Molarity Dilution and p H Main Idea Solution



























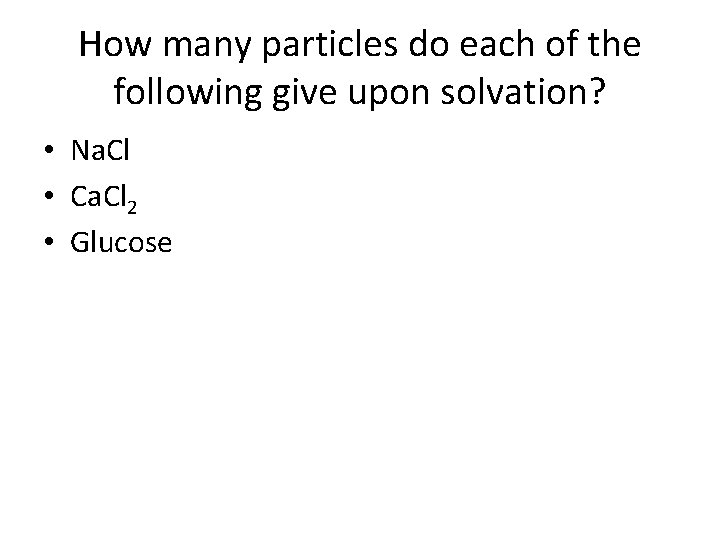














![Calculating the p. H = - log [H+] (The [ ] means Molarity) Example: Calculating the p. H = - log [H+] (The [ ] means Molarity) Example:](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-66.jpg)



![More About Water Autoionization Kw = [H 3 O+] [OH-] = 1. 00 x More About Water Autoionization Kw = [H 3 O+] [OH-] = 1. 00 x](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-70.jpg)
![p. H [H+] [OH-] p. OH 72 p. H [H+] [OH-] p. OH 72](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-72.jpg)
![[H 3 O+], [OH-] and p. H What is the p. H of the [H 3 O+], [OH-] and p. H What is the p. H of the](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-73.jpg)
![[OH-] 4 -1 OH -p 10 0 1 x 0 +]. 1 [H -] [OH-] 4 -1 OH -p 10 0 1 x 0 +]. 1 [H -]](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-74.jpg)


![HOMEWORK 1) How much calcium hydroxide [Ca(OH)2], in grams, is needed to produce 1. HOMEWORK 1) How much calcium hydroxide [Ca(OH)2], in grams, is needed to produce 1.](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-77.jpg)
- Slides: 78

Molarity, Dilution, and p. H Main Idea: Solution concentrations are measured in molarity. Dilution is a useful technique for creating a new solution from a stock solution. p. H is a measure of the concentration of hydronium ions in a solution. 1

Properties of Aqueous Solutions • Solution- a homogeneous mixture of two or more substances. • Solute- a substance in a solution that is present in the smallest amount. • Solvent- a substance in a solution that is present in the largest amount. • In an aqueous solution, the solute is a liquid or solid and the solvent is always water.

Molarity Review • One of the most common units of solution concentration is molarity. • Molarity (M) is the number of moles of solute per liter of solution. • Molarity is also known as molar concentration, and the unit M is read as “molar. ” • A liter of solution containing 1 mol of solute is a 1 M solution, which is read as a “one-molar” solution. • A liter of solution containing 0. 1 mol of solute is a 0. 1 M solution. 3

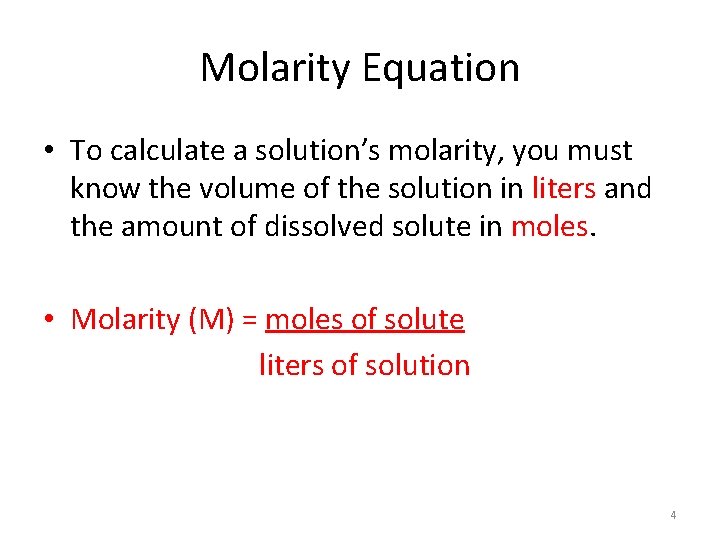
Molarity Equation • To calculate a solution’s molarity, you must know the volume of the solution in liters and the amount of dissolved solute in moles. • Molarity (M) = moles of solute liters of solution 4

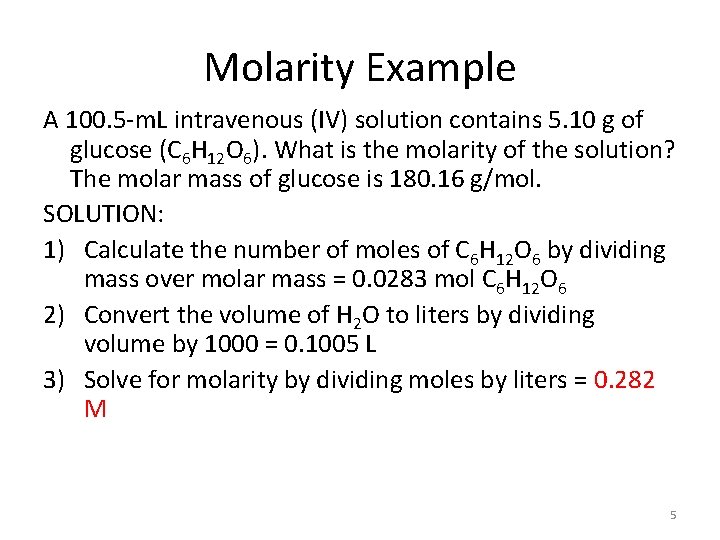
Molarity Example A 100. 5 -m. L intravenous (IV) solution contains 5. 10 g of glucose (C 6 H 12 O 6). What is the molarity of the solution? The molar mass of glucose is 180. 16 g/mol. SOLUTION: 1) Calculate the number of moles of C 6 H 12 O 6 by dividing mass over molar mass = 0. 0283 mol C 6 H 12 O 6 2) Convert the volume of H 2 O to liters by dividing volume by 1000 = 0. 1005 L 3) Solve for molarity by dividing moles by liters = 0. 282 M 5

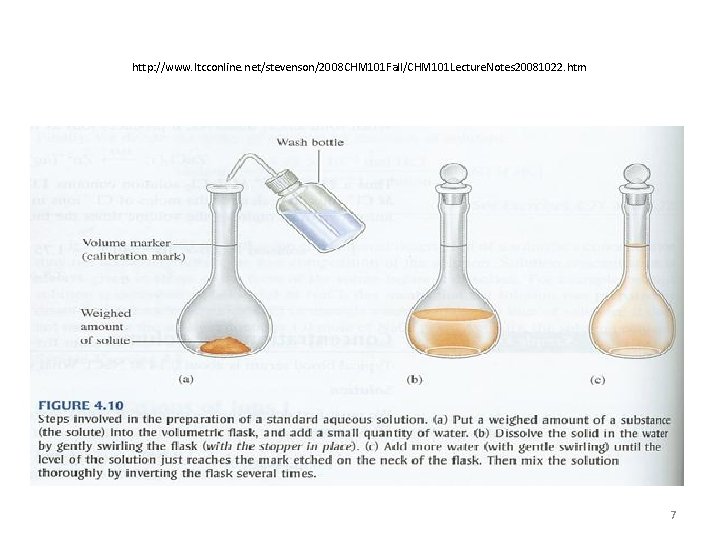
Preparing Molar Solutions • Now that you know how to calculate the molarity of a solution, how would you prepare one in the laboratory? • STEP 1: Calculate the mass of the solute needed using the molarity definition and accounting for the desired concentration and volume. • STEP 2: The mass of the solute is measured on a balance. • STEP 3: The solute is placed in a volumetric flask of the correct volume. • STEP 4: Distilled water is added to the flask to bring the solution level up to the calibration mark. 6

http: //www. ltcconline. net/stevenson/2008 CHM 101 Fall/CHM 101 Lecture. Notes 20081022. htm 7

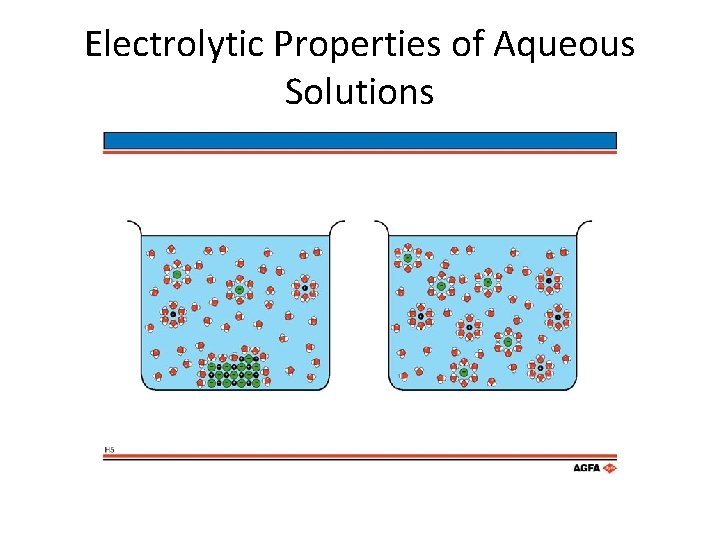
Properties of Aqueous Solutions • All solutes that dissolve in water fit into one of two categories: electrolyte or non-electrolyte. • Electrolyte- a substance that when dissolved in water conducts electricity • Non-electrolyte- a substance that when dissolved in water does not conduct electricity. • To have an electrolyte, ions must be present in water.

Electrolytic Properties of Aqueous Solutions • Na. Cl in water. – What happens? – Na. Cl(s) → Na+(aq) + Cl–(aq) – Completely dissociates

Strong vs. Weak Electrolytes • How do you know when an electrolyte is strong or weak? • Take a look at how HCl dissociates in water. – HCl(s) → H+(aq) + Cl–(aq)

Electrolytic Properties of Aqueous Solutions

Electrolytic Properties of Aqueous Solutions

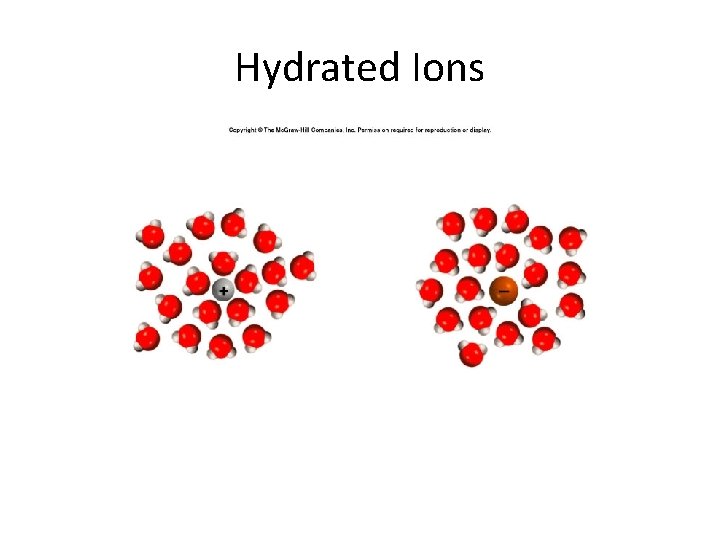
Hydrated Ions

Electrolytic Properties of Aqueous Solutions • What about weak electrolytes? • What makes them weak? – Ionization of acetic acid • CH 3 COOH(aq) ↔ CH 3 COO–(aq) + H+(aq)

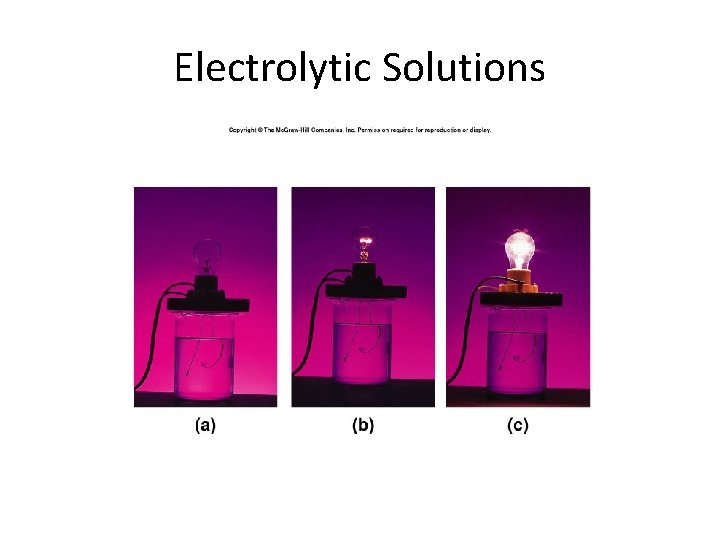
Electrolytic Solutions

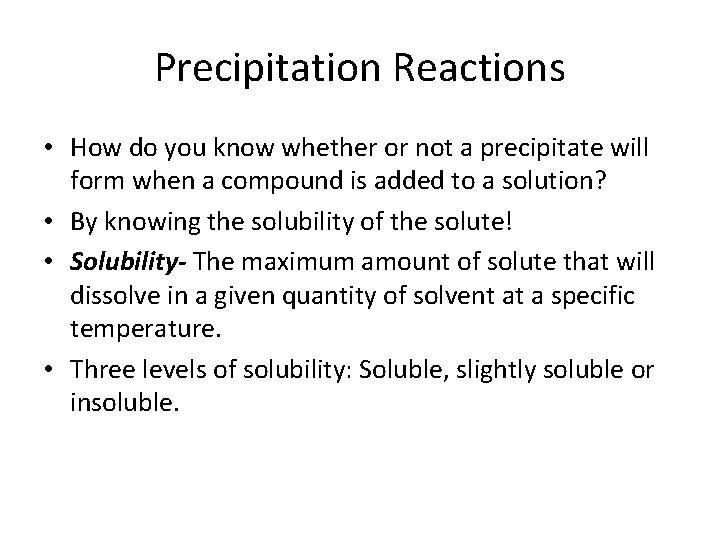
Precipitation Reactions • Precipitation Reaction- a reaction that results in the formation of an insoluble product. • These reactions usually involve ionic compounds. • Formation of Pb. I 2: – Pb(NO 3)2(aq) + 2 KI(aq) → Pb. I 2(s) + 2 KNO 3(aq)

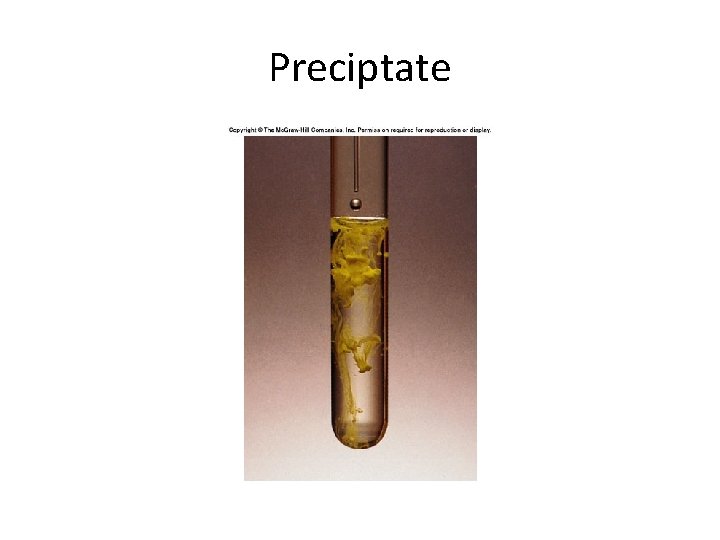
Preciptate

Precipitate

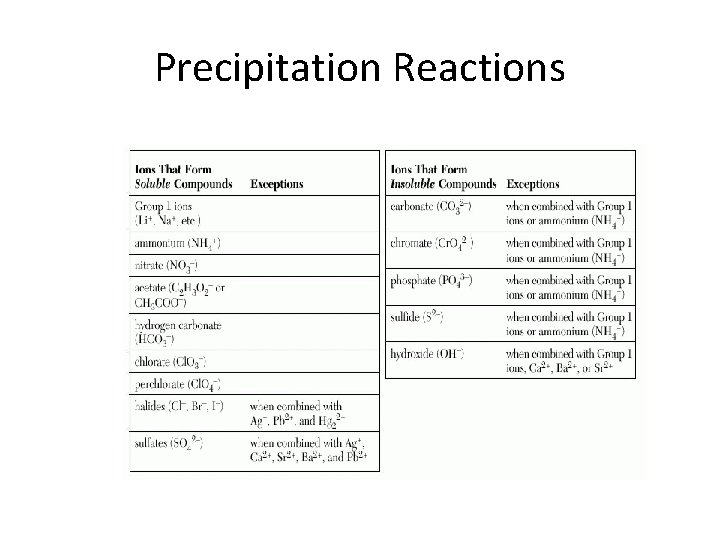
Precipitation Reactions • How do you know whether or not a precipitate will form when a compound is added to a solution? • By knowing the solubility of the solute! • Solubility- The maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature. • Three levels of solubility: Soluble, slightly soluble or insoluble.

Precipitation Reactions

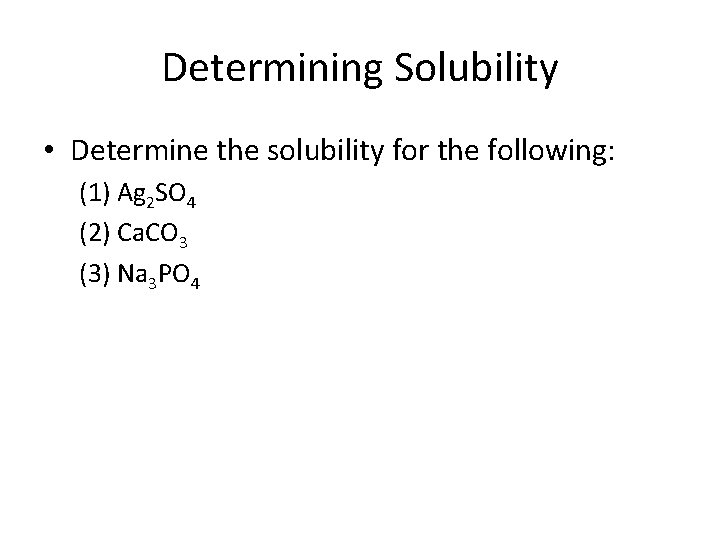
Determining Solubility • Determine the solubility for the following: (1) Ag 2 SO 4 (2) Ca. CO 3 (3) Na 3 PO 4

Diluting Molar Solutions • In the laboratory, you might use concentrated solutions of standard molarities, called stock solutions. – For example, concentrated hydrochloric acid (HCl) is 12 M. • You can prepare a less-concentrated solution by diluting the stock solution with additional solvent. – Dilution is used when a specific concentration is needed and the starting material is already in the form of a solution (i. e. , acids). 22

Dilution of Solutions • When you want to dilute a solution, what happens to the number of moles present in the solution? – Do they increase? – Decrease? – Stay the same?

Dilution of Solutions

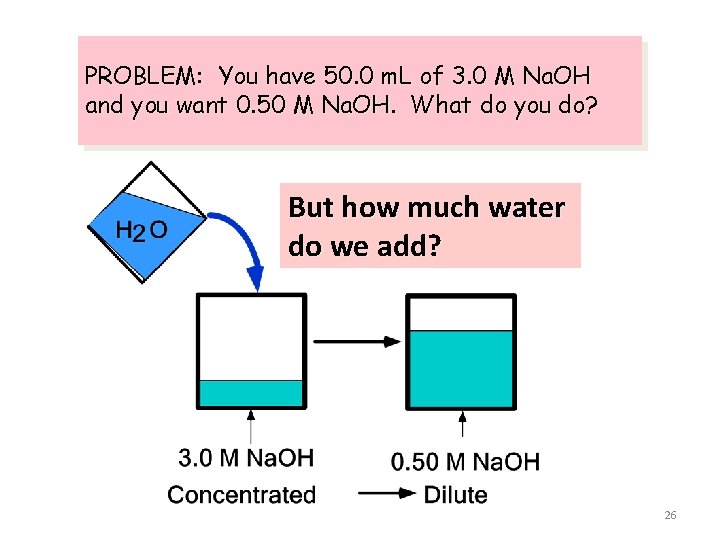
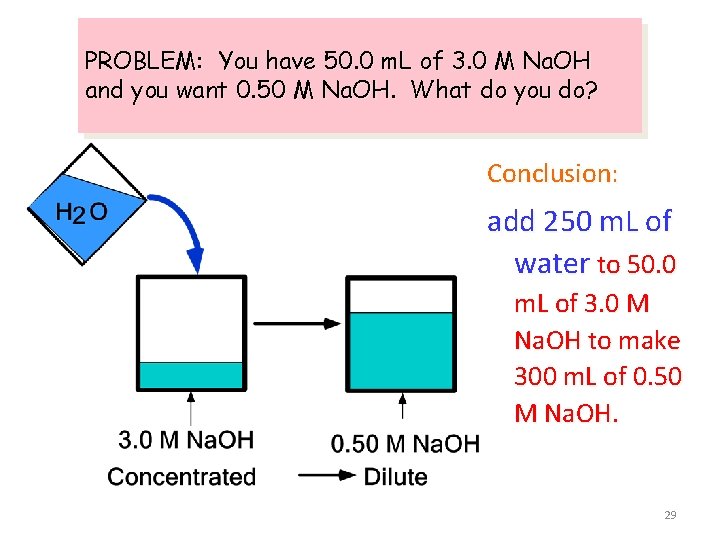
PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? Add water to the 3. 0 M solution to lower its concentration to 0. 50 M Dilute the solution! 25

PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? But how much water do we add? 26

PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? How much water is added? The important point is that ---> moles of Na. OH in ORIGINAL solution = moles of Na. OH in FINAL solution 27

PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? Amount of Na. OH in original solution = M • V = (3. 0 mol/L)(0. 050 L) = 0. 15 mol Na. OH Amount of Na. OH in final solution must also = 0. 15 mol Na. OH Volume of final solution = (0. 15 mol Na. OH) / (0. 50 M) = 0. 30 L or 300 m. L 28

PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? Conclusion: add 250 m. L of water to 50. 0 m. L of 3. 0 M Na. OH to make 300 m. L of 0. 50 M Na. OH. 29

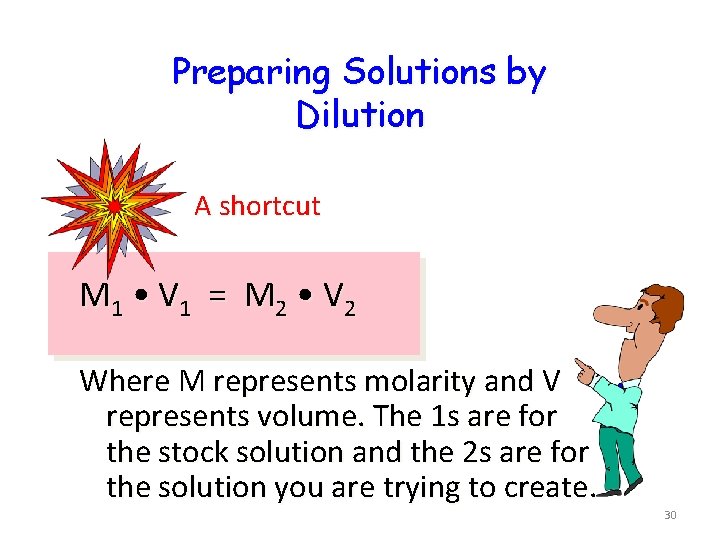
Preparing Solutions by Dilution A shortcut M 1 • V 1 = M 2 • V 2 Where M represents molarity and V represents volume. The 1 s are for the stock solution and the 2 s are for the solution you are trying to create. 30

What factors affect the rate of dissolving? 31

What factors affect the rate of dissolving? • Temperature • You can dissolve more into a warm liquid than you can into a cold liquid 32

What factors affect the rate of dissolving? • Temperature • You can dissolve more into a warm liquid than you can into a cold liquid • Surface area • Which dissolves faster, a cube of sugar or grains of sugar? Why? 33

What factors affect the rate of dissolving? • Temperature • You can dissolve more into a warm liquid than you can into a cold liquid • Surface area • Which dissolves faster, a cube of sugar or grains of sugar? Why? • Concentration • The more solute already dissolved in a solvent, the slower the rate of dissolving. 34

What factors affect the rate of dissolving? • Pressure • What affect would increasing pressure have on the rate of dissolving? Why? 35

What factors affect the rate of dissolving? • Pressure • What affect would increasing pressure have on the rate of dissolving? Why? • Mixing • Describe what happens when you mix a solution. 36

How do you get from this…

…to this?

Add an ionic compound!

Colligative Properties • Properties that depend only on the number of solute particles and not on their identity.

Some Colligative Properties are: • Vapor pressure lowering • Boiling point elevation • Freezing Point depression

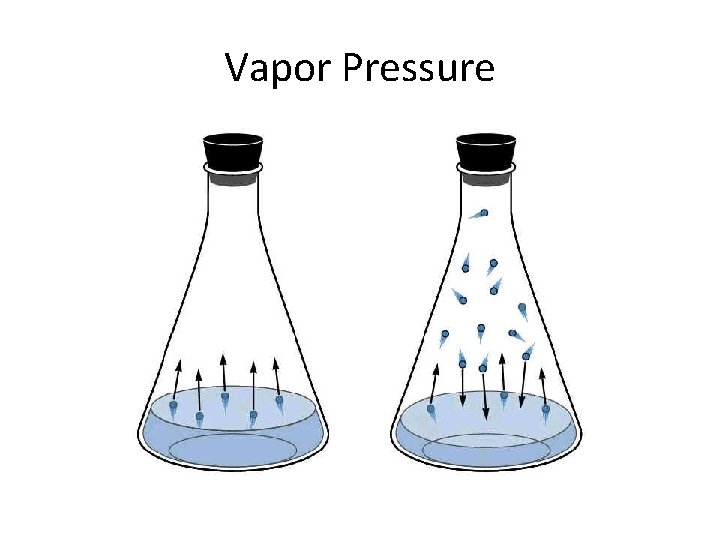
Vapor Pressure

Vapor Pressure Lowering • The particles of solute are surrounded by and attracted to particles of solvent. • Now the solvent particles have less kinetic energy and tend less to escape into the space above the liquid. • So the vapor pressure is less.

Ionic vs Molecular Solutes • Ionic solutes produce two or more ion particles in solution. • They affect the colligative properties proportionately more than molecular solutes (that do not ionize). • The effect is proportional to the number of particles of the solute in the solution.
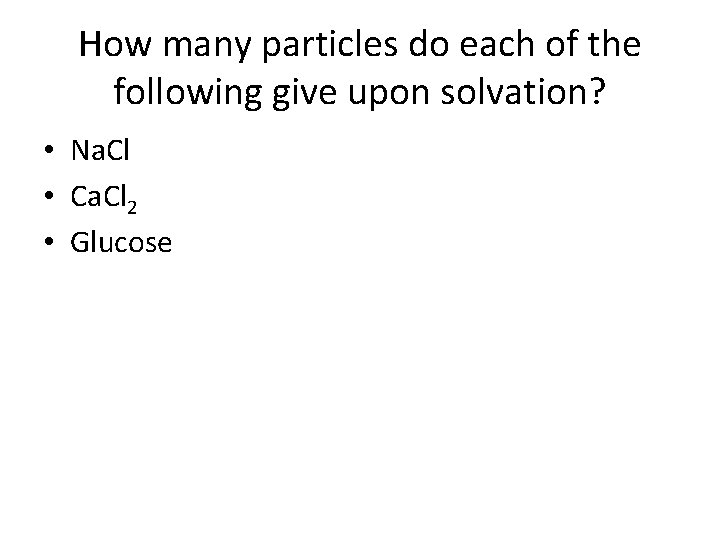
How many particles do each of the following give upon solvation? • Na. Cl • Ca. Cl 2 • Glucose

Freezing Point Depression

Example • Salt is added to melt ice by reducing the freezing point of water.

Boiling Point Elevation

Example • Addition of ethylene glycol C 2 H 6 O 2 (antifreeze) to car radiators.

Ready for a test soon? !? !? ! • It’s review time! • In this unit we studied SOLUTIONS! 50

Freezing Point Depression and Boiling Point Elevation • ∆Tb =mkb (for water kb=0. 51 o. C/m) • Freezing Point Depression o. C/m) • ∆Tf=mkf (for water k =1. 86 f • Note: m is the molality of the particles, so if the solute is ionic, multiply by the #of particles it dissociates to.

Which is more effective for lowering the freezing point of water? • Na. Cl or Ca. Cl 2

Example 1: • Find the new freezing point of 3 m Na. Cl in water.

Example 2: • Find the new boiling point of 3 m Na. Cl in water.

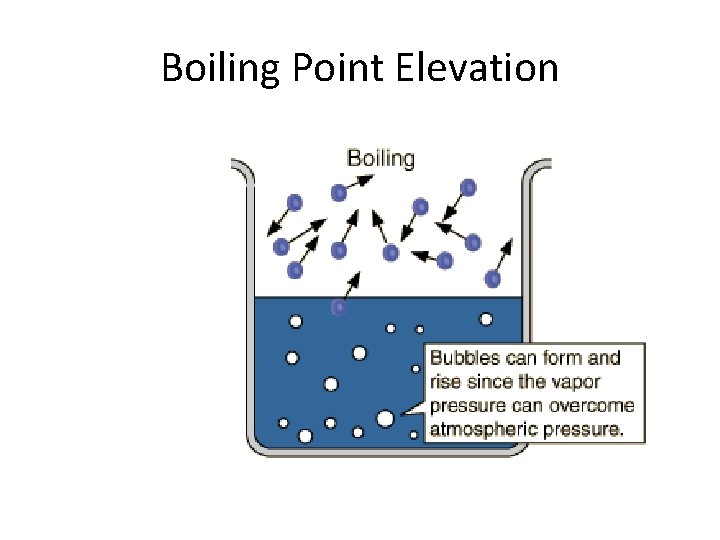
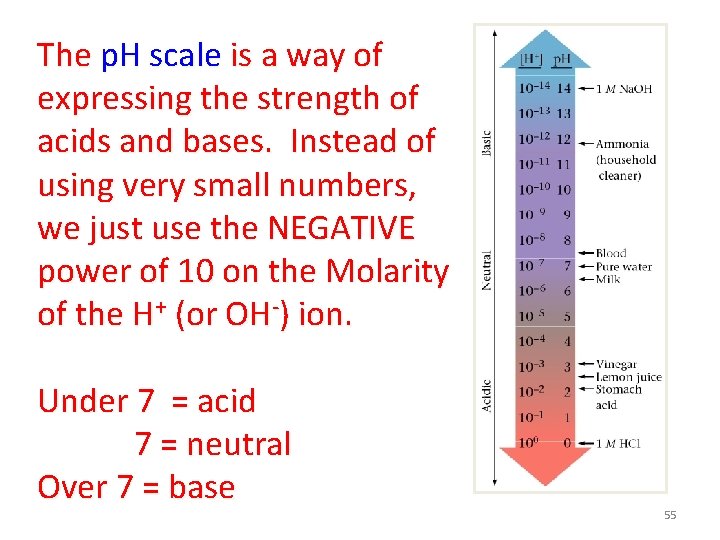
The p. H scale is a way of expressing the strength of acids and bases. Instead of using very small numbers, we just use the NEGATIVE power of 10 on the Molarity of the H+ (or OH-) ion. Under 7 = acid 7 = neutral Over 7 = base 55

Acid-Base Reactions • Acids- generally have a sour taste, change litmus from blue to red, can react with certain metals to produce gas, conduct electricity. • Bases- generally have a bitter taste, change litmus from red to blue, feel slippery, conduct electricity. • BrØnstead Acid- proton donor • BrØnstead Base- proton acceptor

Acid-Base Reactions • Acid or Base? – HCl(aq) + H 2 O(l) → H 3 O+(aq) + Cl–(aq) – NH 3(aq) + H 2 O(l) → NH 4+(aq) + OH–(aq)

Acid-Base Reactions • Look at the following compounds and decide whether they are a BrØnstead Acid or a BrØnstead Base. – HBr – NO 2– – HCO 3–

Arrhenius definition of acids and bases: Acids are compounds that give off H+ ions (also called hydronium ions, H 3 O+ ions, or simply “protons”) when you dissolve them in water. Bases are compounds that give off OH(hydroxide) ions when you dissolve them in water. 59

• Arrhenius acids almost always start with the letter “H” in their formulas – this is the source of the H+ ion that comes off when you dissolve the compound. Common examples include the following: • HNO 3(l) H+(aq) + NO 3(aq) • HCl • HBr • H 2 SO 4 • Arrhenius bases always have “OH” in their formulas, indicating the presence of the hydroxide ion. Common examples include: • Na. OH(s) Na+1(aq) + OH-1(aq) • KOH • Mg(OH)2 60

Acid-Base Reactions

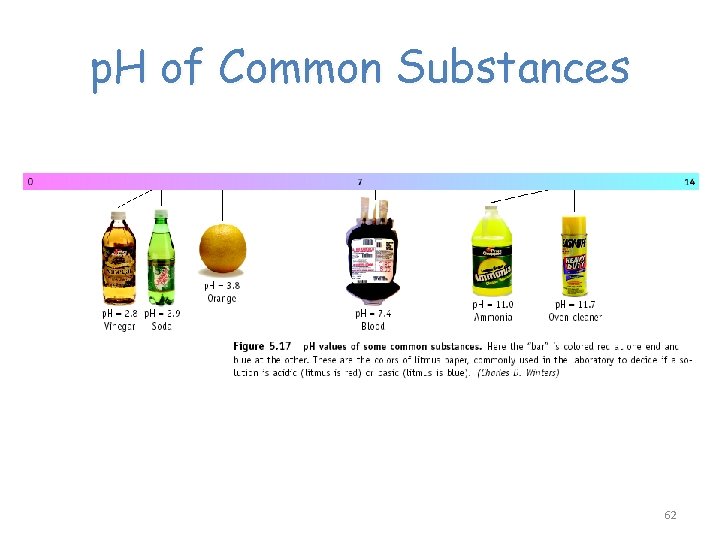
p. H of Common Substances 62

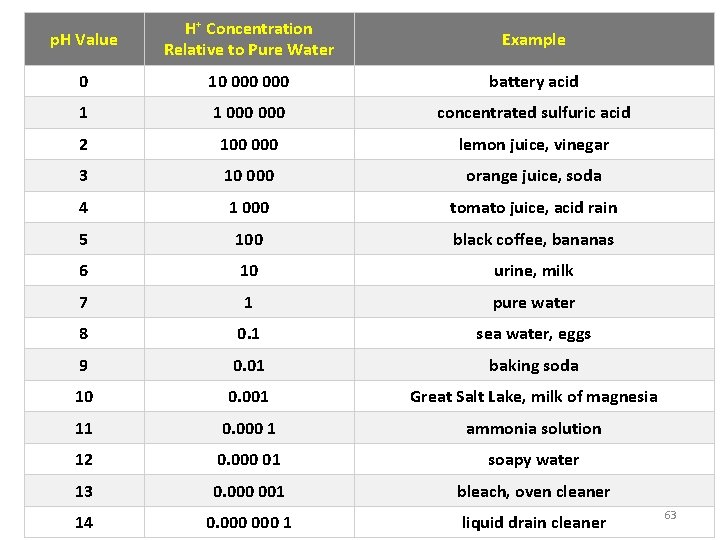
p. H Value H+ Concentration Relative to Pure Water Example 0 10 000 battery acid 1 1 000 concentrated sulfuric acid 2 100 000 lemon juice, vinegar 3 10 000 orange juice, soda 4 1 000 tomato juice, acid rain 5 100 black coffee, bananas 6 10 urine, milk 7 1 pure water 8 0. 1 sea water, eggs 9 0. 01 baking soda 10 0. 001 Great Salt Lake, milk of magnesia 11 0. 000 1 ammonia solution 12 0. 000 01 soapy water 13 0. 000 001 bleach, oven cleaner 14 0. 000 1 liquid drain cleaner 63

Put the following substances in order, from lowest p. H to highest p. H: • • Drain Cleaner Water Vinegar Soap Orange Juice Baking Soda Battery Acid 64

Put the following substances in order, from lowest p. H to highest p. H: • • Battery Acid Vinegar Orange Juice Water Baking Soda Soap Drain Cleaner Lowest p. H (most Acidic) Highest p. H (most Basic) 65
![Calculating the p H log H The means Molarity Example Calculating the p. H = - log [H+] (The [ ] means Molarity) Example:](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-66.jpg)
Calculating the p. H = - log [H+] (The [ ] means Molarity) Example: If [H+] = 1 X 10 -10 p. H = - log 1 X 10 -10 p. H = - (- 10) p. H = 10 Example: If [H+] = 1. 8 X 10 -5 p. H = - log 1. 8 X 10 -5 p. H = - (- 4. 74) p. H = 4. 74 66

p. H calculations – Solving for H+ If the p. H of Coke is 3. 12, [H+] = ? ? ? Because p. H = - log [H+] then - p. H = log [H+] Take antilog (10 x) of both sides and get 10 -p. H = [H+] = 10 -3. 12 = 7. 6 x 10 -4 M *** to find antilog on your calculator, look for “Shift” or “ 2 nd function” and then the log button 67

p. H calculations – Solving for H+ • A solution has a p. H of 8. 5. What is the Molarity of hydrogen ions in the solution? p. H = - log [H+] 8. 5 = - log [H+] -8. 5 = log [H+] Antilog -8. 5 = antilog (log [H+]) 10 -8. 5 = [H+] 3. 16 X 10 -9 = [H+] 68

More About Water H 2 O can function as both an ACID and a BASE…. . water is AMPHOTERIC In pure water there can be AUTOIONIZATION Equilibrium constant for water = Kw Kw = [H 3 O+] [OH-] = 1. 00 x 10 -14 at 25 o. C
![More About Water Autoionization Kw H 3 O OH 1 00 x More About Water Autoionization Kw = [H 3 O+] [OH-] = 1. 00 x](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-70.jpg)
More About Water Autoionization Kw = [H 3 O+] [OH-] = 1. 00 x 10 -14 at 25 o. C In a neutral solution [H 3 O+] = [OH-] so Kw = [H 3 O+]2 = [OH-]2 and so [H 3 O+] = [OH-] = 1. 00 x 10 -7 M 70

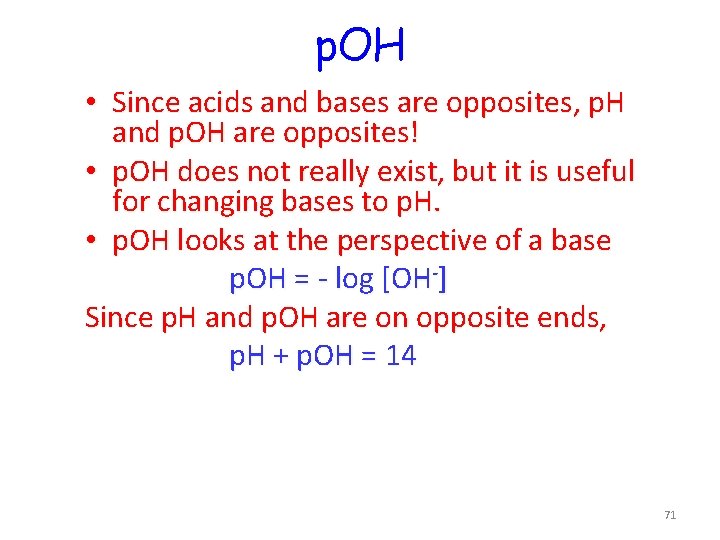
p. OH • Since acids and bases are opposites, p. H and p. OH are opposites! • p. OH does not really exist, but it is useful for changing bases to p. H. • p. OH looks at the perspective of a base p. OH = - log [OH-] Since p. H and p. OH are on opposite ends, p. H + p. OH = 14 71
![p H H OH p OH 72 p. H [H+] [OH-] p. OH 72](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-72.jpg)
p. H [H+] [OH-] p. OH 72
![H 3 O OH and p H What is the p H of the [H 3 O+], [OH-] and p. H What is the p. H of the](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-73.jpg)
[H 3 O+], [OH-] and p. H What is the p. H of the 0. 0010 M Na. OH solution? [OH-] = 0. 0010 (or 1. 0 X 10 -3 M) p. OH = - log 0. 0010 p. OH = 3 p. H = 14 – 3 = 11 OR Kw = [H 3 O+] [OH-] [H 3 O+] = 1. 0 x 10 -11 M p. H = - log (1. 0 x 10 -11) = 11. 00 73
![OH 4 1 OH p 10 0 1 x 0 1 H [OH-] 4 -1 OH -p 10 0 1 x 0 +]. 1 [H -]](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-74.jpg)
[OH-] 4 -1 OH -p 10 0 1 x 0 +]. 1 [H -] [H+] 4 -1 H g[O -Lo 0 1 x - ] 0 1. OH [ p. OH +] g[H -Lo H -p 10 H O p 4 1 p. H H p 14 74

Oxidation Reduction Reactions • Oxidation Reaction- refers to the half-reaction that involves the loss of electrons. • Reduction Reaction- refers to the half-reaction that involves the gain of electrons. OILRIG • Oxidizing agent- the compound or ion in a redox reaction that donates electrons. • Reducing agent- the compound or ion in a redox reaction that accepts electrons.

Oxidation-Reduction Reactions
![HOMEWORK 1 How much calcium hydroxide CaOH2 in grams is needed to produce 1 HOMEWORK 1) How much calcium hydroxide [Ca(OH)2], in grams, is needed to produce 1.](https://slidetodoc.com/presentation_image/5fb420eafe7093e538a5ab20777b101d/image-77.jpg)
HOMEWORK 1) How much calcium hydroxide [Ca(OH)2], in grams, is needed to produce 1. 5 L of a 0. 25 M solution? 2) What volume of a 3. 00 M KI stock solution would you use to make 0. 300 L of a 1. 25 M KI solution? 3) How many m. L of a 5. 0 M H 2 SO 4 stock solution would you need to prepare 100. 0 m. L of 0. 25 M H 2 SO 4? 4) If 0. 50 L of 5. 00 M stock solution is diluted to make 2. 0 L of solution, how much HCl, in grams, is in the solution? 77

HOMEWORK 5) Calculate the p. H of solutions having the following ion concentrations at 298 K. a) [H+] = 1. 0 x 10 -2 M b) [H+] = 3. 0 x 10 -6 M 6) Calculate the p. H of a solution having [OH-] = 8. 2 x 10 -6 M. 7) Calculate p. H and p. OH for an aqueous solution containing 1. 0 x 10 -3 mol of HCl dissolved in 5. 0 L of solution. 8) Calculate the [H+] and [OH-] in a sample of seawater with a p. OH = 5. 60. 78