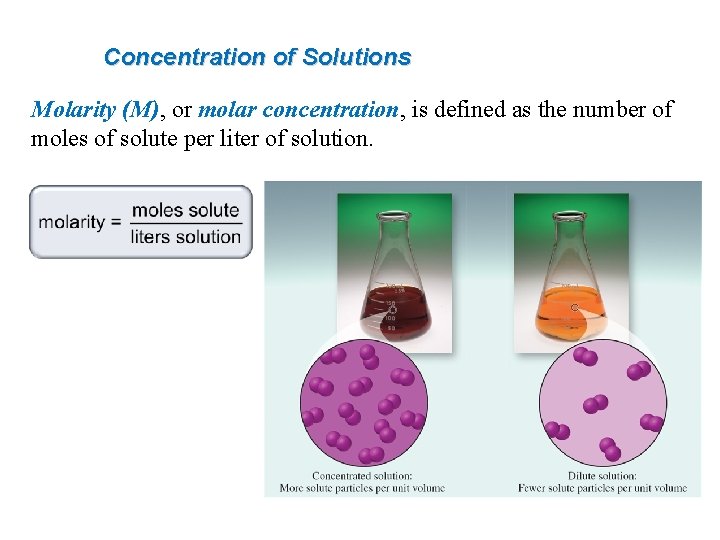
Concentration of Solutions Molarity M or molar concentration





- Slides: 12

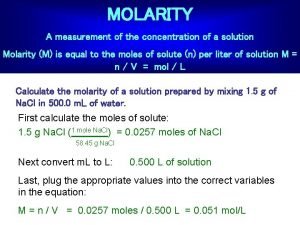
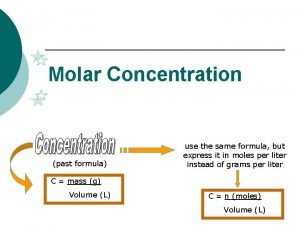
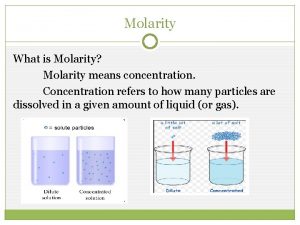
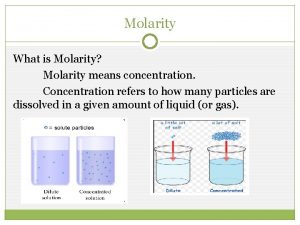
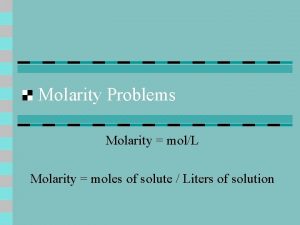
Concentration of Solutions Molarity (M), or molar concentration, is defined as the number of moles of solute per liter of solution.

Worked Example 9. 8 For an aqueous solution of glucose (C 6 H 12 O 6), determine (a) the molarity of 2. 00 L of a solution that contains 50. 0 g of glucose, (b) the volume of this solution that would contain 0. 250 mole of glucose, and (c) the number of moles of glucose in 0. 500 L of this solution. Strategy Convert the mass of glucose given to moles, and use the equations for interconversions of M, liters, and moles to calculate the answers. moles of glucose = 50. 0 g = 0. 277 mol 180. 2 g/mol Solution 0. 277 mol C 6 H 12 O 6 (a) molarity = = 0. 139 M 2. 00 L solution 0. 250 mol C 6 H 12 O 6 (b) volume = = 1. 80 L 0. 139 M solution (c) moles of C 6 H 12 O 6 in 0. 500 L = 0. 500 L× 0. 139 M = 0. 0695 mol

4. 5

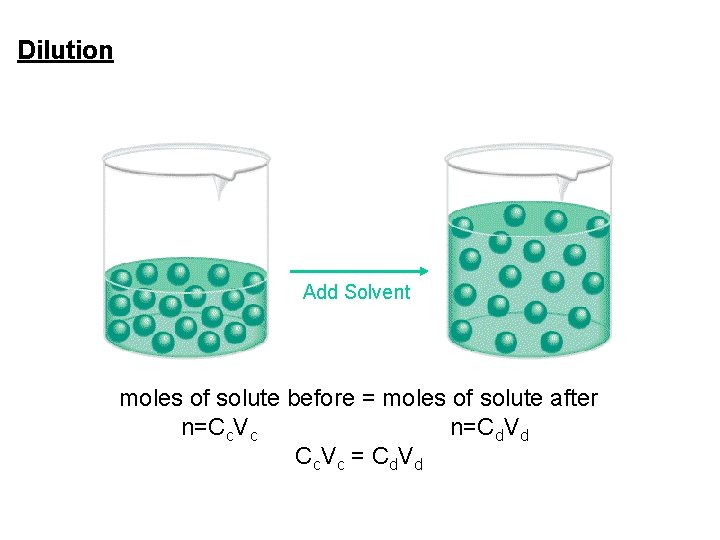
Concentration of Solutions Dilution is the process of preparing a less concentrated solution from a more concentrated one. moles of solute before dilution = moles of solute after dilution

Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent molesofofsolute before = moles Moles of solute after Moles of solute n=Cc. Vc(i) n=Cd. V before dilution after dilution (f) d Cc. Vc = Cd. Vd = Mi V i = Mf V f 4. 5

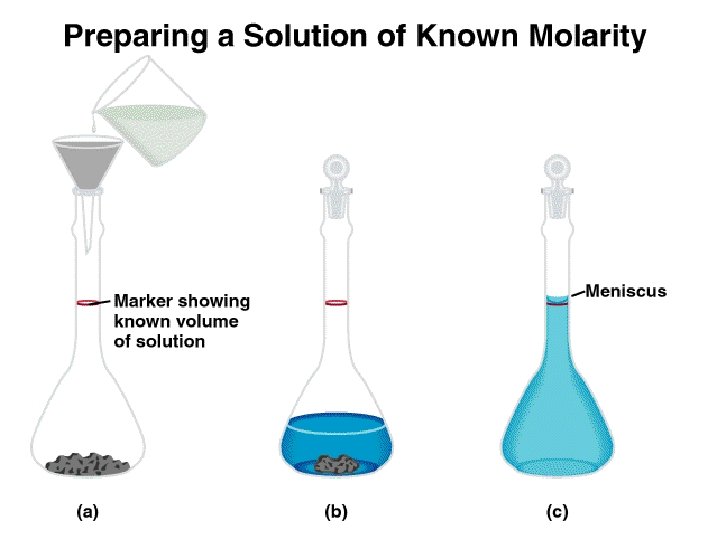
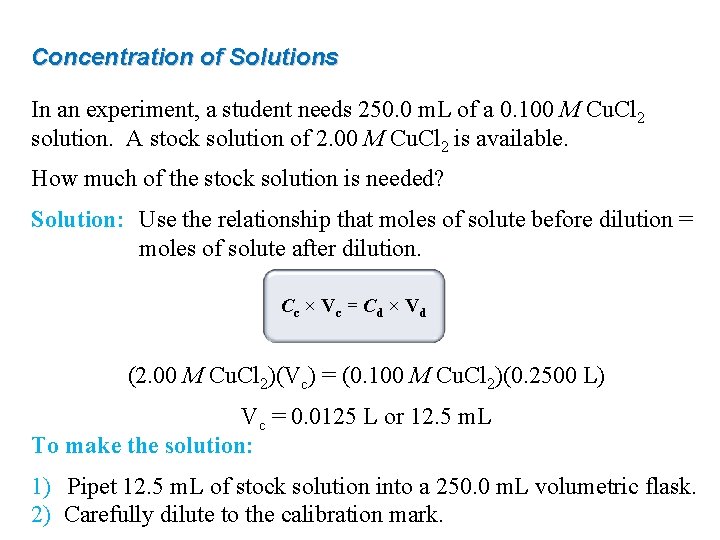
Concentration of Solutions In an experiment, a student needs 250. 0 m. L of a 0. 100 M Cu. Cl 2 solution. A stock solution of 2. 00 M Cu. Cl 2 is available. How much of the stock solution is needed? Solution: Use the relationship that moles of solute before dilution = moles of solute after dilution. Cc × Vc = Cd × Vd (2. 00 M Cu. Cl 2)(Vc) = (0. 100 M Cu. Cl 2)(0. 2500 L) Vc = 0. 0125 L or 12. 5 m. L To make the solution: 1) Pipet 12. 5 m. L of stock solution into a 250. 0 m. L volumetric flask. 2) Carefully dilute to the calibration mark.

How would you prepare 60. 0 m. L of 0. 2 M HNO 3 from a stock solution of 4. 00 M HNO 3? Cc. Vc = Cd. Vd Cc = 4. 00 M Cd = 0. 200 M Vd = 0. 06 L Vc = ? L Cd. Vd 0. 200 M x 0. 06 L Vc = = 0. 003 L = 3 m. L = 4. 00 M Cc 3 m. L of acid + 57 m. L of water = 60 m. L of solution

Concentration of Solutions Because most volumes measured in the laboratory are in milliliters rather than liters, it is worth pointing out that the equation can also be used with m. L as the volume as long as both volume measurements are the SAME unit.

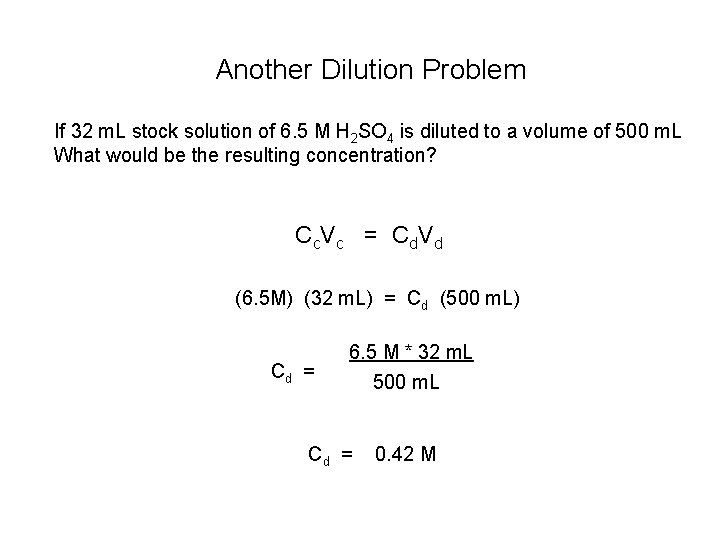
Another Dilution Problem If 32 m. L stock solution of 6. 5 M H 2 SO 4 is diluted to a volume of 500 m. L What would be the resulting concentration? C c. V c = C d V d (6. 5 M) (32 m. L) = Cd (500 m. L) Cd = 6. 5 M * 32 m. L 500 m. L Cd = 0. 42 M

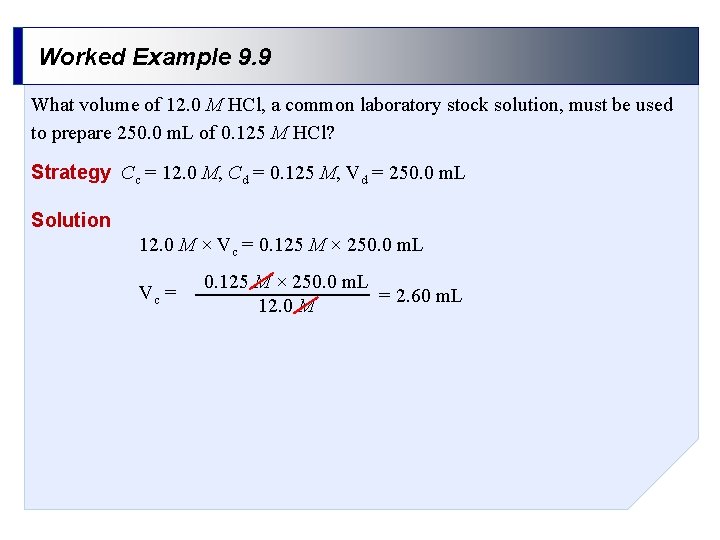
Worked Example 9. 9 What volume of 12. 0 M HCl, a common laboratory stock solution, must be used to prepare 250. 0 m. L of 0. 125 M HCl? Strategy Cc = 12. 0 M, Cd = 0. 125 M, Vd = 250. 0 m. L Solution 12. 0 M × Vc = 0. 125 M × 250. 0 m. L = 2. 60 m. L 12. 0 M

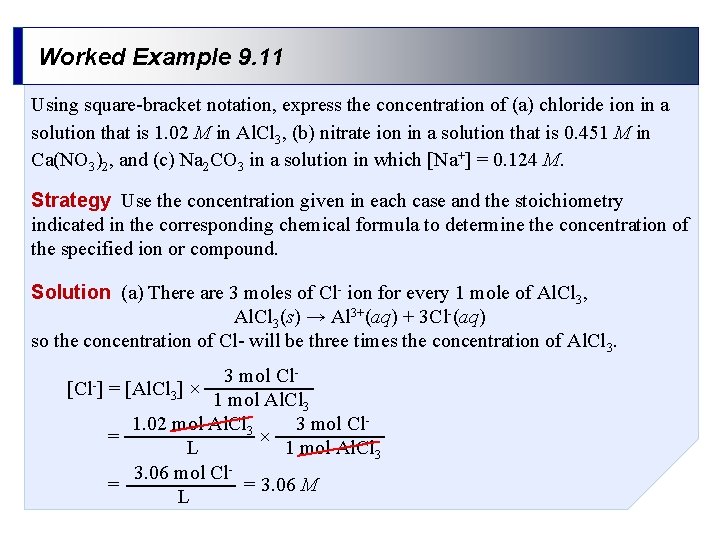
Worked Example 9. 11 Using square-bracket notation, express the concentration of (a) chloride ion in a solution that is 1. 02 M in Al. Cl 3, (b) nitrate ion in a solution that is 0. 451 M in Ca(NO 3)2, and (c) Na 2 CO 3 in a solution in which [Na+] = 0. 124 M. Strategy Use the concentration given in each case and the stoichiometry indicated in the corresponding chemical formula to determine the concentration of the specified ion or compound. Solution (a) There are 3 moles of Cl- ion for every 1 mole of Al. Cl 3, Al. Cl 3(s) → Al 3+(aq) + 3 Cl-(aq) so the concentration of Cl- will be three times the concentration of Al. Cl 3. [Cl-] 3 mol Cl= [Al. Cl 3] × 1 mol Al. Cl 3 1. 02 mol Al. Cl 3 3 mol Cl= × L 1 mol Al. Cl 3 3. 06 mol Cl= = 3. 06 M L

Homework Read section 8. 7 Page 405 (top)#1 -4 Page 405 (bottom)#2 -5
 Molar concentration
Molar concentration What is molarity a measurement of
What is molarity a measurement of Molarity to concentration
Molarity to concentration Measures of concentration: molarity
Measures of concentration: molarity Calculate the molarity
Calculate the molarity Measures of concentration molarity quiz
Measures of concentration molarity quiz Concentration moles equation
Concentration moles equation Is concentration and molarity the same
Is concentration and molarity the same Molality to molarity
Molality to molarity Concentration of solution formula
Concentration of solution formula Ligation
Ligation Molarity is equal to
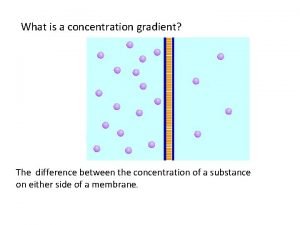
Molarity is equal to Whats a concentration gradient
Whats a concentration gradient