MOLARITY MOLALITY DILUTIONS PERCENTS Molarity Molarity involves a



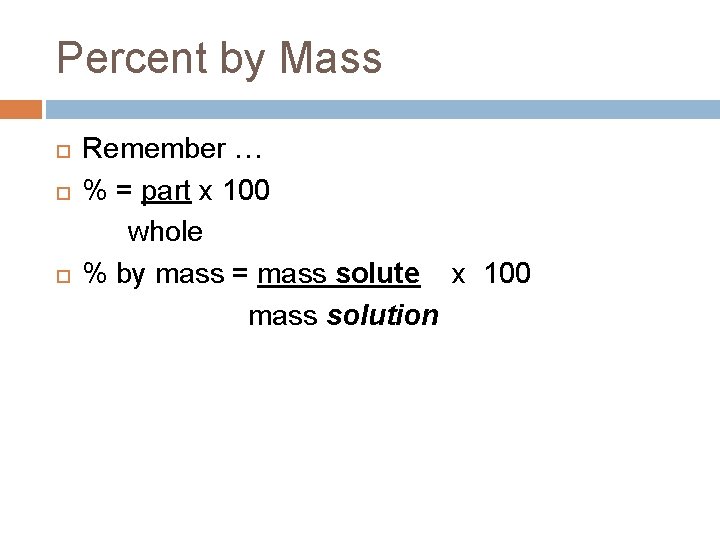
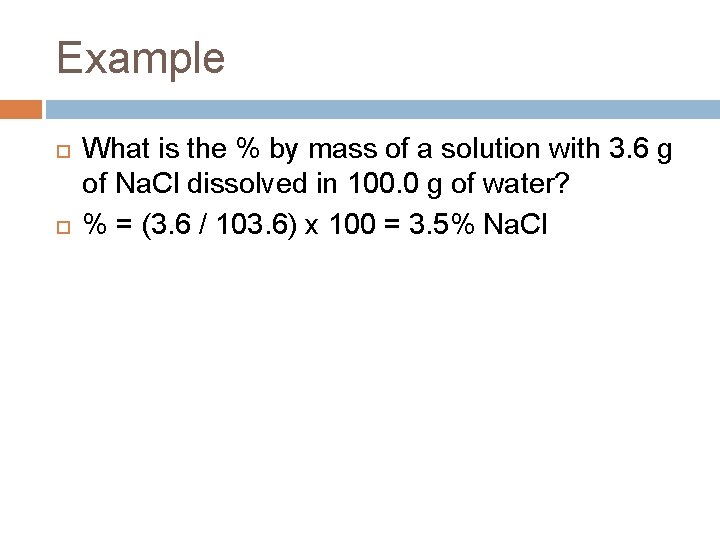


- Slides: 10

MOLARITY, MOLALITY, DILUTIONS & PERCENTS

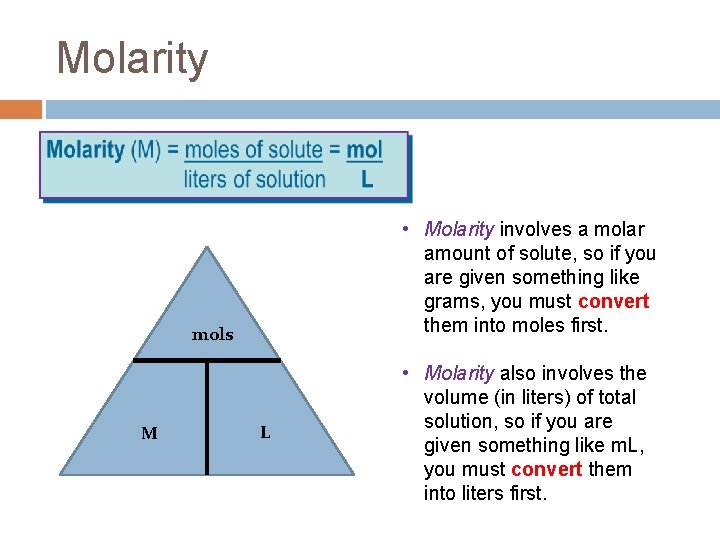
Molarity • Molarity involves a molar amount of solute, so if you are given something like grams, you must convert them into moles first. mols M L • Molarity also involves the volume (in liters) of total solution, so if you are given something like m. L, you must convert them into liters first.

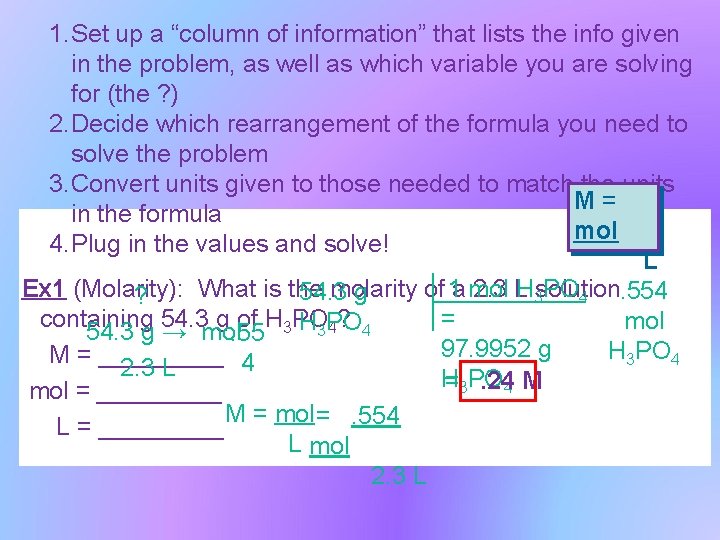
1. Set up a “column of information” that lists the info given in the problem, as well as which variable you are solving for (the ? ) 2. Decide which rearrangement of the formula you need to solve the problem 3. Convert units given to those needed to match the units M= in the formula mol 4. Plug in the values and solve! L H 3 solution PO 4. 554 Ex 1 (Molarity): What is the molarity of 1 a mol 2. 3 L 54. 3 g ? = containing 54. 3 g of H 3 PO mol 4? 4 54. 3 g → mol. 55 H 3 PO 97. 9952 g H 3 PO 4 M = _____ 4 2. 3 L H =3 PO. 244 M mol = _____ M = mol =. 554 L = _____ L mol 2. 3 L

Dilution When chemists purchase solutions, they generally purchase “stock solutions” which are extremely concentrated solutions This way a chemist can dilute the strong solution to any concentration that they wish. This stops the chemist from having to buy several concentrations M 1 V 1 = M 2 V 2 There are four parts to the dilution equation: 1. M 1 = Molarity of the diluted (or desired) solution 2. M 2 = Molarity of the concentrated (or stock) solution 3. V 1 = volume of the diluted (or desired) solution 4. V 2 = volume of the concentrated (or stock)

1. Set up a “column of information” that lists the info given in the problem, as well as which variable you are solving for (the ? ) 2. Decide which rearrangement you need to solve the problem V 2 = M 1 V 1 3. Convert units given to those needed to match the units. Min 2 the formula 4. Plug in the values and solve! 3. 8 M Ex 3 (Dilution): How many m. L of 12 M HCl is needed to 121. 5 M L of. Va M 1 V 1 that V 2 is = (3. 8 M)(1. 5 L). 475 L produce solution 3. 8 M? 2 = = 1. 5 L = M 1 = _____ M 2 12 M ? M 2 = _____. 475 L 1000 m. L 475 m. L = HCl V 1 = _____ 1 L V 2 = _____

Concentration The amount of solute in a solution. Describing Concentration % by mass - medicated creams % by volume- rubbing alcohol
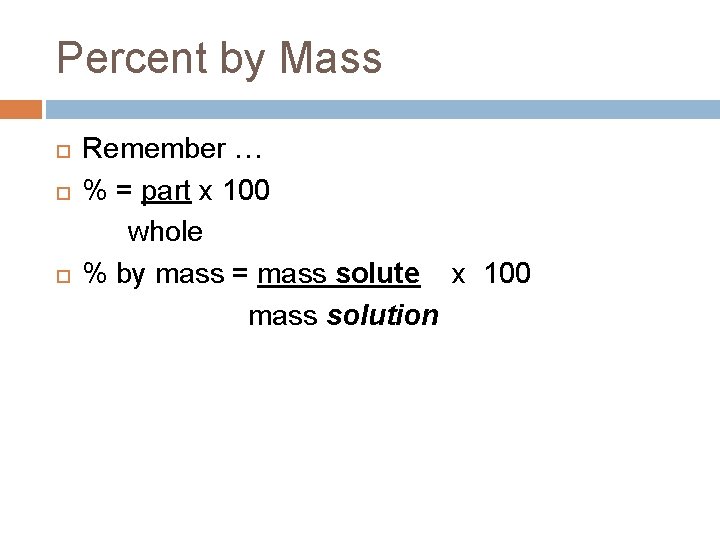
Percent by Mass Remember … % = part x 100 whole % by mass = mass solute x 100 mass solution
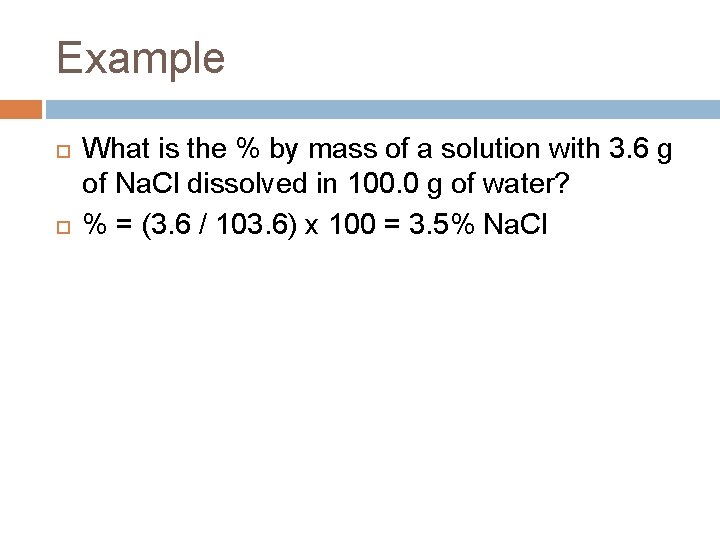
Example What is the % by mass of a solution with 3. 6 g of Na. Cl dissolved in 100. 0 g of water? % = (3. 6 / 103. 6) x 100 = 3. 5% Na. Cl

Percent by Volume u. Remember … u% = part x 100 whole u% by volume = volume solute x 100 volume solution

Example u. What is the % by volume of 75. 0 m. L of ethanol dissolved in 200. 0 m. L of water? u% = (75. 0 / 275. 0) x 100 = 27. 3%