Tonicity The relative concentration of solutions Hypertonic Isotonic











- Slides: 14

Tonicity: The relative concentration of solutions. Hypertonic, Isotonic and Hypotonic.

What is a solution? • There are two components or parts to a solution. • The first part of the solution is called the solvent which is often a liquid. • In most biological systems (like you and me) the solution is water. • In fact, water is called the universal solvent.

What is a solution? • The second part of the solution is called the solute. • This can be a gas, liquid or solid. • The solute is dissolved in the solvent. • Salt (Na. Cl), and starch are all examples of solutes.

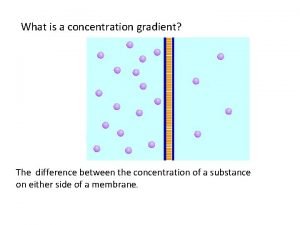
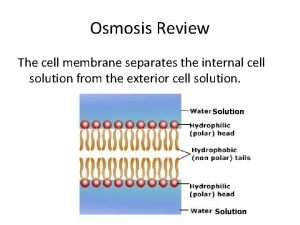
Effect of solution on osmosis. • Osmosis is the term used to describe the movement (diffusion) of water molecules across a cell membrane. • The water moves from areas of high concentration to areas of low concentration.

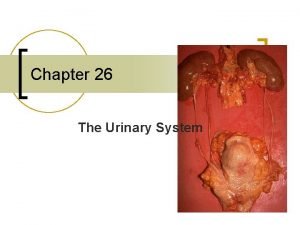
Types of solutions • To determine the type of solution you cell is in, you compare it to the concentration of the solution within the cell. • If the concentration of solution outside the cell is higher than inside the cell it is called a hypertonic solution. • If the concentration of solution outside the cell is the same as inside the cell it is called an isotonic solution. • If the concentration of solution outside the cell is lower than inside the cell it is called a hypotonic solution.

Hypertonic solution • What happens to a cell when it is placed in a hypertonic solution? • If the cytoplasm of a cell is 95% water and 5% Na. Cl and it is placed in a solution that is 90% and 10% water, what would happen? • Will water move into the cell, or will the water move outside the cell?

Isotonic solution • When a cell is placed in a solution that has the same concentration as the cytoplasm what will happen? • In which direction will osmosis occur?

Hypotonic solution • What would happen to a cell placed in a hypotonic solution? • Consider a case where a cell with 95% water and 5% salt was placed in distilled water (100% water). • What would happen to this cell?

Results

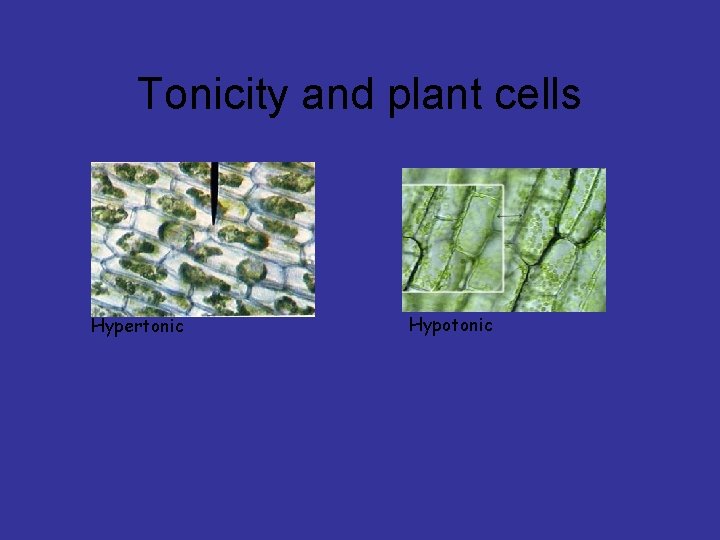
Summary • When a cell is placed in a hypertonic solution, water diffuses out of the cell, and it shrivels up. In plant cells, you can see the cell membrane pull away from the cell wall. • In an isotonic solution, the cell remains the same. The rate of osmosis is the same in both directions. This is equilibrium.

Summary continued. • When a cell is placed in a hypotonic solution water diffuses into the cell and causes it to expand, and then burst (lyse). • The cell wall in plants prevents it from bursting. This contidion is called turgidity.

Effect of tonicity • These are red blood cells. Isotonic Hypertonic Hypotonic

Tonicity and plant cells Hypertonic Hypotonic

What happens to a salted slug?
 Tonicity
Tonicity Hypertonic hypotonic isotonic red blood cells
Hypertonic hypotonic isotonic red blood cells Plants cells _________ water and start to _w_ ___ ___ ___.
Plants cells _________ water and start to _w_ ___ ___ ___. Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Hypertonic
Hypertonic Osmosis
Osmosis Modified ashworth scale
Modified ashworth scale Principal cells
Principal cells Hypertonic solution
Hypertonic solution Nurses responsibility of oxytocin
Nurses responsibility of oxytocin Mild moderate severe dehydration
Mild moderate severe dehydration Isotonic solution
Isotonic solution Third spacing
Third spacing Water potential gcse
Water potential gcse