Solutions Molarity 1 Molarity M A concentration that








- Slides: 13

Solutions Molarity 1

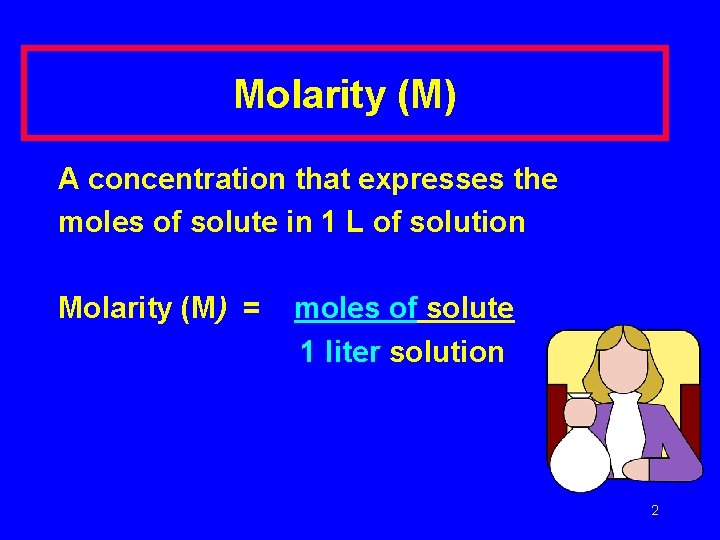
Molarity (M) A concentration that expresses the moles of solute in 1 L of solution Molarity (M) = moles of solute 1 liter solution 2

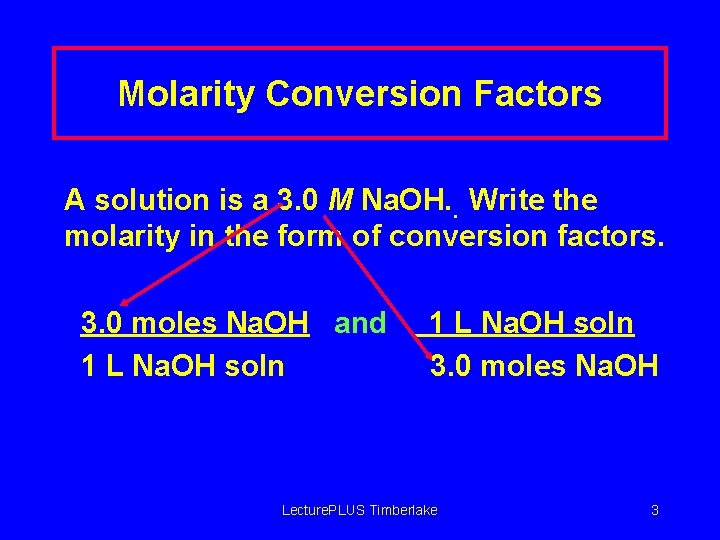
Molarity Conversion Factors A solution is a 3. 0 M Na. OH. . Write the molarity in the form of conversion factors. 3. 0 moles Na. OH and 1 L Na. OH soln 3. 0 moles Na. OH Lecture. PLUS Timberlake 3

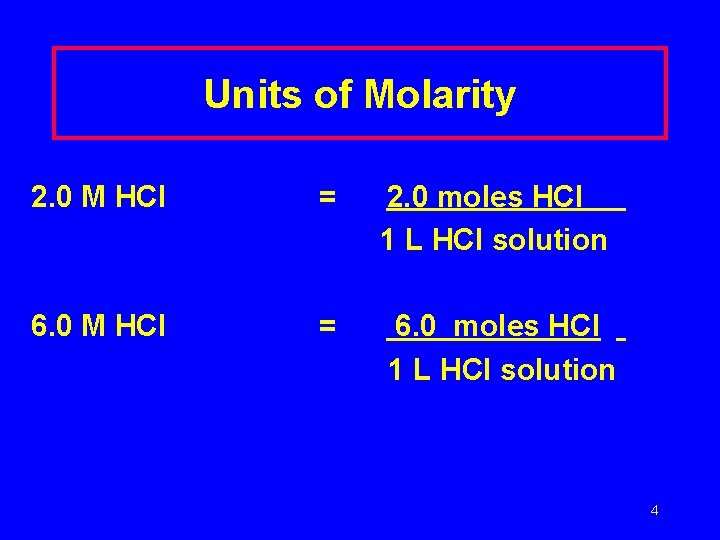
Units of Molarity 2. 0 M HCl = 2. 0 moles HCl 1 L HCl solution 6. 0 M HCl = 6. 0 moles HCl 1 L HCl solution 4

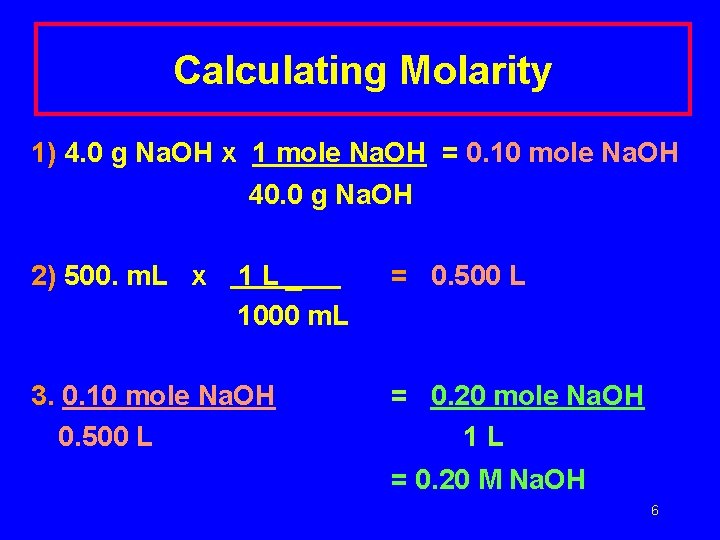
Molarity Calculation If 4. 0 g Na. OH are used to make 500. m. L of Na. OH solution, what is the molarity (M) of the solution? 5

Calculating Molarity 1) 4. 0 g Na. OH x 1 mole Na. OH = 0. 10 mole Na. OH 40. 0 g Na. OH 2) 500. m. L x 1 L_ 1000 m. L 3. 0. 10 mole Na. OH 0. 500 L = 0. 20 mole Na. OH 1 L = 0. 20 M Na. OH 6

Check for Understanding an Dr 1) 8 M 2) 5 M 3) 2 M o 1) A KOH solution with a volume of 400 m. L contains 2 mole KOH. What is the molarity of the solution? 7

Solution #1 an Dr 2) 5 M M = 2 mole KOH = 5 M 0. 4 L o A KOH solution with a volume of 400 m. L contains 2 moles of KOH. What is the molarity of the solution? 8

Checking for Understanding 2) A glucose solution with a volume of 2. 0 L contains 72 g glucose (C 6 H 12 O 6). If glucose has a molar mass of 180. g/mole, what is the molarity of the glucose solution? 1) 0. 20 M 2) 5. 0 M 3) 36 M 9

Solution #2 A glucose solution with a volume of 2. 0 L contains 72 g glucose (C 6 H 12 O 6). If glucose has a molar mass of 180. g/mole, what is the molarity of the glucose solution? 1) 72 g x 1 mole x 180. g 1 = 2. 0 L 0. 20 M 10

Making dilutions M 1 V 1 = M 2 V 2 Equation explained…. M 1 x V 1 = moles before dilution Moles before dilution = moles after dilution M 2 x V 2 = moles after dilution 11

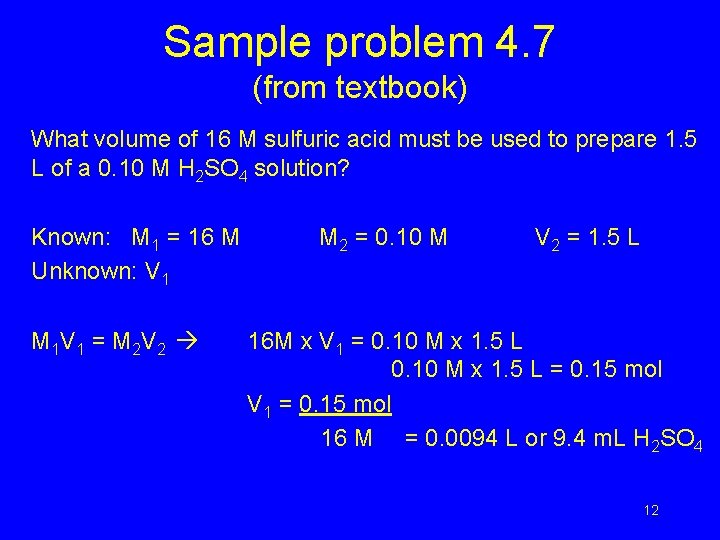
Sample problem 4. 7 (from textbook) What volume of 16 M sulfuric acid must be used to prepare 1. 5 L of a 0. 10 M H 2 SO 4 solution? Known: M 1 = 16 M Unknown: V 1 M 1 V 1 = M 2 V 2 M 2 = 0. 10 M V 2 = 1. 5 L 16 M x V 1 = 0. 10 M x 1. 5 L = 0. 15 mol V 1 = 0. 15 mol 16 M = 0. 0094 L or 9. 4 m. L H 2 SO 4 12

Practice! What volume of 12. 0 M HCl would you need to prepare 200 m. L of 3. 0 M HCl? 13