Stoichiometry with Molar Concentration Molar Concentration Recall that








- Slides: 15

Stoichiometry with Molar Concentration

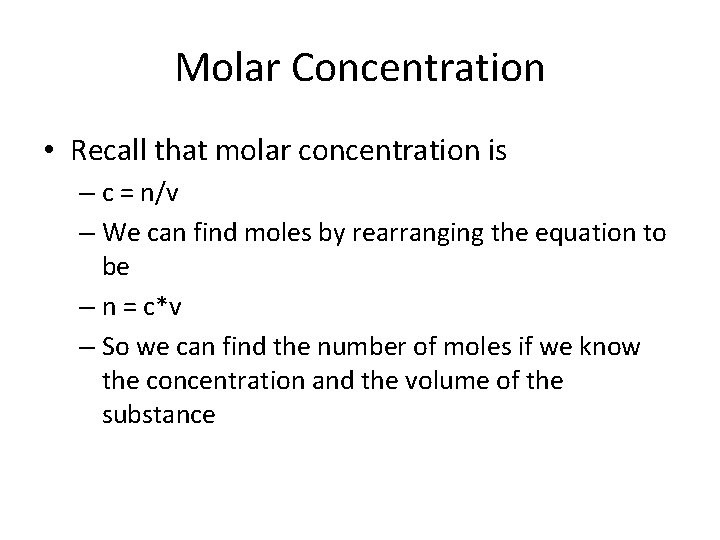
Molar Concentration • Recall that molar concentration is – c = n/v – We can find moles by rearranging the equation to be – n = c*v – So we can find the number of moles if we know the concentration and the volume of the substance

Note! • If a volume is mentioned and the problem is about molarity, do not assume 22. 4 L as that is only for GAS at STP.

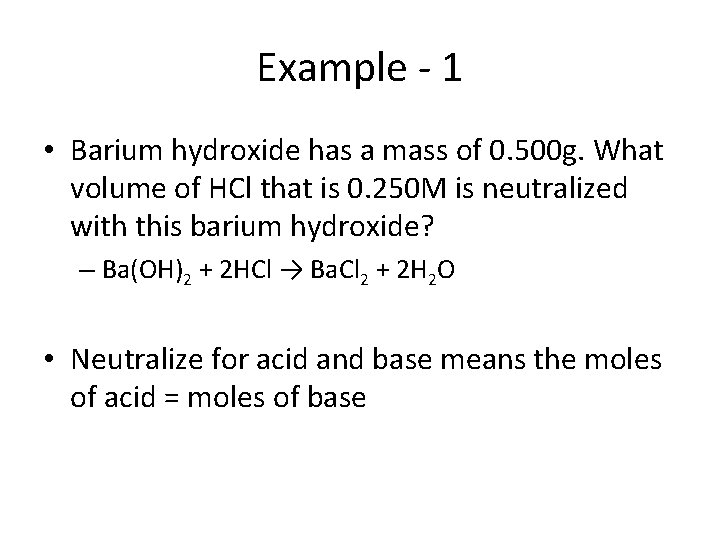
Example - 1 • Barium hydroxide has a mass of 0. 500 g. What volume of HCl that is 0. 250 M is neutralized with this barium hydroxide? – Ba(OH)2 + 2 HCl → Ba. Cl 2 + 2 H 2 O • Neutralize for acid and base means the moles of acid = moles of base

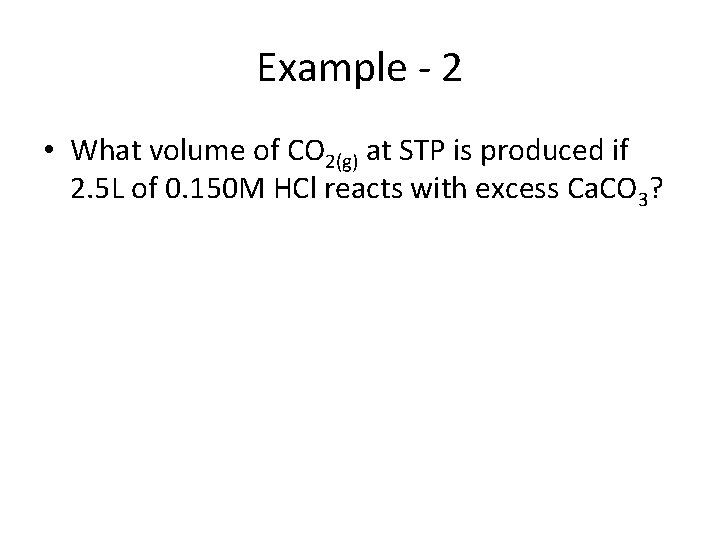
Example - 2 • What volume of CO 2(g) at STP is produced if 2. 5 L of 0. 150 M HCl reacts with excess Ca. CO 3?

Titration • It is a process we use in chemistry to find the concentration of an acid or base • We can perform this process when we know the exact volume and concentration of an acid or base and the volume of the substance we are neutralizing it • We use a tool called a burette to perform this as it is highly accurate and easy to control the volume down to 0. 1 m. L


Titration • What we do – We have an unknown substance in an Erlenmeyer flask with a known volume – We add our known concentration substance into the burette – We add an indicator into our unknown substance • Phenolphthalein – indicator turns pink at around p. H of 8. 5 – We slowly add or known substance into the flask and stop when our Erlenmeyer flask of solution is pink • This is our equivalence point

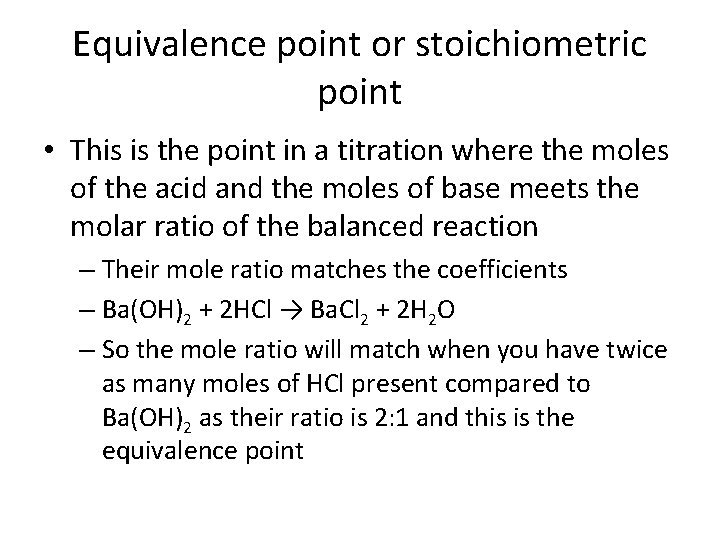
Equivalence point or stoichiometric point • This is the point in a titration where the moles of the acid and the moles of base meets the molar ratio of the balanced reaction – Their mole ratio matches the coefficients – Ba(OH)2 + 2 HCl → Ba. Cl 2 + 2 H 2 O – So the mole ratio will match when you have twice as many moles of HCl present compared to Ba(OH)2 as their ratio is 2: 1 and this is the equivalence point

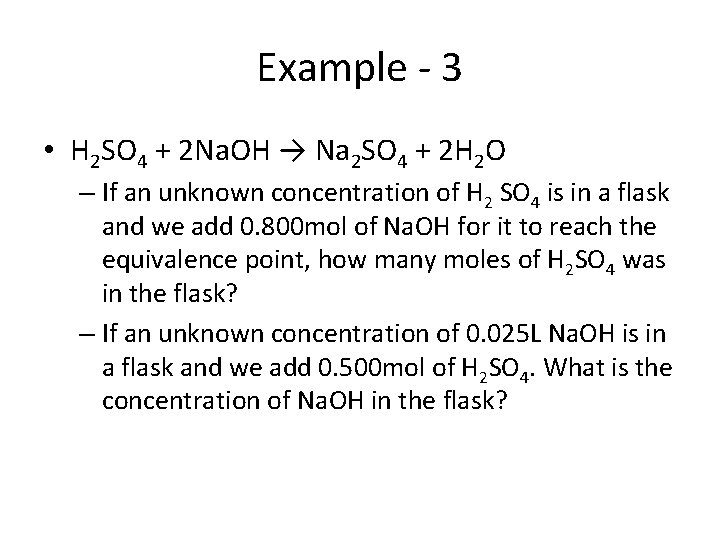
Example - 3 • H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O – If an unknown concentration of H 2 SO 4 is in a flask and we add 0. 800 mol of Na. OH for it to reach the equivalence point, how many moles of H 2 SO 4 was in the flask? – If an unknown concentration of 0. 025 L Na. OH is in a flask and we add 0. 500 mol of H 2 SO 4. What is the concentration of Na. OH in the flask?

Note! • Equivalence point is met when the moles are equal based on their molar ratio, not their concentration!

Example - 4 • H 2 SO 4 + 2 Na. OH → Na 2 SO 4 + 2 H 2 O – It takes 21. 55 m. L of Na. OH at 0. 500 M to titrate 25. 00 m. L of H 2 SO 4. What is the molarity of H 2 SO 4 present?

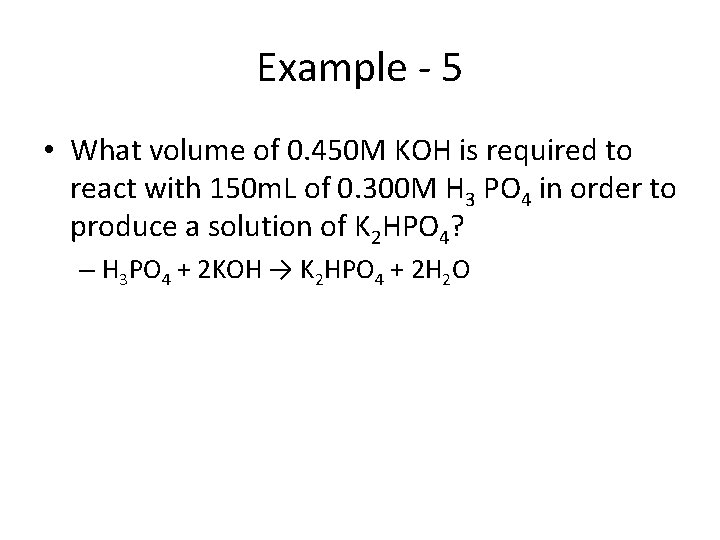
Example - 5 • What volume of 0. 450 M KOH is required to react with 150 m. L of 0. 300 M H 3 PO 4 in order to produce a solution of K 2 HPO 4? – H 3 PO 4 + 2 KOH → K 2 HPO 4 + 2 H 2 O

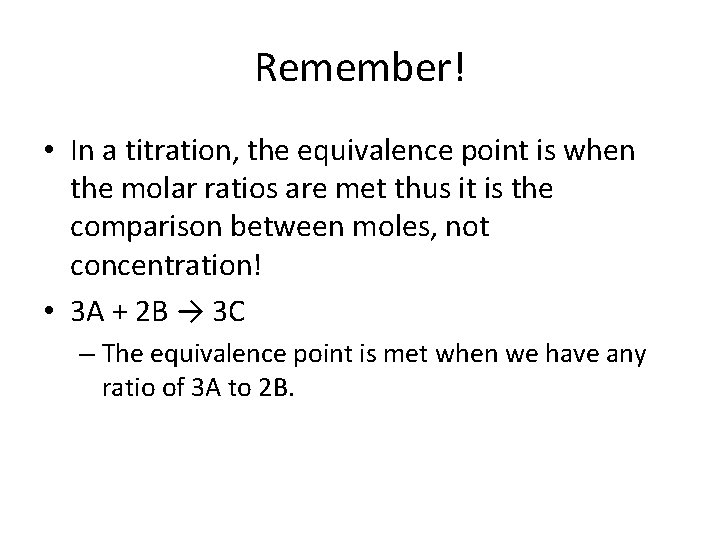
Remember! • In a titration, the equivalence point is when the molar ratios are met thus it is the comparison between moles, not concentration! • 3 A + 2 B → 3 C – The equivalence point is met when we have any ratio of 3 A to 2 B.

Practice - 1 • Page 131 - #17 -25