Concentration of a Solution The concentration of a





- Slides: 10

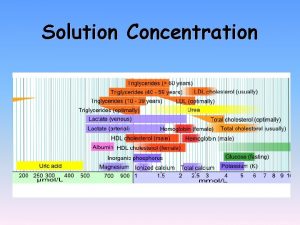
Concentration of a Solution • The concentration of a solution is a measure of the amount of solute in a given amount of solvent or solution. • Chemists commonly use several different ways to quantitatively express the concentration of a solution. These include: q Percent Composition by mass q Parts per million / Part per billion q Molarity (M) q Molality (m)

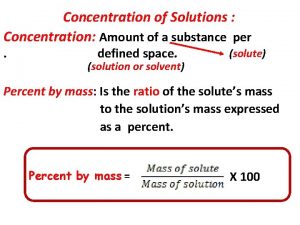
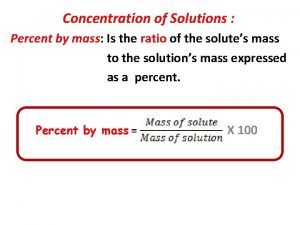
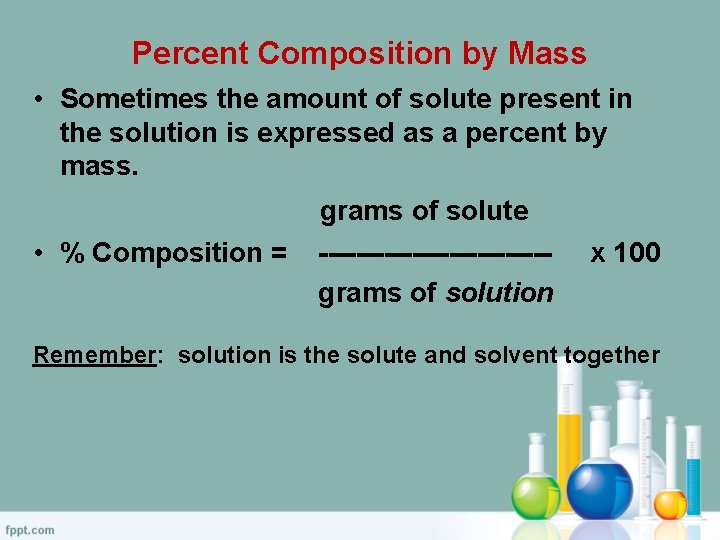
Percent Composition by Mass • Sometimes the amount of solute present in the solution is expressed as a percent by mass. • % Composition = grams of solute ------------grams of solution x 100 Remember: solution is the solute and solvent together

Percent Composition Sample Problems • What is the percent composition of a solution that contains 115 g Na. Cl dissolved in 500 g of H 2 O? • How many grams of KMn. O 4 are needed to make 500 g of a 12. 0% KMn. O 4 solution?

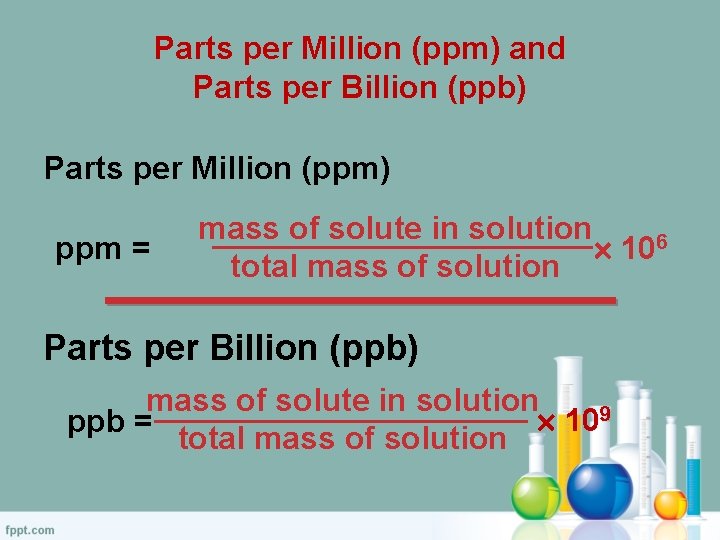
Parts per Million (ppm) and Parts per Billion (ppb) Parts per Million (ppm) ppm = mass of solute in solution 106 total mass of solution Parts per Billion (ppb) mass of solute in solution 109 ppb = total mass of solution

What Do You Think? • A 900. 0 g sample of sea water is found to contain 6. 7 x 10 -3 g Zn. Express this concentration in ppm.

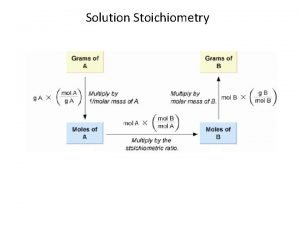
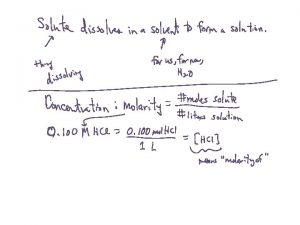
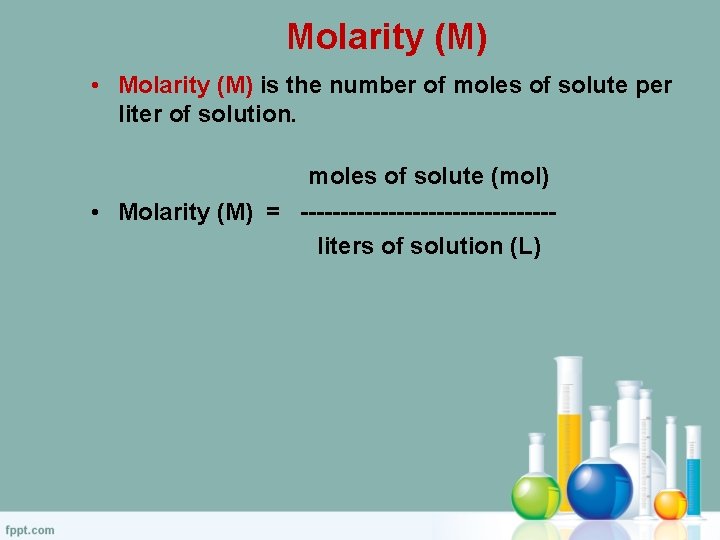
Molarity (M) • Molarity (M) is the number of moles of solute per liter of solution. moles of solute (mol) • Molarity (M) = ----------------liters of solution (L)

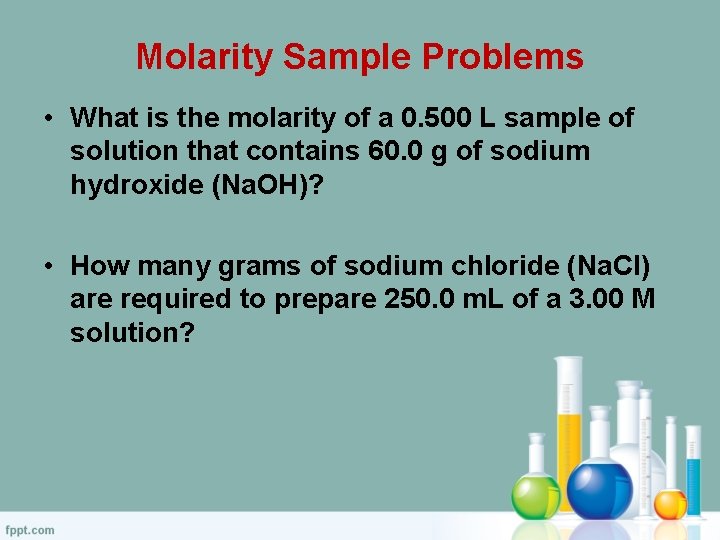
Molarity Sample Problems • What is the molarity of a 0. 500 L sample of solution that contains 60. 0 g of sodium hydroxide (Na. OH)? • How many grams of sodium chloride (Na. Cl) are required to prepare 250. 0 m. L of a 3. 00 M solution?

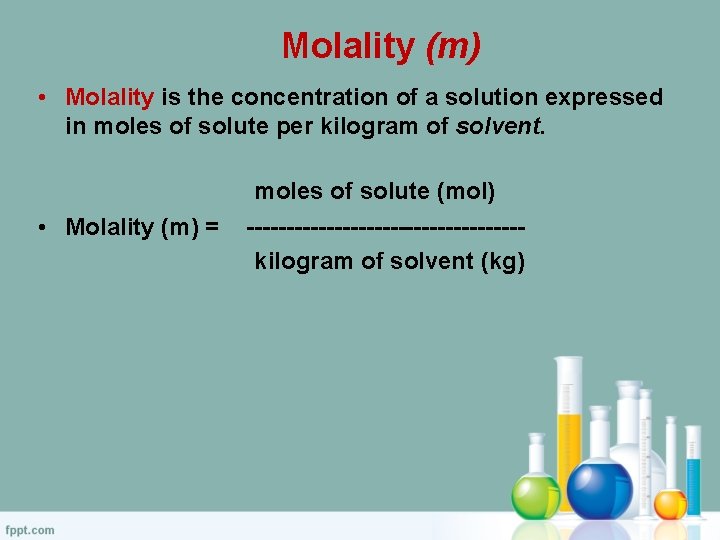
Molality (m) • Molality is the concentration of a solution expressed in moles of solute per kilogram of solvent. • Molality (m) = moles of solute (mol) -----------------kilogram of solvent (kg)

Molality Sample Problem • A solution was prepared by dissolving 17. 1 g of glucose, C 6 H 12 O 6, in 275 g of water. What is the molality (m) of this solution?

Wrapping It Up • Reflect on what you have learned concerning the today’s topic. • Respond to the following… – What is the most important thing you learned today? – What is one question you would still like answered? – What is a way what you have learned today connects with what you knew before?
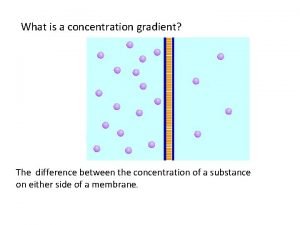
 Movement of high concentration to low concentration
Movement of high concentration to low concentration Whats a concentration gradient
Whats a concentration gradient How to calculate concentration of solution
How to calculate concentration of solution How to determine concentration of a solution
How to determine concentration of a solution Percent by mass
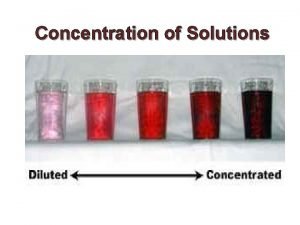
Percent by mass Concentration of solution
Concentration of solution Concentration of solution formula
Concentration of solution formula Living by chemistry solutions
Living by chemistry solutions Lesson 80 bearly alive solution concentration
Lesson 80 bearly alive solution concentration What is the formula of molarity
What is the formula of molarity Solute solvent
Solute solvent