Unit 5 Lesson 6 Measuring Concentration the Chemists







- Slides: 11

Unit 5, Lesson 6 Measuring Concentration the Chemist’s Way: MOLARITY

Review: What is concentration? • The amount of solute that dissolves in the solvent

Review • Concentration can be measured many ways: Ø Unsat’d, supersat’d Ø Percent volume Ø Percent mass Ø Molarity

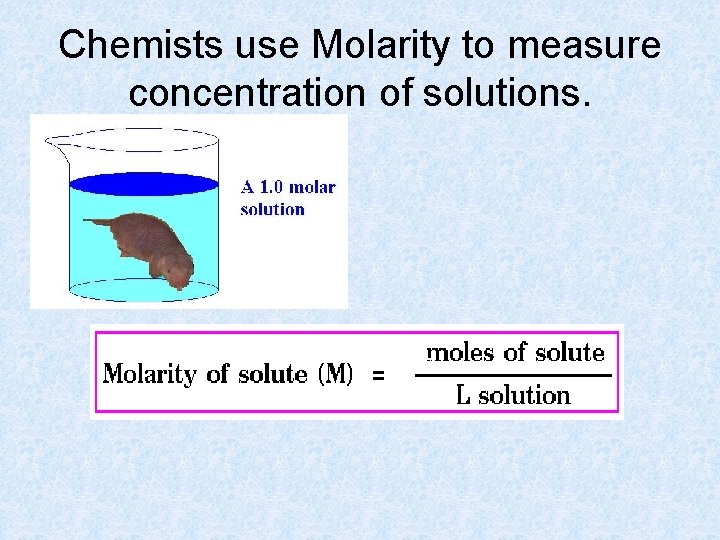
Chemists use Molarity to measure concentration of solutions.

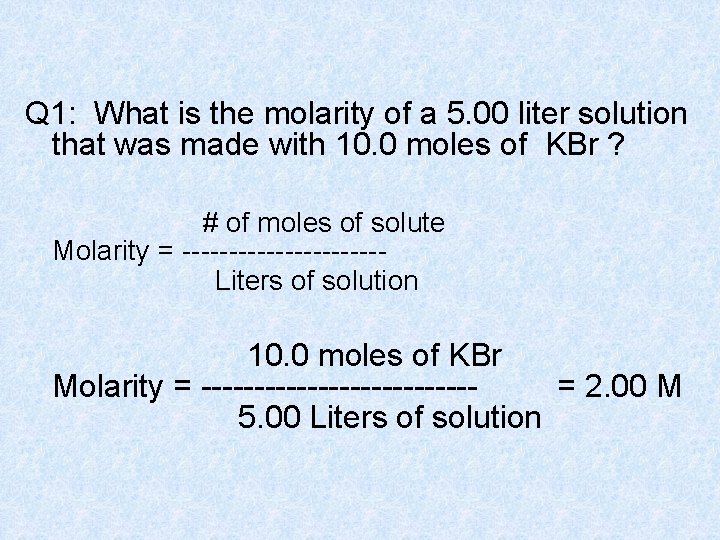
Q 1: What is the molarity of a 5. 00 liter solution that was made with 10. 0 moles of KBr ? # of moles of solute Molarity = ----------- Liters of solution 10. 0 moles of KBr Molarity = ------------- = 2. 00 M 5. 00 Liters of solution

Q 2: What is the molarity of a 6. 00 liter solution that was made with 3. 0 moles of KBr ? # of moles of solute Molarity = ----------- Liters of solution 3. 0 moles of KBr Molarity = ------------- = 0. 5 M 6. 00 Liters of solution

But how do you measure moles? • Does a balance or scale measure in moles or grams? • Grams • So, how do you measure out moles? !? • You have to convert from moles to grams. • How do you do that? • Using molar mass • How do you do that? • EASY!

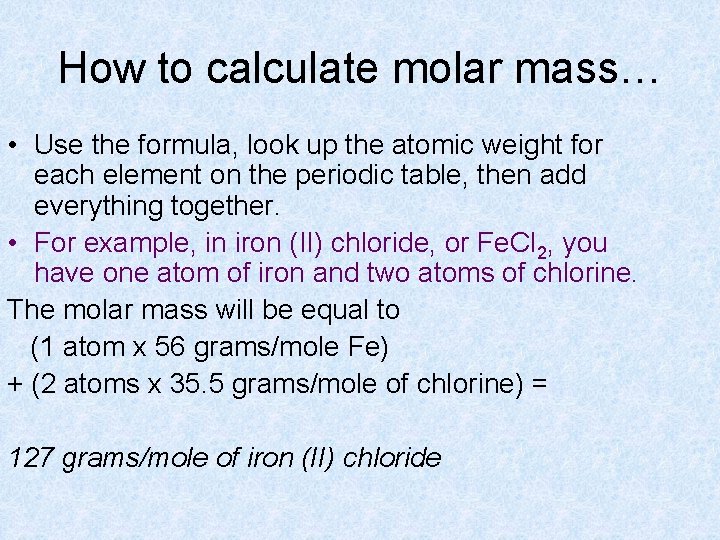
How to calculate molar mass… • Use the formula, look up the atomic weight for each element on the periodic table, then add everything together. • For example, in iron (II) chloride, or Fe. Cl 2, you have one atom of iron and two atoms of chlorine. The molar mass will be equal to (1 atom x 56 grams/mole Fe) + (2 atoms x 35. 5 grams/mole of chlorine) = 127 grams/mole of iron (II) chloride

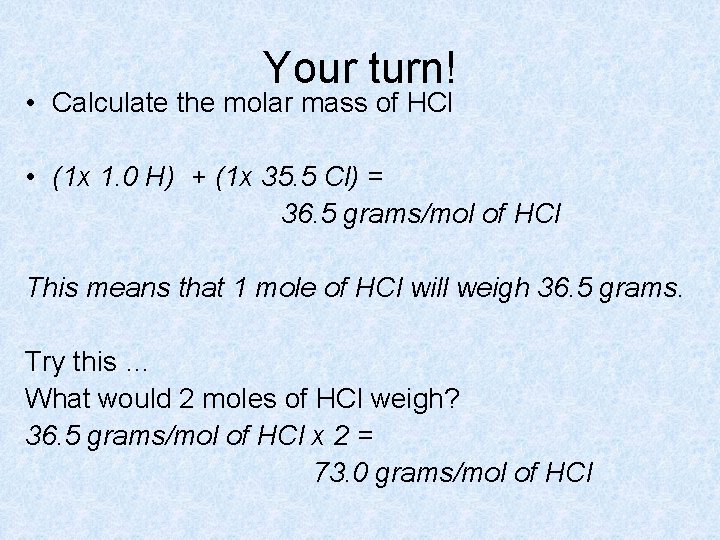
Your turn! • Calculate the molar mass of HCl • (1 x 1. 0 H) + (1 x 35. 5 Cl) = 36. 5 grams/mol of HCl This means that 1 mole of HCl will weigh 36. 5 grams. Try this … What would 2 moles of HCl weigh? 36. 5 grams/mol of HCl x 2 = 73. 0 grams/mol of HCl

Putting it all together • The lab says to make a 6. 0 HCl solution, what do you do? • 6 M means 6 moles per liter, • So 6 moles of HCl • 35. 5 grams/mol of HCl x 6 = 213. 0 grams/mol of HCl Weigh out 213. 0 grams of HCl and mix with 1 liter of water!

Your turn again… • The lab says to make a 2. 0 M Na. OH solution, what do you do?