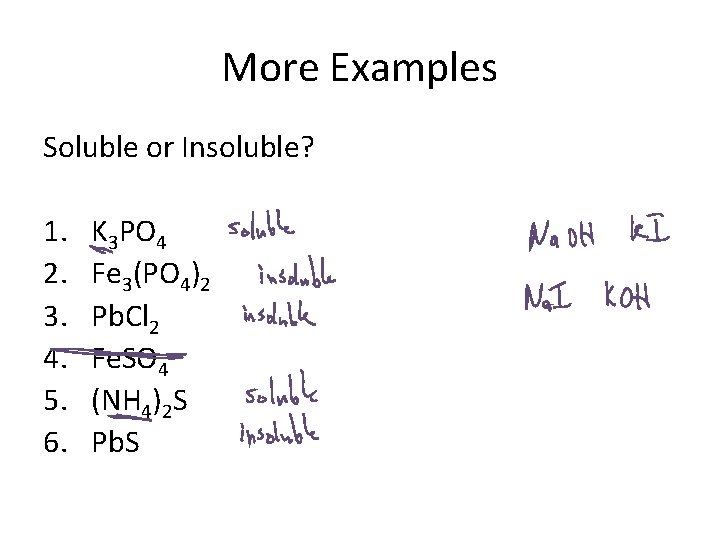
More Examples Soluble or Insoluble? 1. 2. 3. 4. 5. 6. K 3 PO 4 Fe 3(PO 4)2 Pb. Cl 2 Fe. SO 4 (NH 4)2 S Pb. S

To Determine Solubility: 1. identify the two ions 2. soluble ions with no “exceptions” never form precipitates 3. use listing under soluble/insoluble, but check to see if it’s an exception

PRS Example 1 Which of the following is soluble? 1. 2. 3. 4. Al. PO 4 Pb. Br 2 Al(OH)3 Fe. SO 4

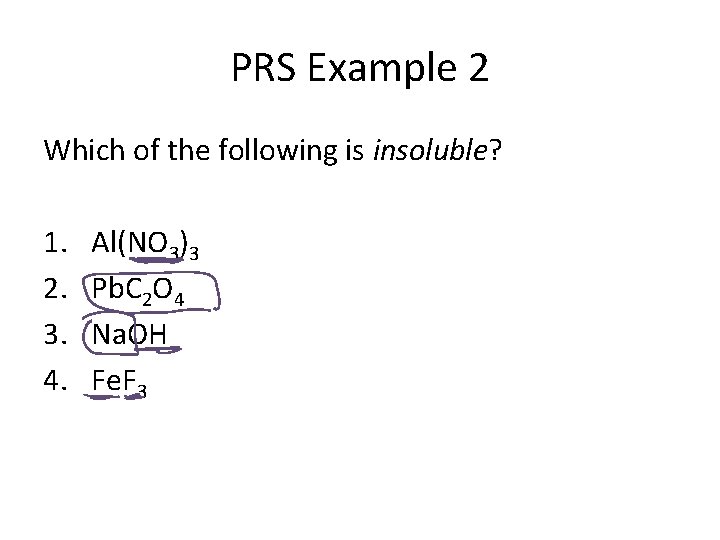
PRS Example 2 Which of the following is insoluble? 1. 2. 3. 4. Al(NO 3)3 Pb. C 2 O 4 Na. OH Fe. F 3

To Determine Solubility: 1. identify the two ions 2. soluble ions with no “exceptions” never form precipitates 3. use listing under soluble/insoluble, but check to see if it’s an exception

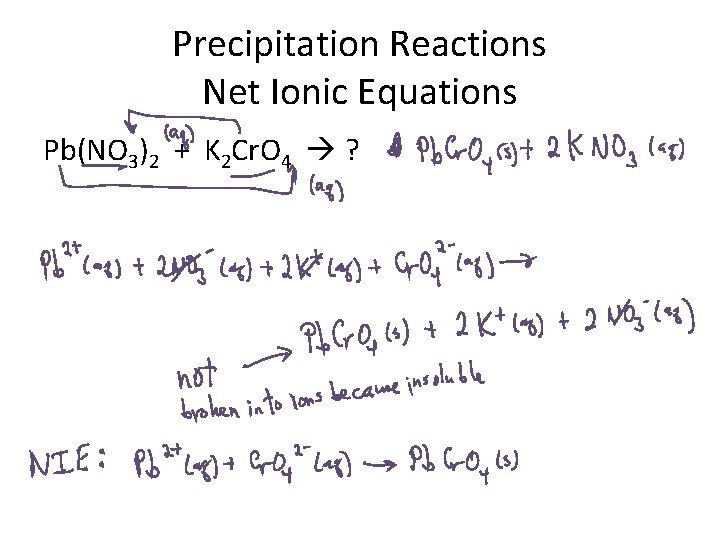
Precipitation Reactions Net Ionic Equations Pb(NO 3)2 + K 2 Cr. O 4 ?

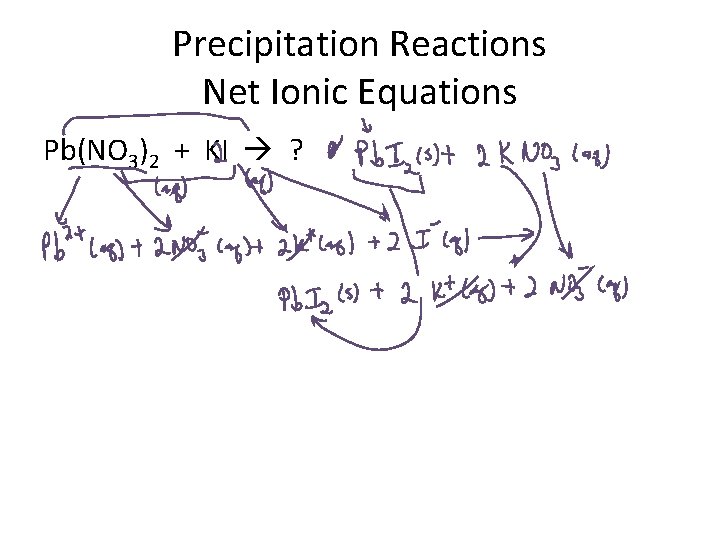
Precipitation Reactions Net Ionic Equations Pb(NO 3)2 + KI ?

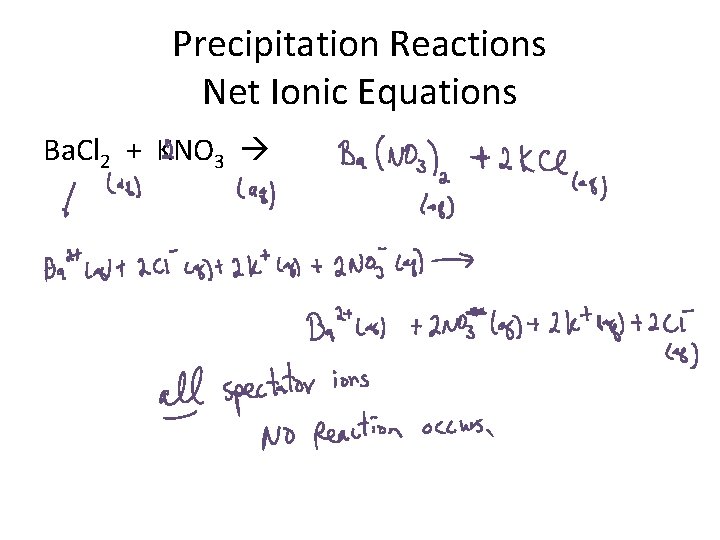
Precipitation Reactions Net Ionic Equations Ba. Cl 2 + KNO 3

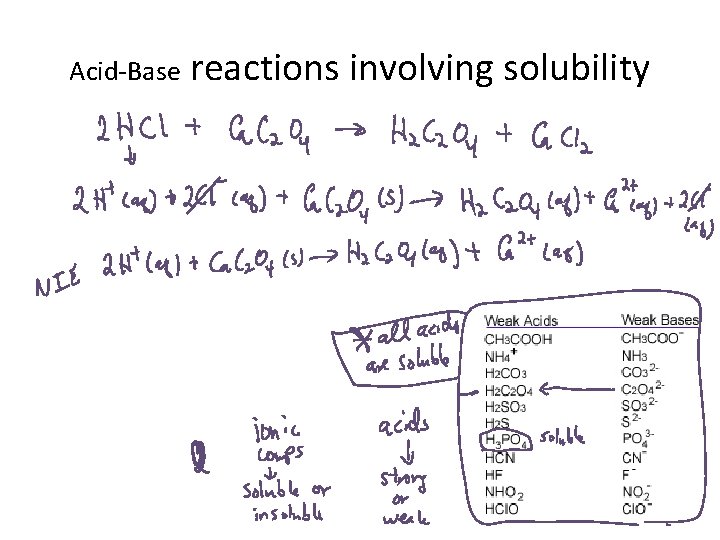
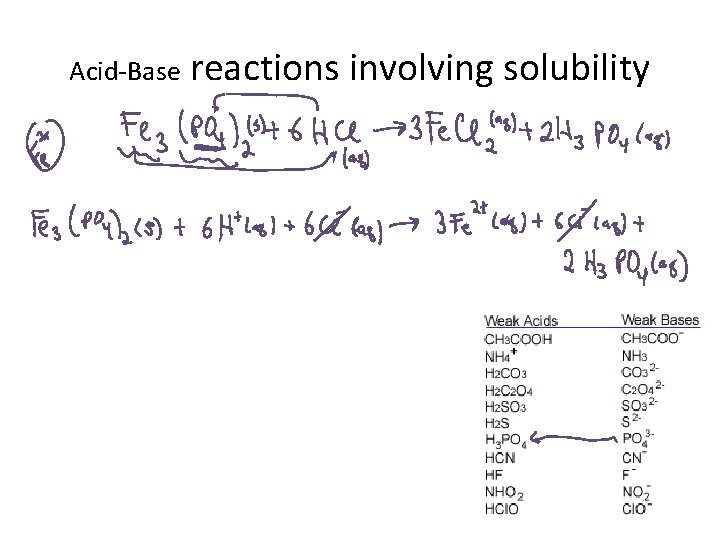
Acid-Base reactions involving solubility

Acid-Base reactions involving solubility


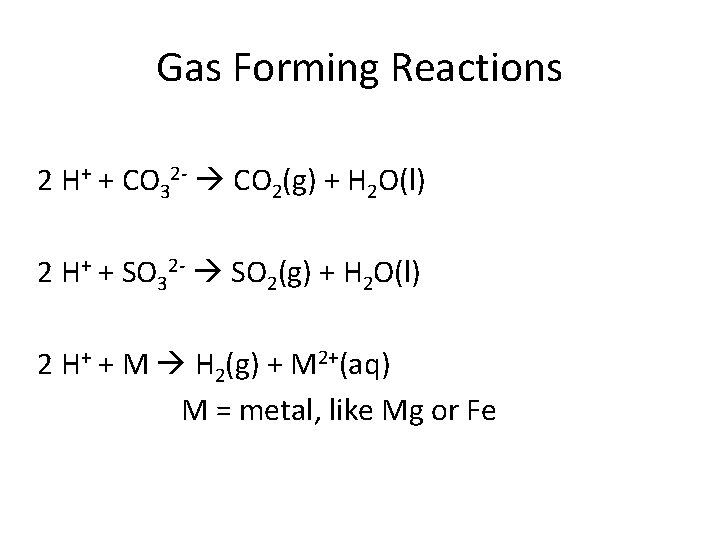
Gas Forming Reactions 2 H+ + CO 32 - CO 2(g) + H 2 O(l) 2 H+ + SO 32 - SO 2(g) + H 2 O(l) 2 H+ + M H 2(g) + M 2+(aq) M = metal, like Mg or Fe

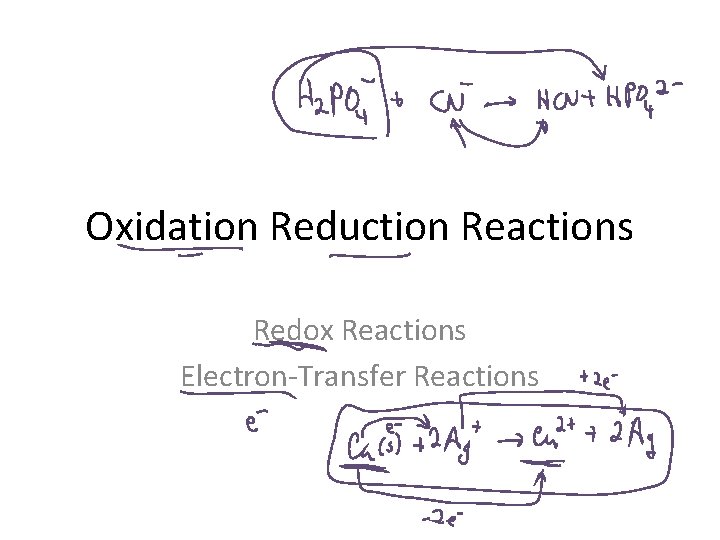
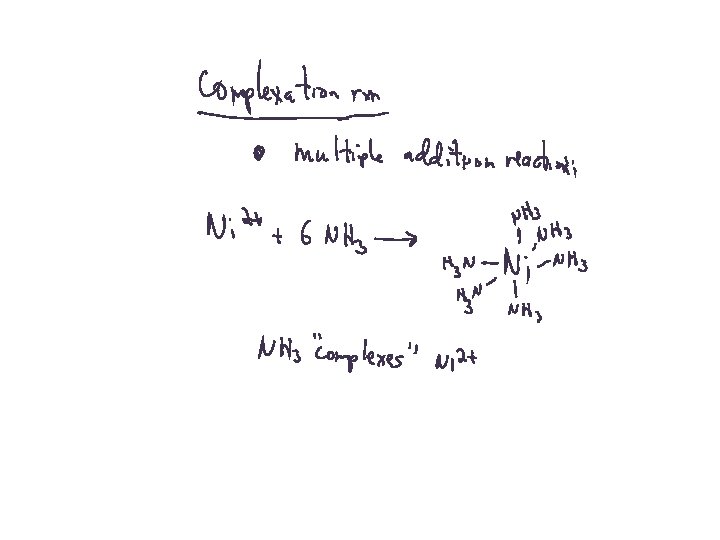
Oxidation Reduction Reactions Redox Reactions Electron-Transfer Reactions

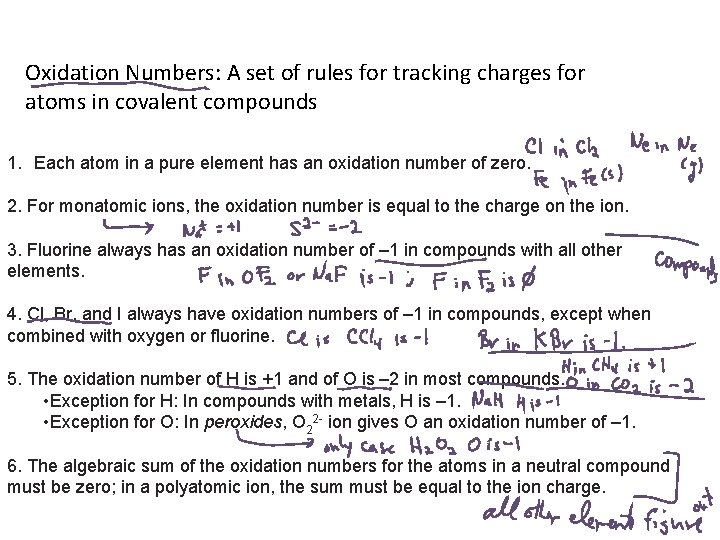
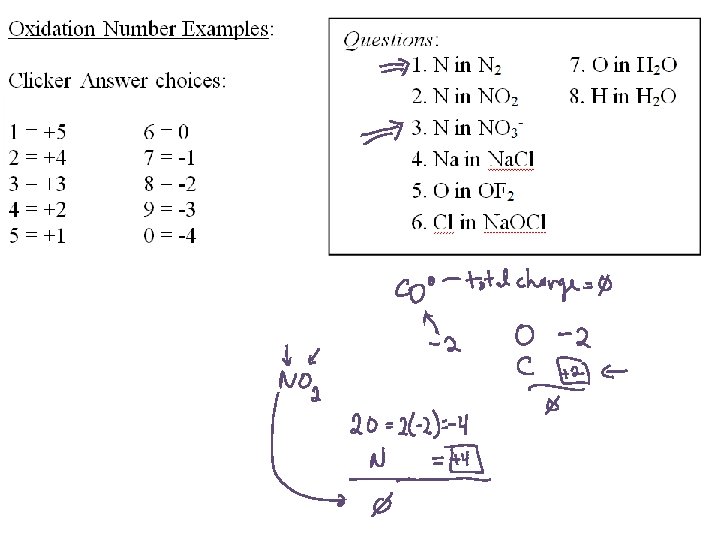
Oxidation Numbers: A set of rules for tracking charges for atoms in covalent compounds 1. Each atom in a pure element has an oxidation number of zero. 2. For monatomic ions, the oxidation number is equal to the charge on the ion. 3. Fluorine always has an oxidation number of – 1 in compounds with all other elements. 4. Cl, Br, and I always have oxidation numbers of – 1 in compounds, except when combined with oxygen or fluorine. 5. The oxidation number of H is +1 and of O is – 2 in most compounds. • Exception for H: In compounds with metals, H is – 1. • Exception for O: In peroxides, O 22 - ion gives O an oxidation number of – 1. 6. The algebraic sum of the oxidation numbers for the atoms in a neutral compound must be zero; in a polyatomic ion, the sum must be equal to the ion charge.


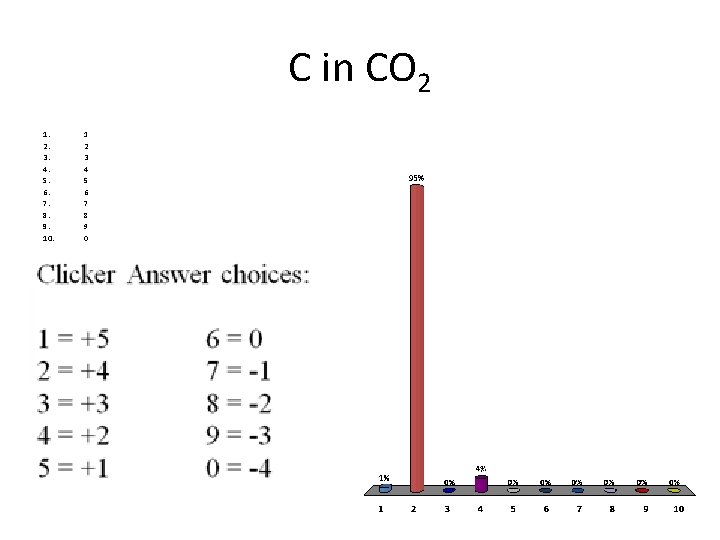
C in CO 2 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 1 2 3 4 5 6 7 8 9 0

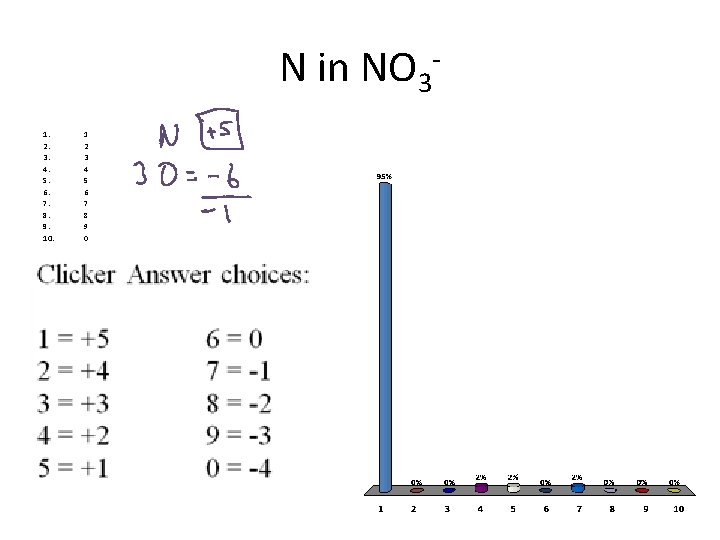
N in NO 31. 2. 3. 4. 5. 6. 7. 8. 9. 10. 1 2 3 4 5 6 7 8 9 0

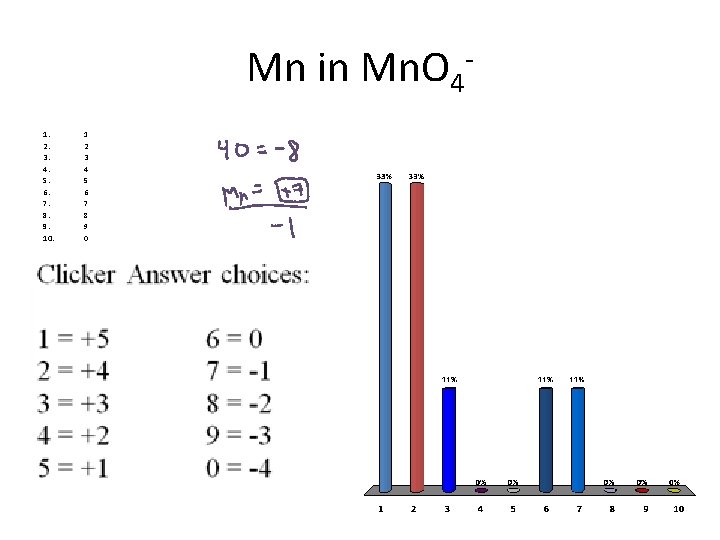
Mn in Mn. O 41. 2. 3. 4. 5. 6. 7. 8. 9. 10. 1 2 3 4 5 6 7 8 9 0

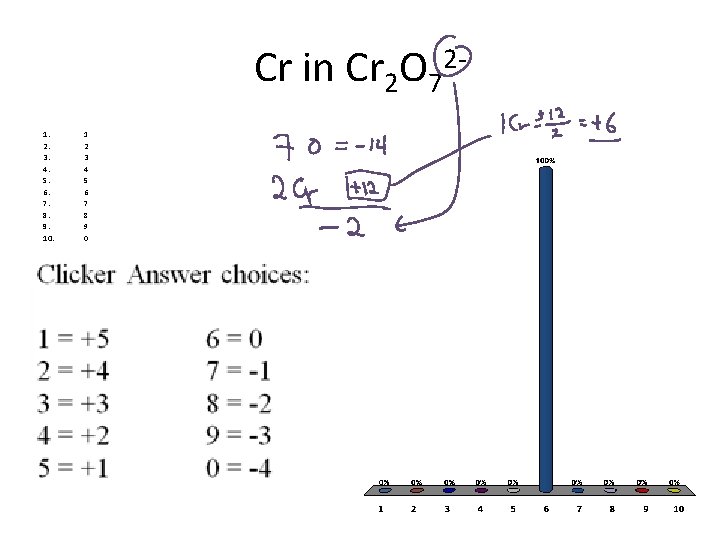
Cr in Cr 2 O 721. 2. 3. 4. 5. 6. 7. 8. 9. 10. 1 2 3 4 5 6 7 8 9 0

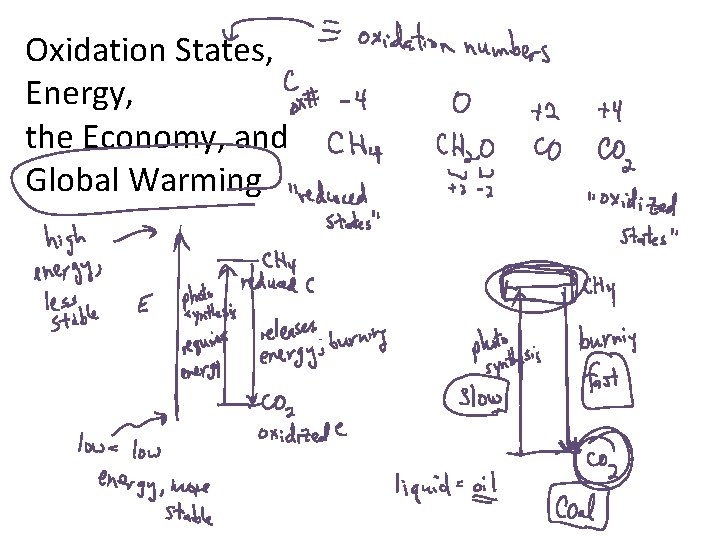
Oxidation States, Energy, the Economy, and Global Warming

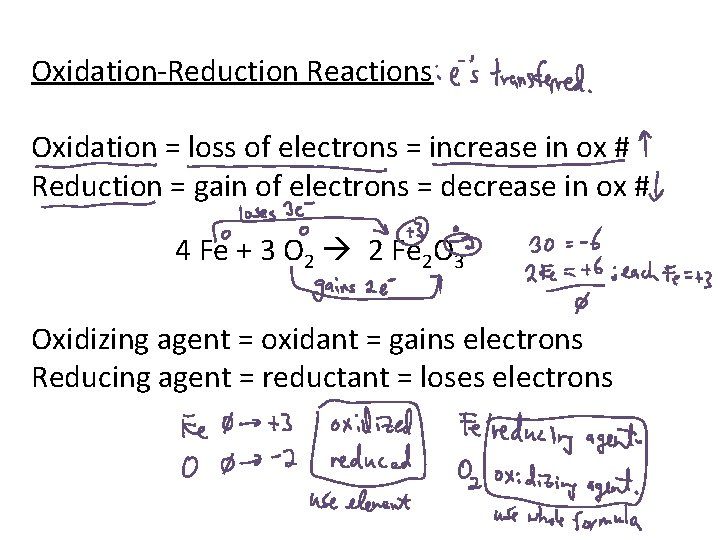
Oxidation-Reduction Reactions Oxidation = loss of electrons = increase in ox # Reduction = gain of electrons = decrease in ox # 4 Fe + 3 O 2 2 Fe 2 O 3 Oxidizing agent = oxidant = gains electrons Reducing agent = reductant = loses electrons

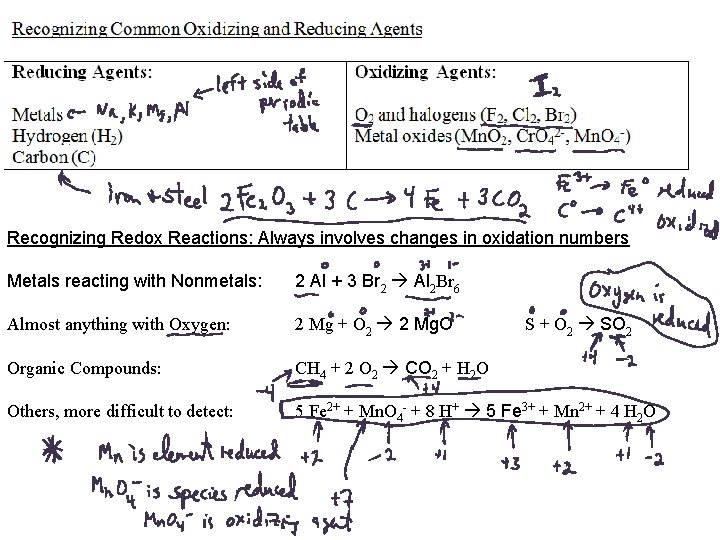
Recognizing Redox Reactions: Always involves changes in oxidation numbers Metals reacting with Nonmetals: 2 Al + 3 Br 2 Al 2 Br 6 Almost anything with Oxygen: 2 Mg + O 2 2 Mg. O Organic Compounds: CH 4 + 2 O 2 CO 2 + H 2 O Others, more difficult to detect: 5 Fe 2+ + Mn. O 4 - + 8 H+ 5 Fe 3+ + Mn 2+ + 4 H 2 O S + O 2 SO 2

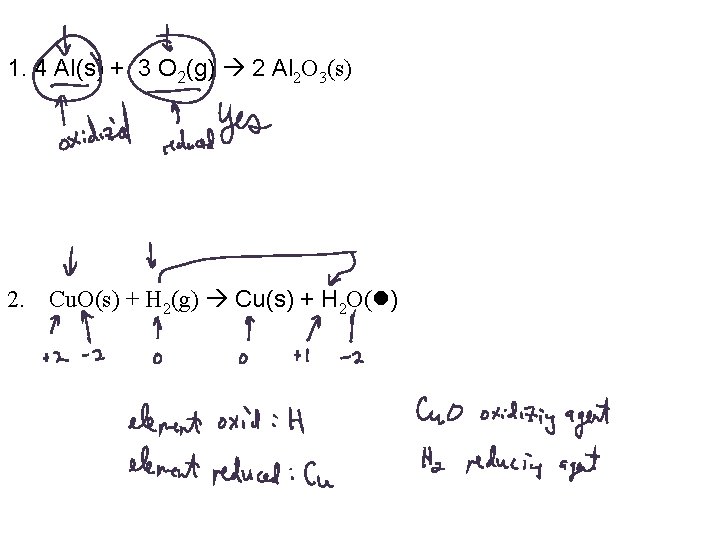
1. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) 2. Cu. O(s) + H 2(g) Cu(s) + H 2 O( )

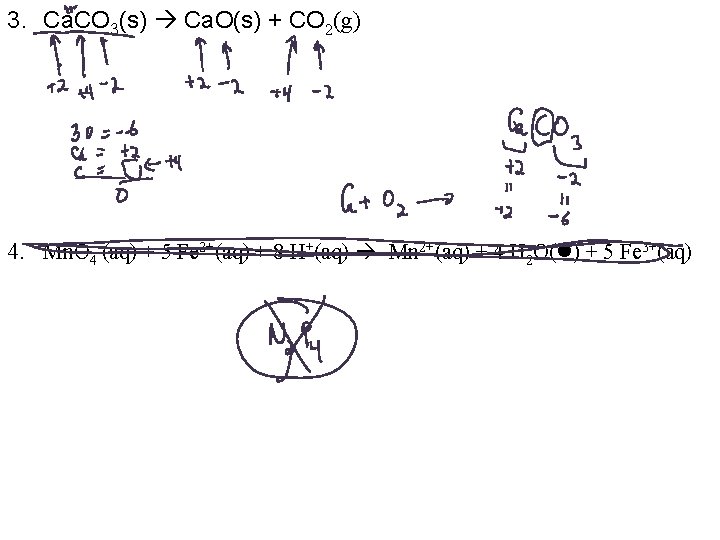
3. Ca. CO 3(s) Ca. O(s) + CO 2(g) 4. Mn. O 4 -(aq) + 5 Fe 2+(aq) + 8 H+(aq) Mn 2+(aq) + 4 H 2 O( ) + 5 Fe 3+(aq)

5. 2 H 2 O 2(aq) 2 H 2 O( ) + O 2(g) 6. Ca. CO 3(s) + 2 H+(aq) CO 2(g) + H 2 O( ) + Ca 2+(aq)

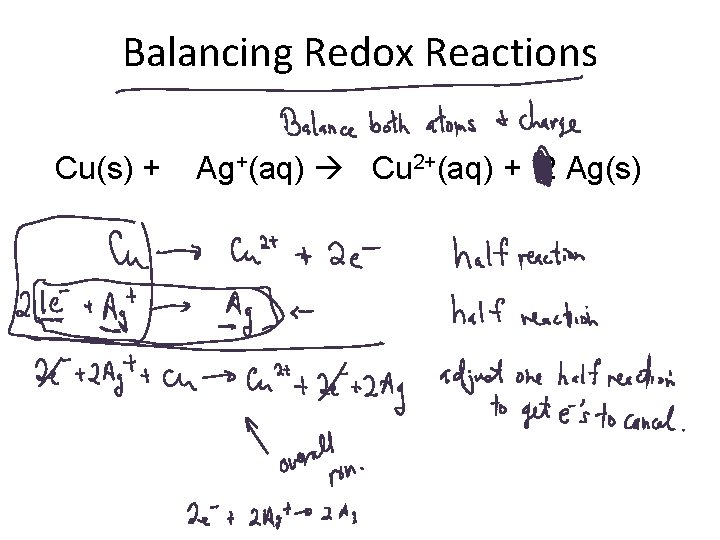
Balancing Redox Reactions Cu(s) + Ag+(aq) Cu 2+(aq) + 2 Ag(s)

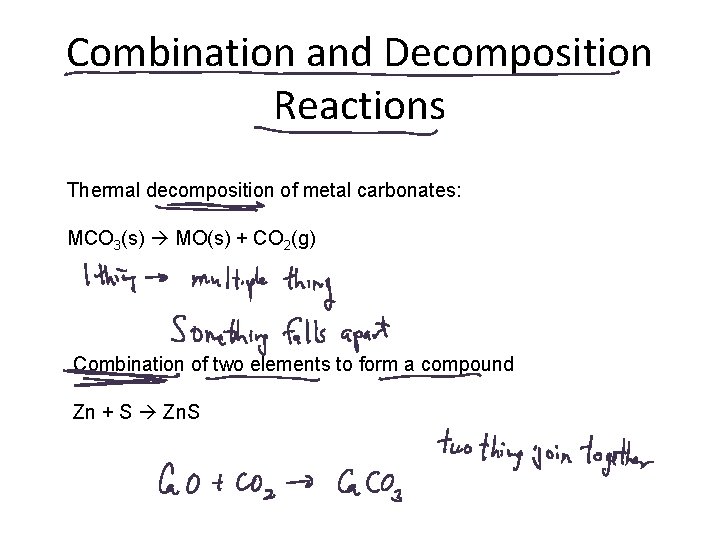
Combination and Decomposition Reactions Thermal decomposition of metal carbonates: MCO 3(s) MO(s) + CO 2(g) Combination of two elements to form a compound Zn + S Zn. S

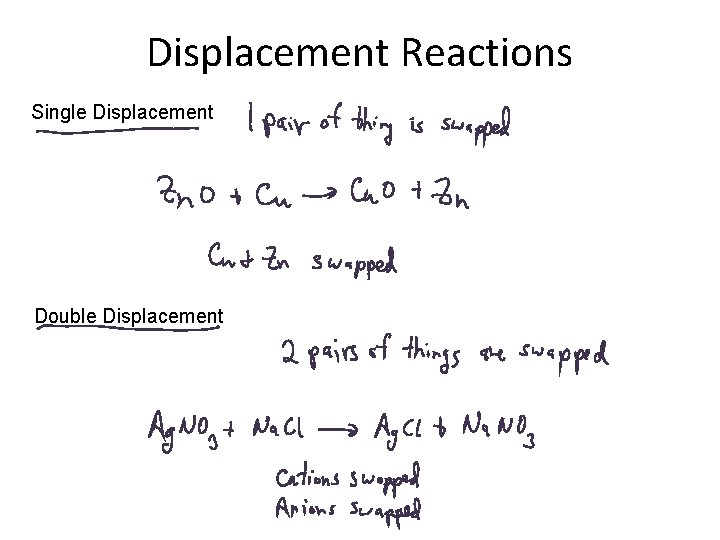
Displacement Reactions Single Displacement Double Displacement


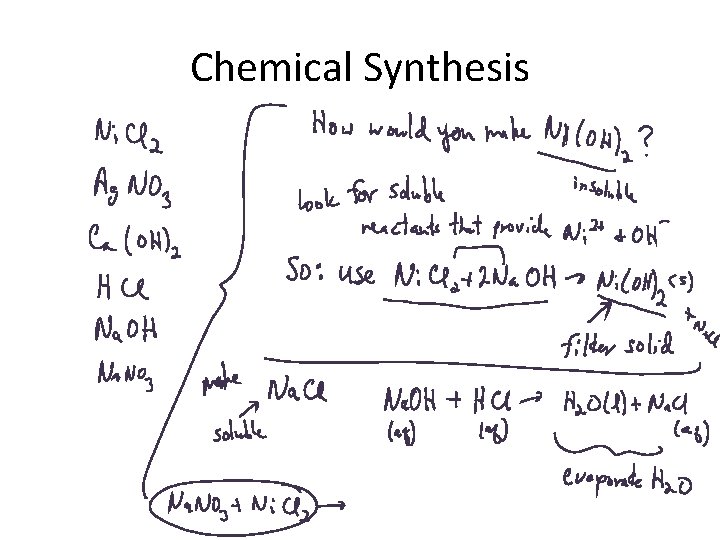
Chemical Synthesis




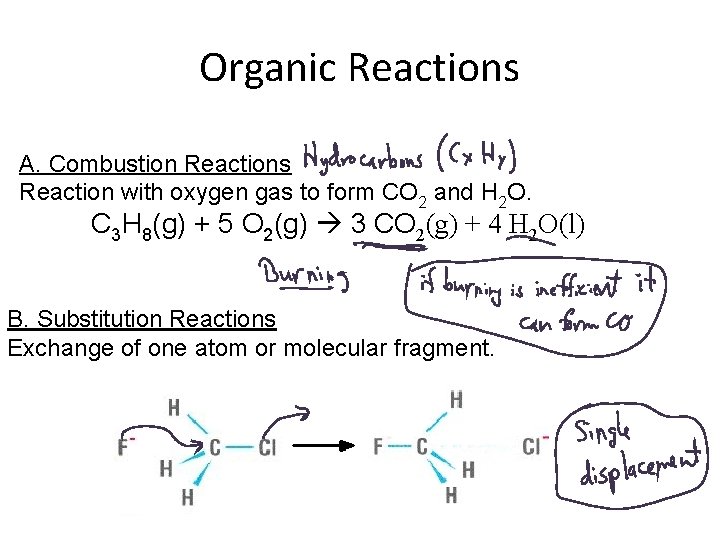
Organic Reactions A. Combustion Reactions Reaction with oxygen gas to form CO 2 and H 2 O. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) B. Substitution Reactions Exchange of one atom or molecular fragment.

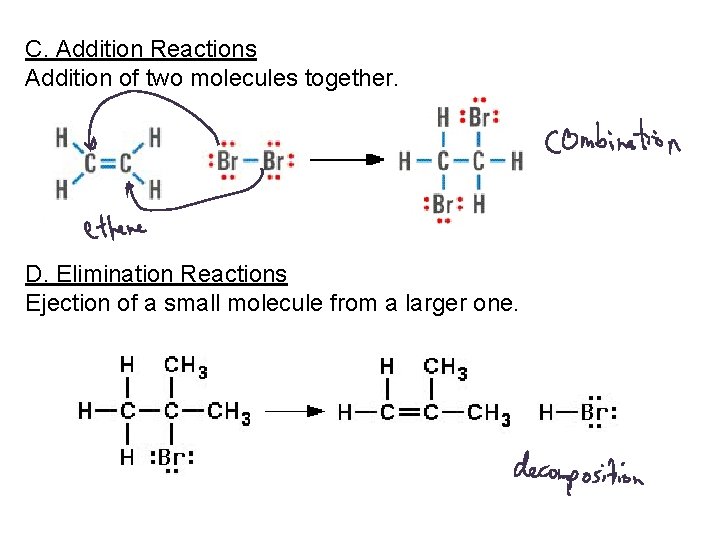
C. Addition Reactions Addition of two molecules together. D. Elimination Reactions Ejection of a small molecule from a larger one.

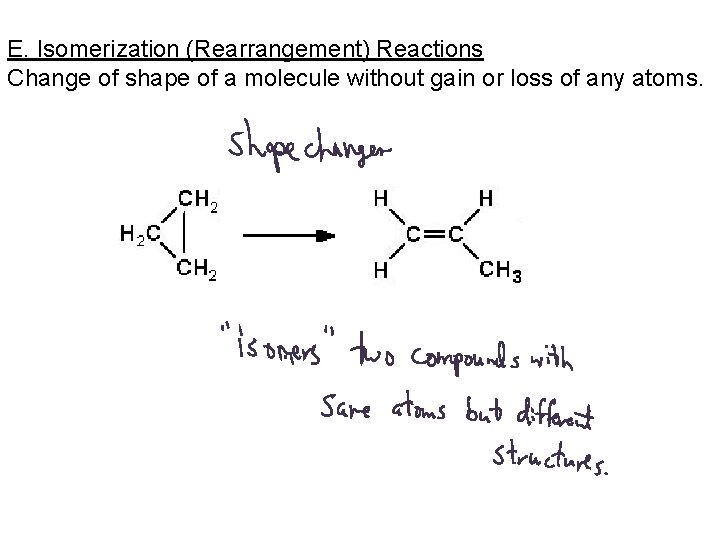
E. Isomerization (Rearrangement) Reactions Change of shape of a molecule without gain or loss of any atoms.