12 4 NOTES Solution Concentration II Solution Concentration











- Slides: 17

12. 4 NOTES Solution Concentration

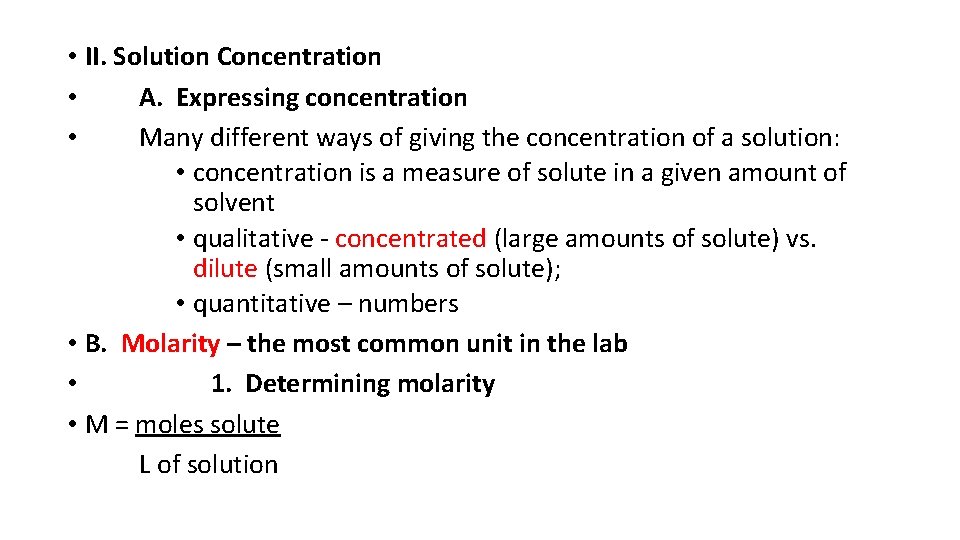
• II. Solution Concentration • A. Expressing concentration • Many different ways of giving the concentration of a solution: • concentration is a measure of solute in a given amount of solvent • qualitative - concentrated (large amounts of solute) vs. dilute (small amounts of solute); • quantitative – numbers • B. Molarity – the most common unit in the lab • 1. Determining molarity • M = moles solute L of solution

• Examples: a. 47. 5 grams of Na. OH are dissolved in enough water to make 1500 m. L of solution. M? • 47. 5 g ( 1 mol Na. OH) = 1. 19 mol = 0. 79 M • 40. 0 g 1. 5 L •

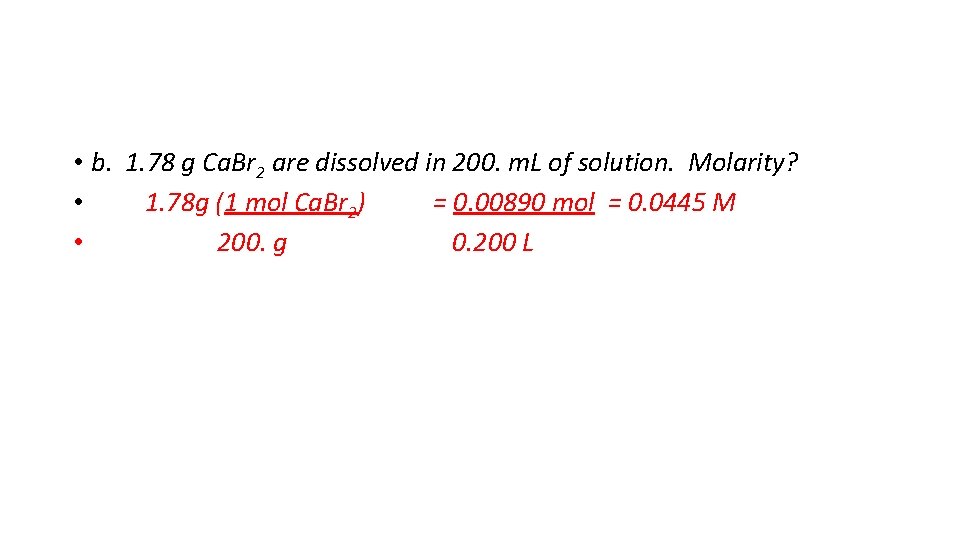
• b. 1. 78 g Ca. Br 2 are dissolved in 200. m. L of solution. Molarity? • 1. 78 g (1 mol Ca. Br 2) = 0. 00890 mol = 0. 0445 M • 200. g 0. 200 L

• 2. Preparing molar solutions to make solution, dissolve solute in volume smaller than desired final volume (b/c solute particles will also take up space) and then add solvent to raise the volume to the desired value; • If M = moles solute , then M x L = moles solute, or MV = mol (V is in L) • L solution

• Examples: a. How many grams of Ca. Br 2 are needed to prepare 2. 5 L of a 1. 0 M solution? • 2. 5 L x 1. 0 M = 2. 5 mol (199. 9 g Ca. Br 2) = 500 g • 1 mol

• b. What mass of Na. OH is present in 500. m. L of a 0. 75 M solution? • 0. 500 L x 0. 75 M = 0. 38 mol (40. 0 g Na. OH) = 15. g • 1 mol

• c. How many grams of HCl are dissolved in 350. 0 m. L of a 2. 0 M solution? • 0. 3500 L x 2. 0 M = 0. 70 mol (36. 5 g HCl) = 26 g • 1 mol

• 3. Diluting solutions • When diluting a solution, the number of moles of solute has not been changed. Therefore, we can use the relationship M 1 V 1 = M 2 V 2. Since volume appears on both sides of the equation, V does not have to be in liters.

• Examples: a. What volume of 6. 0 M HCl is needed to make 1. 5 liters of 1. 0 M solution? • (6. 0)(V) = (1. 5)(1. 0) • V = 0. 25 L •

• b. If you dilute 25. 0 m. L of 12. 0 M HCl to 500. m. L of solution, what is the new molarity? • (25. 0)(12. 0) = (500. )(M) • 0. 60 = M

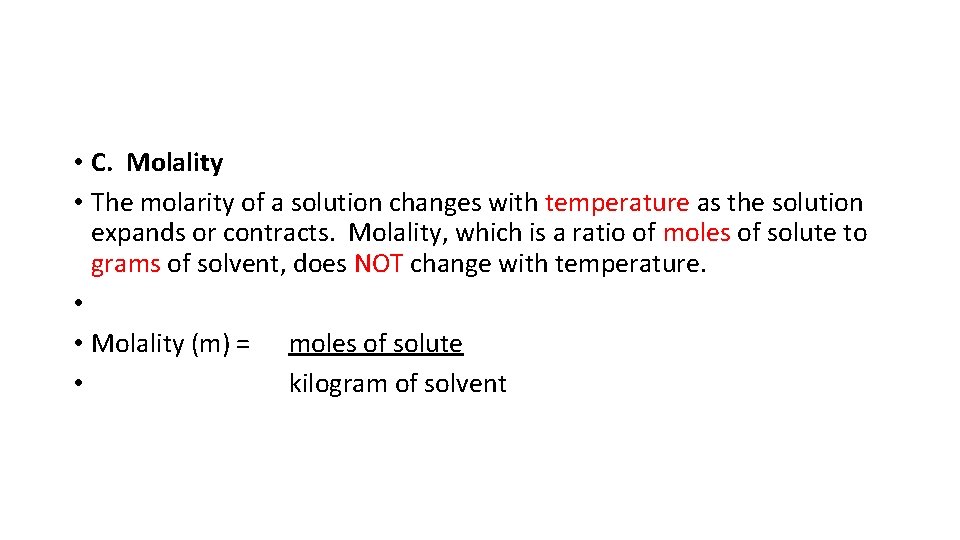
• C. Molality • The molarity of a solution changes with temperature as the solution expands or contracts. Molality, which is a ratio of moles of solute to grams of solvent, does NOT change with temperature. • • Molality (m) = moles of solute • kilogram of solvent

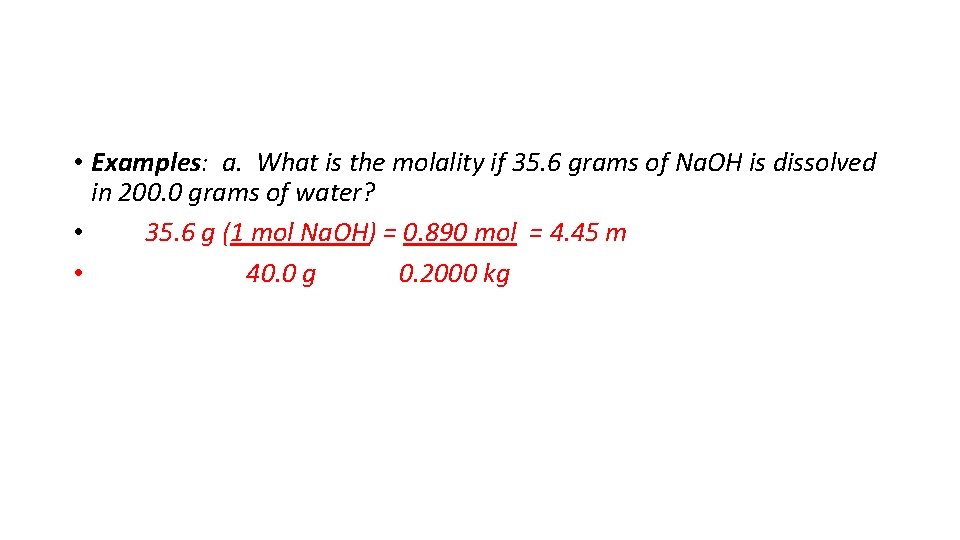
• Examples: a. What is the molality if 35. 6 grams of Na. OH is dissolved in 200. 0 grams of water? • 35. 6 g (1 mol Na. OH) = 0. 890 mol = 4. 45 m • 40. 0 g 0. 2000 kg

• b. What is molality of a solution if 2. 3 grams of KNO 3 is dissolved in 50. 0 grams of water? • 2. 3 g (1 mol KNO 3) = 0. 023 mol = 0. 46 m • 101. 1 g 0. 050 kg •

• D. Mole Fraction • Mole fraction is denoted by an X. Xa = moles of a • Total moles = na na + nb

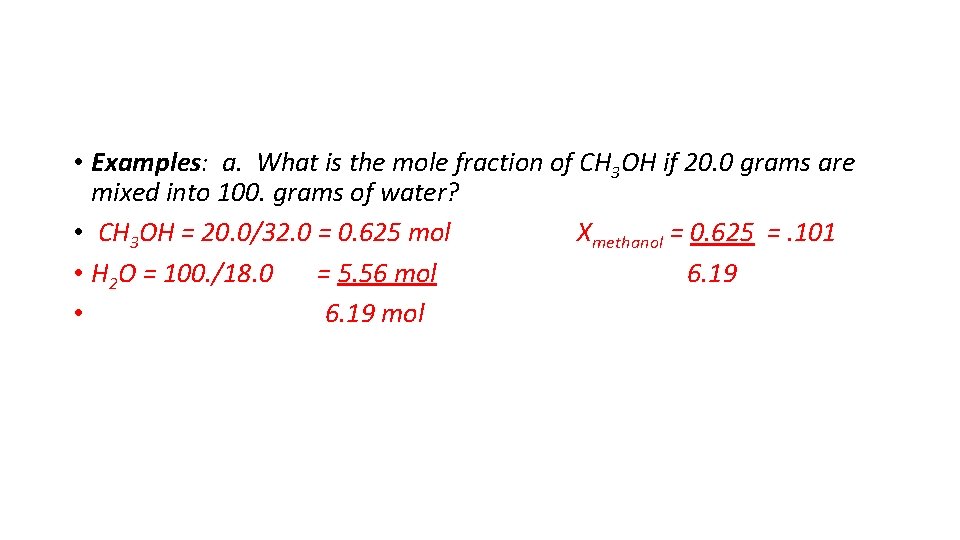
• Examples: a. What is the mole fraction of CH 3 OH if 20. 0 grams are mixed into 100. grams of water? • CH 3 OH = 20. 0/32. 0 = 0. 625 mol Xmethanol = 0. 625 =. 101 • H 2 O = 100. /18. 0 = 5. 56 mol 6. 19 • 6. 19 mol

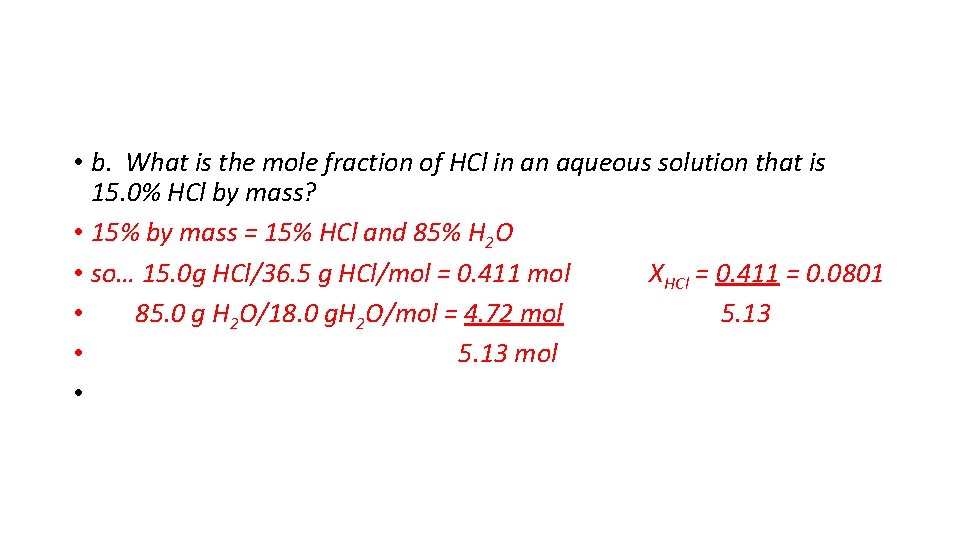
• b. What is the mole fraction of HCl in an aqueous solution that is 15. 0% HCl by mass? • 15% by mass = 15% HCl and 85% H 2 O • so… 15. 0 g HCl/36. 5 g HCl/mol = 0. 411 mol XHCl = 0. 411 = 0. 0801 • 85. 0 g H 2 O/18. 0 g. H 2 O/mol = 4. 72 mol 5. 13 • 5. 13 mol •