Solution Concentration Units Units of Concentration 1 Molality

- Slides: 10

Solution Concentration Units

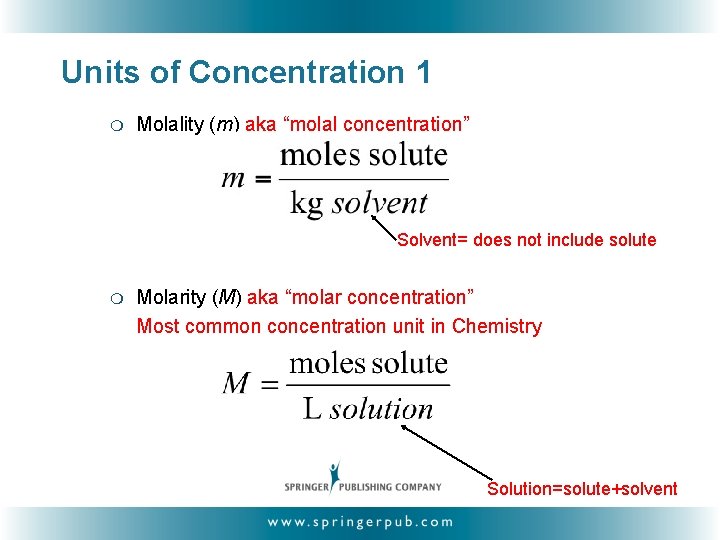
Units of Concentration 1 ❍ Molality (m) aka “molal concentration” Solvent= does not include solute ❍ Molarity (M) aka “molar concentration” Most common concentration unit in Chemistry Solution=solute+solvent

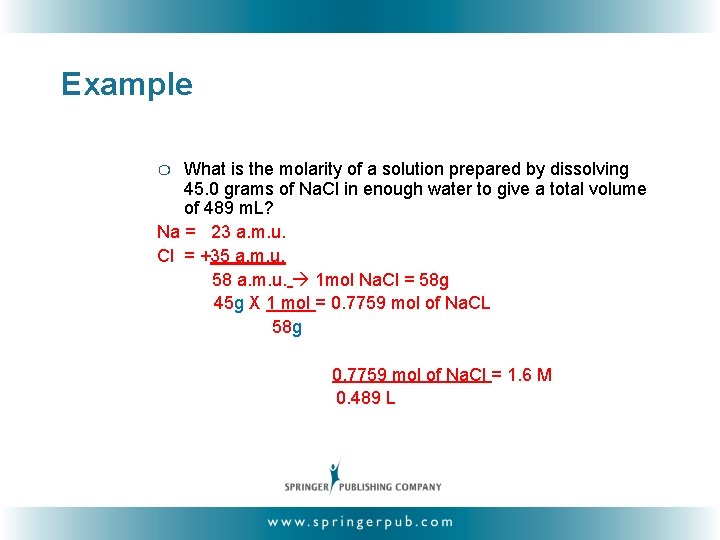
Example What is the molarity of a solution prepared by dissolving 45. 0 grams of Na. Cl in enough water to give a total volume of 489 m. L? Na = 23 a. m. u. Cl = +35 a. m. u. 58 a. m. u. 1 mol Na. Cl = 58 g 45 g X 1 mol = 0. 7759 mol of Na. CL 58 g ❍ 0. 7759 mol of Na. Cl = 1. 6 M 0. 489 L

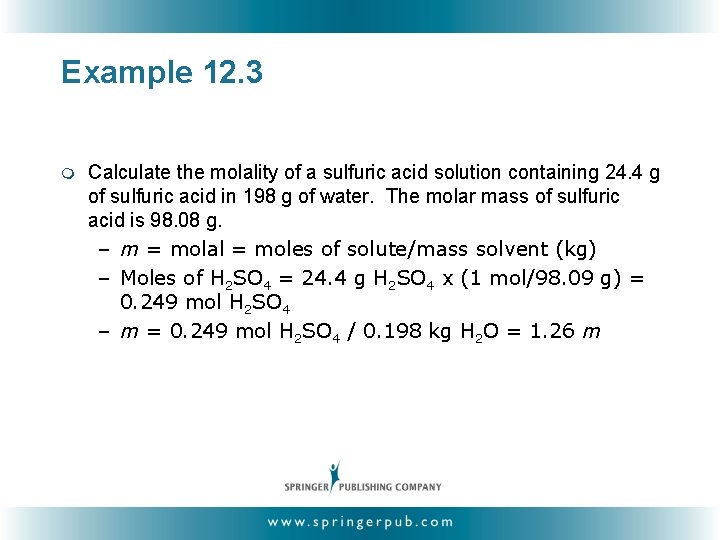
Example 12. 3 m Calculate the molality of a sulfuric acid solution containing 24. 4 g of sulfuric acid in 198 g of water. The molar mass of sulfuric acid is 98. 08 g. – m = molal = moles of solute/mass solvent (kg) – Moles of H 2 SO 4 = 24. 4 g H 2 SO 4 x (1 mol/98. 09 g) = 0. 249 mol H 2 SO 4 – m = 0. 249 mol H 2 SO 4 / 0. 198 kg H 2 O = 1. 26 m

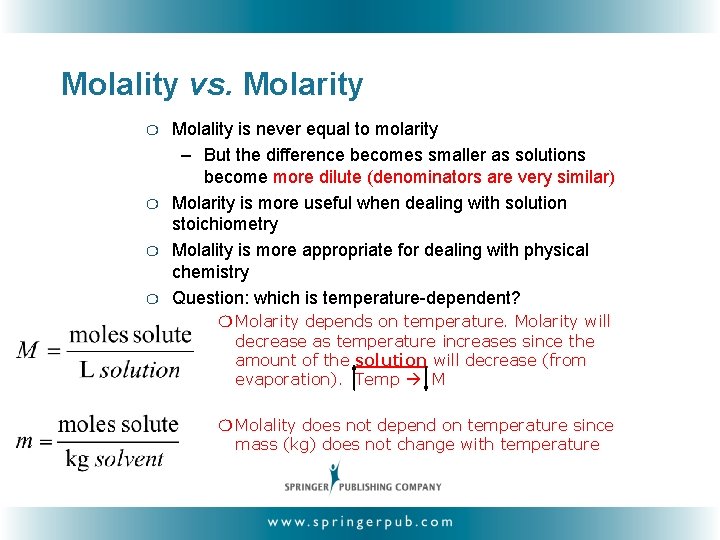
Molality vs. Molarity ❍ ❍ Molality is never equal to molarity – But the difference becomes smaller as solutions become more dilute (denominators are very similar) Molarity is more useful when dealing with solution stoichiometry Molality is more appropriate for dealing with physical chemistry Question: which is temperature-dependent? ❍ Molarity depends on temperature. Molarity will decrease as temperature increases since the amount of the solution will decrease (from evaporation). Temp M ❍ Molality does not depend on temperature since mass (kg) does not change with temperature

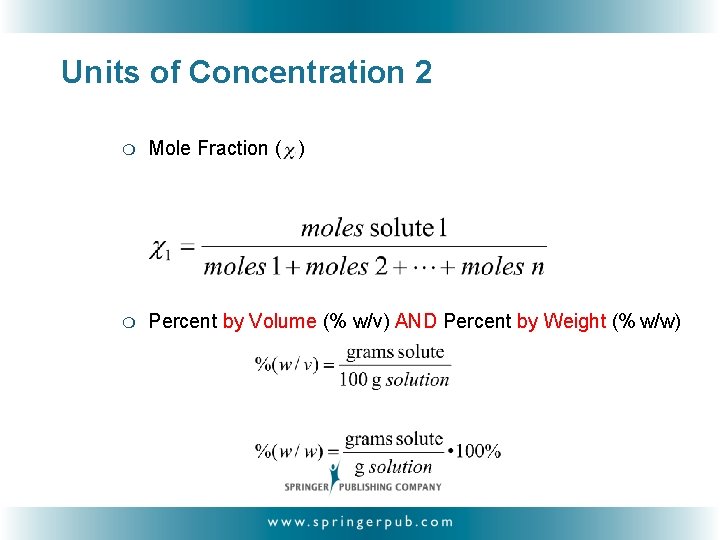
Units of Concentration 2 ❍ Mole Fraction ( ) ❍ Percent by Volume (% w/v) AND Percent by Weight (% w/w)

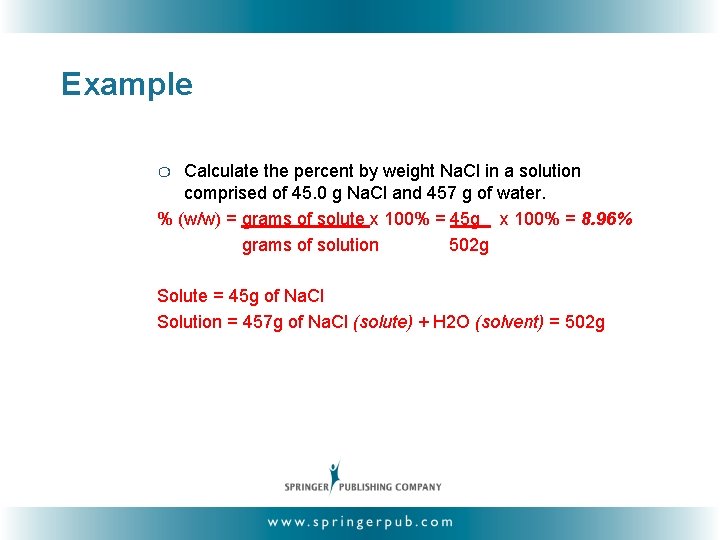
Example Calculate the percent by weight Na. Cl in a solution comprised of 45. 0 g Na. Cl and 457 g of water. % (w/w) = grams of solute x 100% = 45 g x 100% = 8. 96% grams of solution 502 g ❍ Solute = 45 g of Na. Cl Solution = 457 g of Na. Cl (solute) + H 2 O (solvent) = 502 g

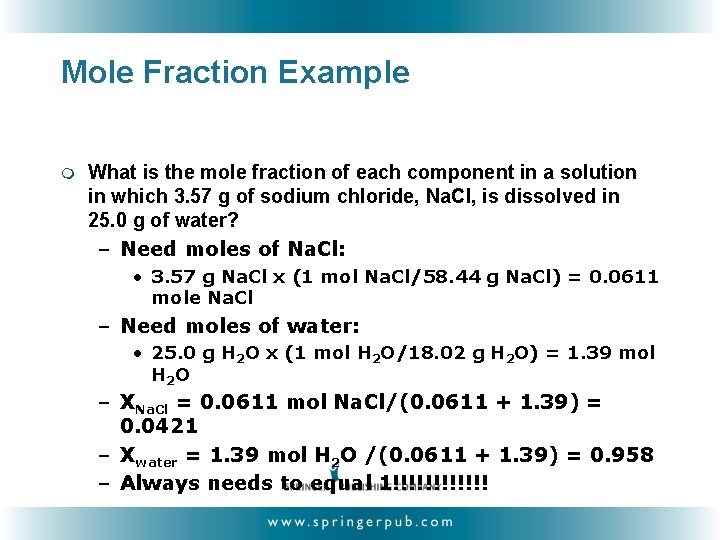
Mole Fraction Example m What is the mole fraction of each component in a solution in which 3. 57 g of sodium chloride, Na. Cl, is dissolved in 25. 0 g of water? – Need moles of Na. Cl: • 3. 57 g Na. Cl x (1 mol Na. Cl/58. 44 g Na. Cl) = 0. 0611 mole Na. Cl – Need moles of water: • 25. 0 g H 2 O x (1 mol H 2 O/18. 02 g H 2 O) = 1. 39 mol H 2 O – XNa. Cl = 0. 0611 mol Na. Cl/(0. 0611 + 1. 39) = 0. 0421 – Xwater = 1. 39 mol H 2 O /(0. 0611 + 1. 39) = 0. 958 – Always needs to equal 1!!!!!!

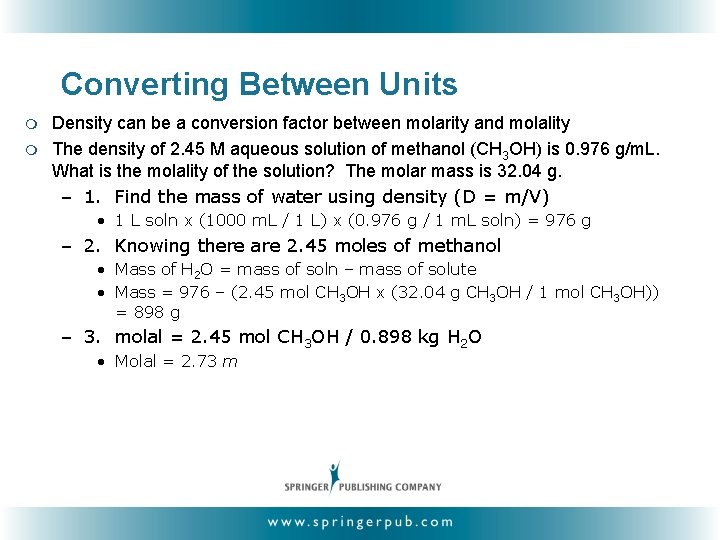
Converting Between Units m m Density can be a conversion factor between molarity and molality The density of 2. 45 M aqueous solution of methanol (CH 3 OH) is 0. 976 g/m. L. What is the molality of the solution? The molar mass is 32. 04 g. – 1. Find the mass of water using density (D = m/V) • 1 L soln x (1000 m. L / 1 L) x (0. 976 g / 1 m. L soln) = 976 g – 2. Knowing there are 2. 45 moles of methanol • Mass of H 2 O = mass of soln – mass of solute • Mass = 976 – (2. 45 mol CH 3 OH x (32. 04 g CH 3 OH / 1 mol CH 3 OH)) = 898 g – 3. molal = 2. 45 mol CH 3 OH / 0. 898 kg H 2 O • Molal = 2. 73 m

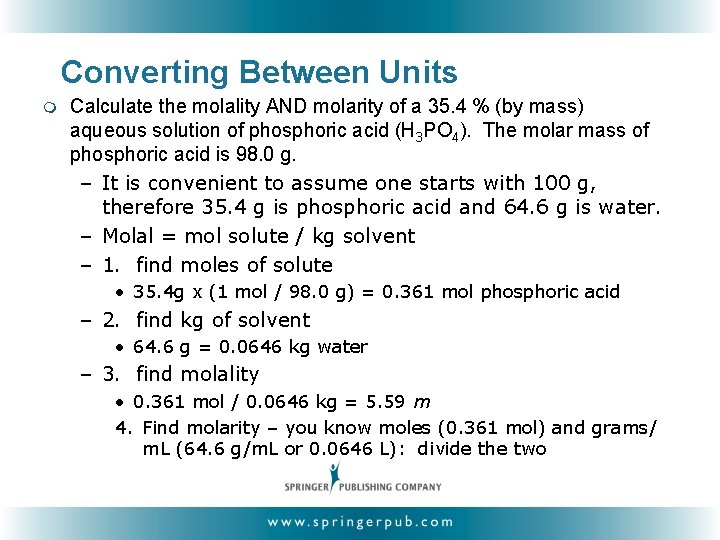
Converting Between Units m Calculate the molality AND molarity of a 35. 4 % (by mass) aqueous solution of phosphoric acid (H 3 PO 4). The molar mass of phosphoric acid is 98. 0 g. – It is convenient to assume one starts with 100 g, therefore 35. 4 g is phosphoric acid and 64. 6 g is water. – Molal = mol solute / kg solvent – 1. find moles of solute • 35. 4 g x (1 mol / 98. 0 g) = 0. 361 mol phosphoric acid – 2. find kg of solvent • 64. 6 g = 0. 0646 kg water – 3. find molality • 0. 361 mol / 0. 0646 kg = 5. 59 m 4. Find molarity – you know moles (0. 361 mol) and grams/ m. L (64. 6 g/m. L or 0. 0646 L): divide the two