Chapter 6 Solutions Percent Concentration General Organic and



- Slides: 27

Chapter 6 Solutions Percent Concentration General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

Concentration The concentration of a solution § is the amount of solute dissolved in a specific amount of solution concentration = amount of solute amount of solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

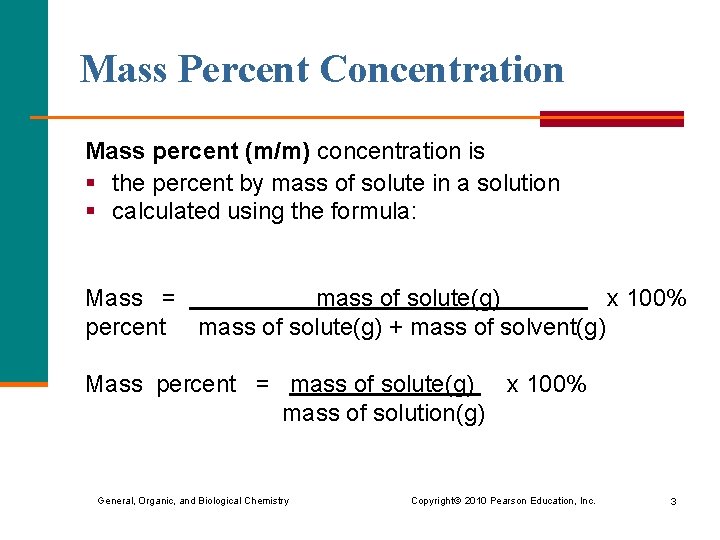
Mass Percent Concentration Mass percent (m/m) concentration is § the percent by mass of solute in a solution § calculated using the formula: Mass = mass of solute(g) x 100% percent mass of solute(g) + mass of solvent(g) Mass percent = mass of solute(g) x 100% mass of solution(g) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

Mass of Solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

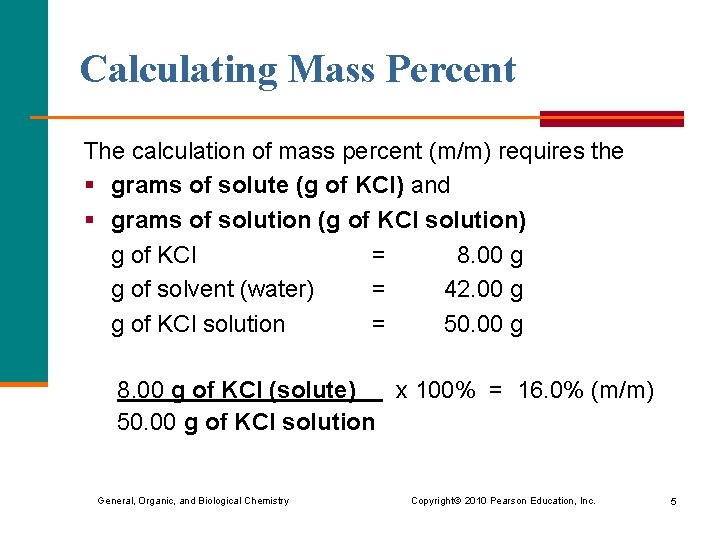
Calculating Mass Percent The calculation of mass percent (m/m) requires the § grams of solute (g of KCl) and § grams of solution (g of KCl solution) g of KCl = 8. 00 g g of solvent (water) = 42. 00 g g of KCl solution = 50. 00 g 8. 00 g of KCl (solute) x 100% = 16. 0% (m/m) 50. 00 g of KCl solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

Guide to Calculating Solution Concentrations General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

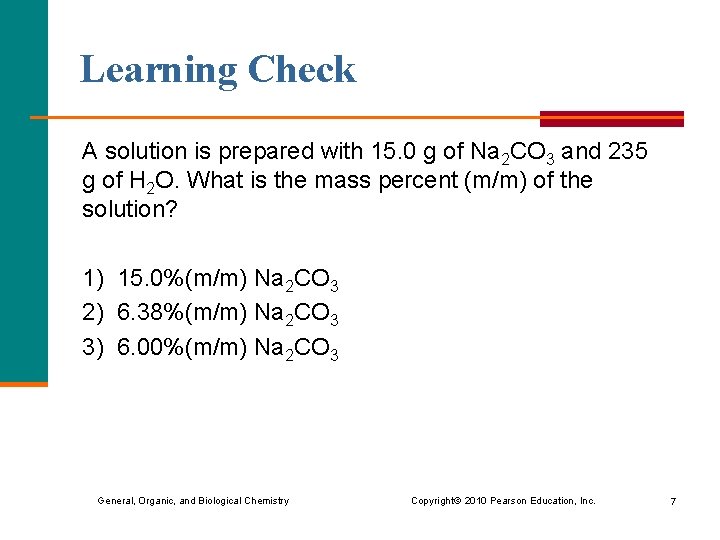
Learning Check A solution is prepared with 15. 0 g of Na 2 CO 3 and 235 g of H 2 O. What is the mass percent (m/m) of the solution? 1) 15. 0%(m/m) Na 2 CO 3 2) 6. 38%(m/m) Na 2 CO 3 3) 6. 00%(m/m) Na 2 CO 3 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

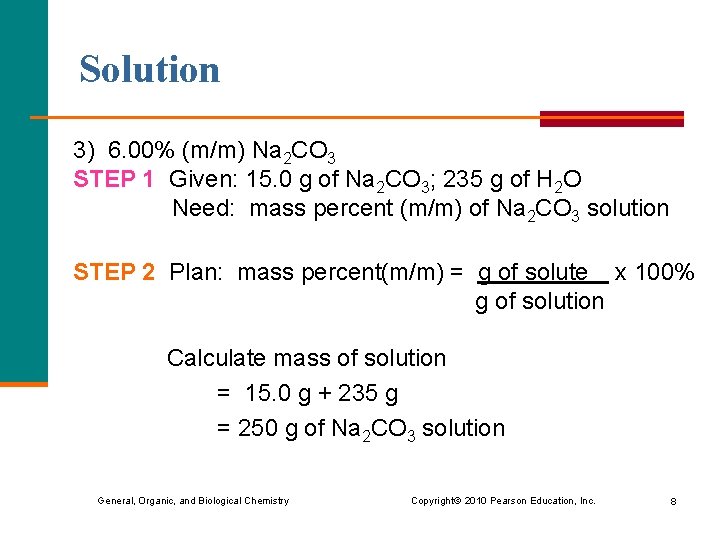
Solution 3) 6. 00% (m/m) Na 2 CO 3 STEP 1 Given: 15. 0 g of Na 2 CO 3; 235 g of H 2 O Need: mass percent (m/m) of Na 2 CO 3 solution STEP 2 Plan: mass percent(m/m) = g of solute x 100% g of solution Calculate mass of solution = 15. 0 g + 235 g = 250 g of Na 2 CO 3 solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

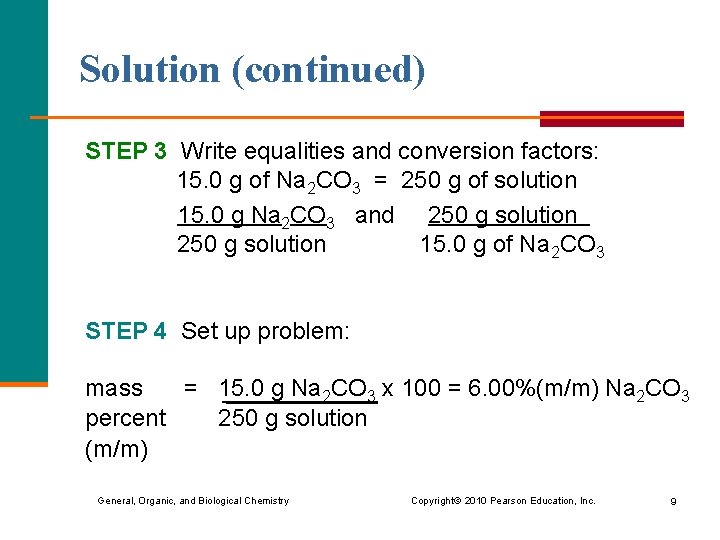
Solution (continued) STEP 3 Write equalities and conversion factors: 15. 0 g of Na 2 CO 3 = 250 g of solution 15. 0 g Na 2 CO 3 and 250 g solution 15. 0 g of Na 2 CO 3 STEP 4 Set up problem: mass = 15. 0 g Na 2 CO 3 x 100 = 6. 00%(m/m) Na 2 CO 3 percent 250 g solution (m/m) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

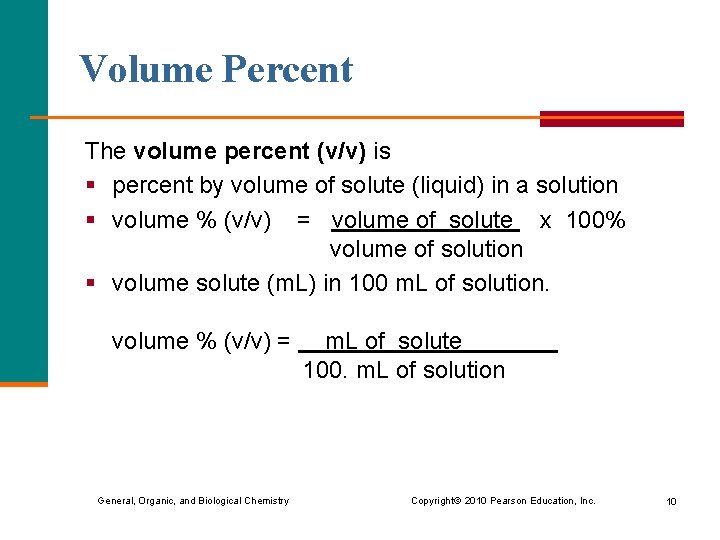
Volume Percent The volume percent (v/v) is § percent by volume of solute (liquid) in a solution § volume % (v/v) = volume of solute x 100% volume of solution § volume solute (m. L) in 100 m. L of solution. volume % (v/v) = m. L of solute 100. m. L of solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

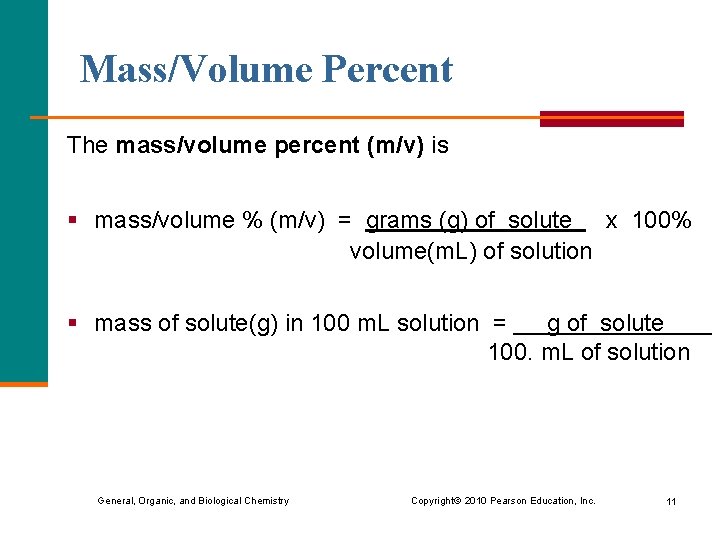
Mass/Volume Percent The mass/volume percent (m/v) is § mass/volume % (m/v) = grams (g) of solute x 100% volume(m. L) of solution § mass of solute(g) in 100 m. L solution = g of solute 100. m. L of solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

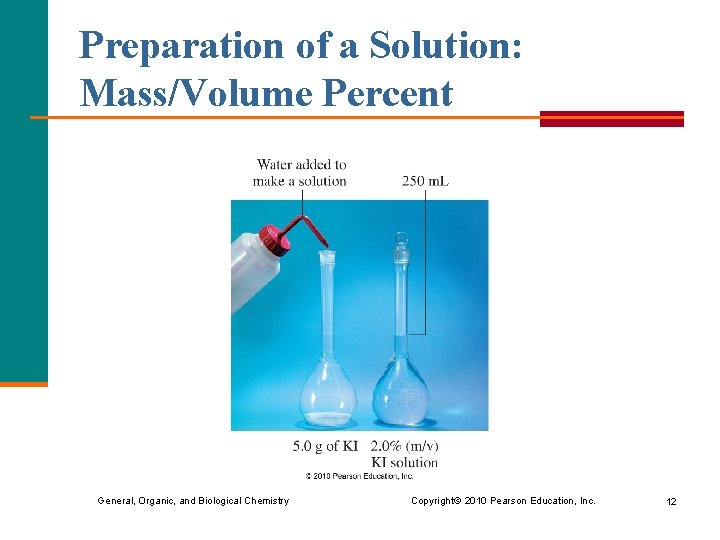
Preparation of a Solution: Mass/Volume Percent General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

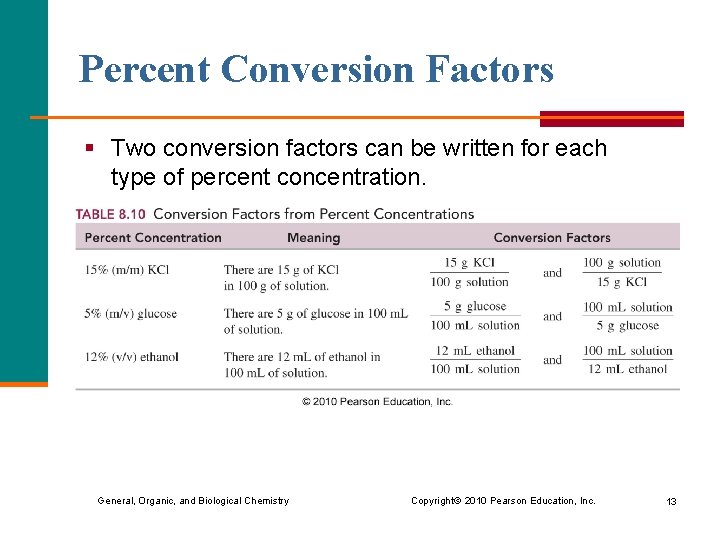
Percent Conversion Factors § Two conversion factors can be written for each type of percent concentration. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13

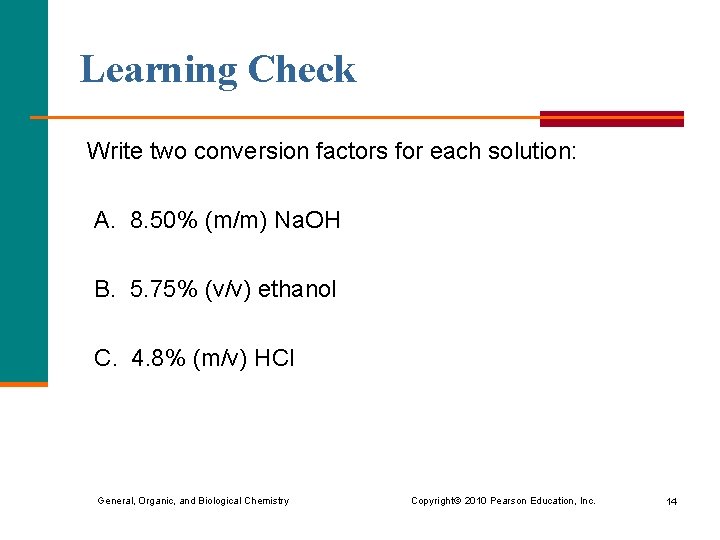
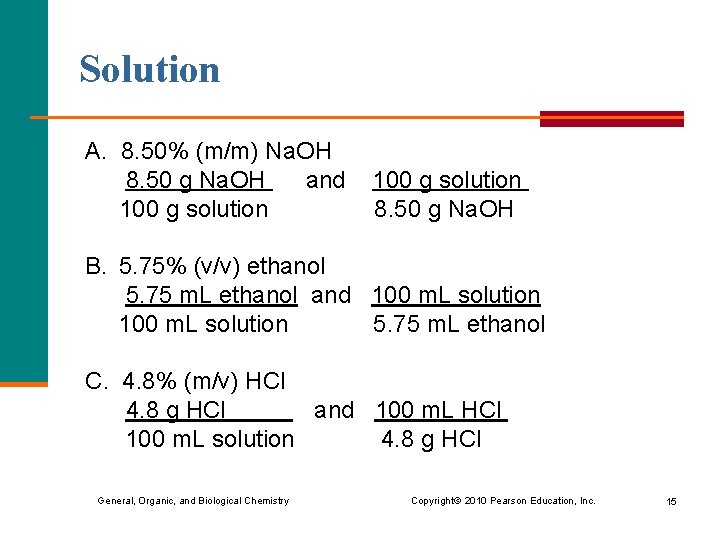
Learning Check Write two conversion factors for each solution: A. 8. 50% (m/m) Na. OH B. 5. 75% (v/v) ethanol C. 4. 8% (m/v) HCl General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

Solution A. 8. 50% (m/m) Na. OH 8. 50 g Na. OH and 100 g solution 8. 50 g Na. OH B. 5. 75% (v/v) ethanol 5. 75 m. L ethanol and 100 m. L solution 5. 75 m. L ethanol C. 4. 8% (m/v) HCl 4. 8 g HCl and 100 m. L HCl 100 m. L solution 4. 8 g HCl General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

Guide to Using Concentration to Calculate Mass or Volume General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

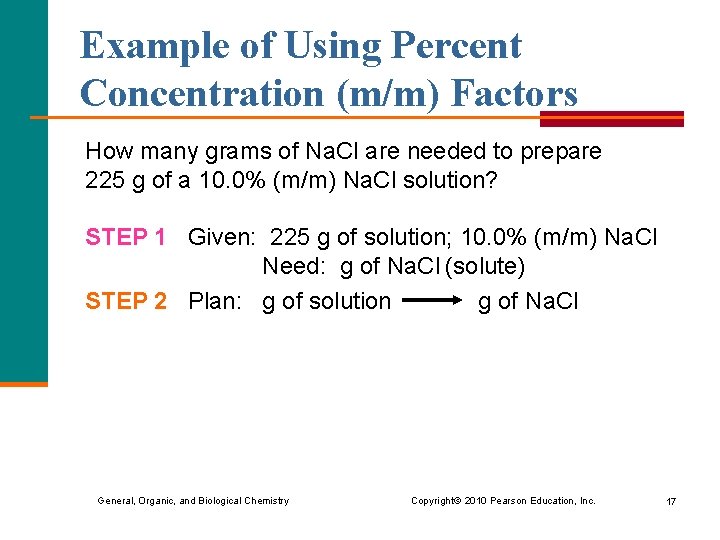
Example of Using Percent Concentration (m/m) Factors How many grams of Na. Cl are needed to prepare 225 g of a 10. 0% (m/m) Na. Cl solution? STEP 1 Given: 225 g of solution; 10. 0% (m/m) Na. Cl Need: g of Na. Cl (solute) STEP 2 Plan: g of solution g of Na. Cl General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

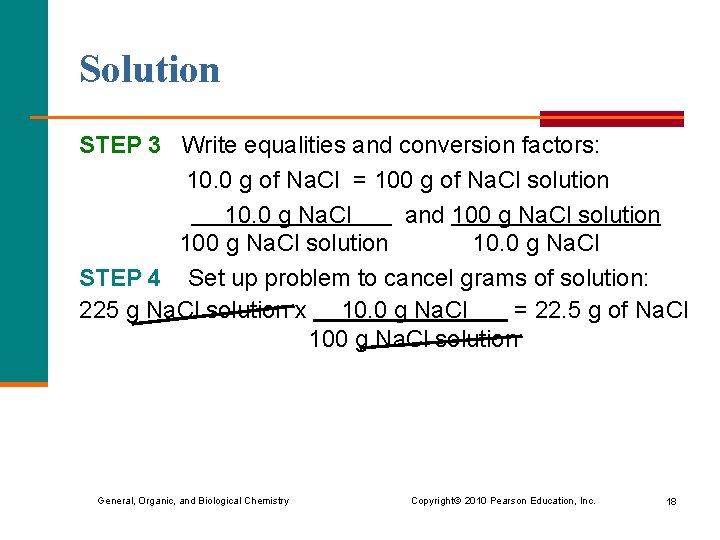
Solution STEP 3 Write equalities and conversion factors: 10. 0 g of Na. Cl = 100 g of Na. Cl solution 10. 0 g Na. Cl and 100 g Na. Cl solution 10. 0 g Na. Cl STEP 4 Set up problem to cancel grams of solution: 225 g Na. Cl solution x 10. 0 g Na. Cl = 22. 5 g of Na. Cl 100 g Na. Cl solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

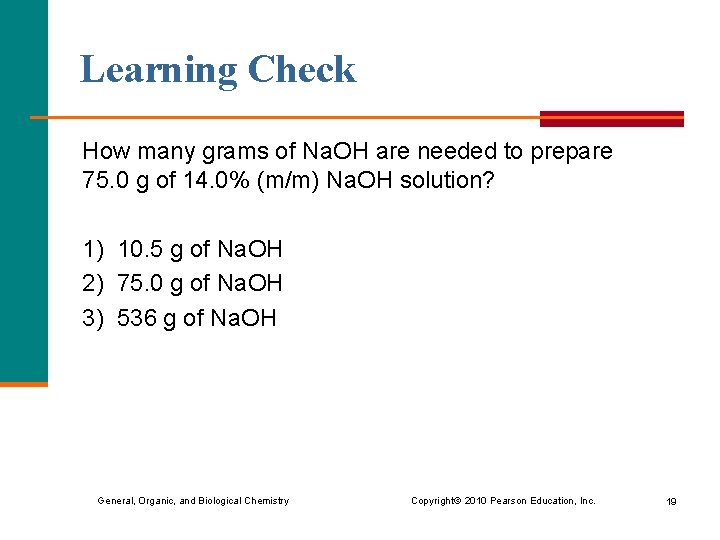
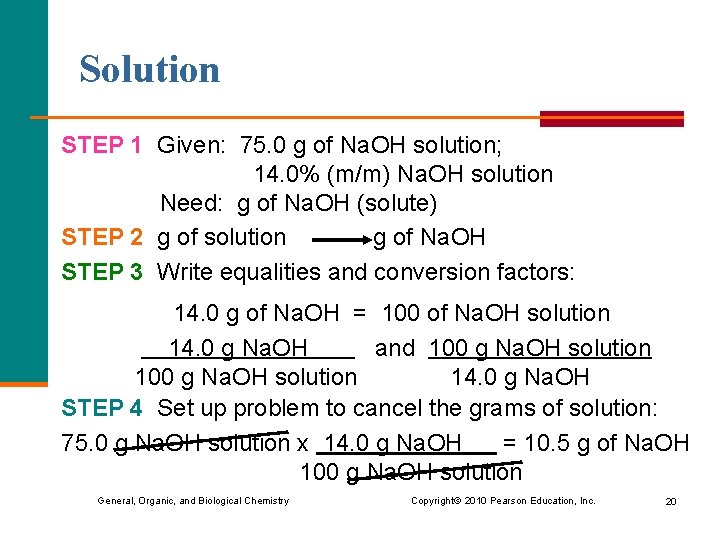
Learning Check How many grams of Na. OH are needed to prepare 75. 0 g of 14. 0% (m/m) Na. OH solution? 1) 10. 5 g of Na. OH 2) 75. 0 g of Na. OH 3) 536 g of Na. OH General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

Solution STEP 1 Given: 75. 0 g of Na. OH solution; 14. 0% (m/m) Na. OH solution Need: g of Na. OH (solute) STEP 2 g of solution g of Na. OH STEP 3 Write equalities and conversion factors: 14. 0 g of Na. OH = 100 of Na. OH solution 14. 0 g Na. OH and 100 g Na. OH solution 14. 0 g Na. OH STEP 4 Set up problem to cancel the grams of solution: 75. 0 g Na. OH solution x 14. 0 g Na. OH = 10. 5 g of Na. OH 100 g Na. OH solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20

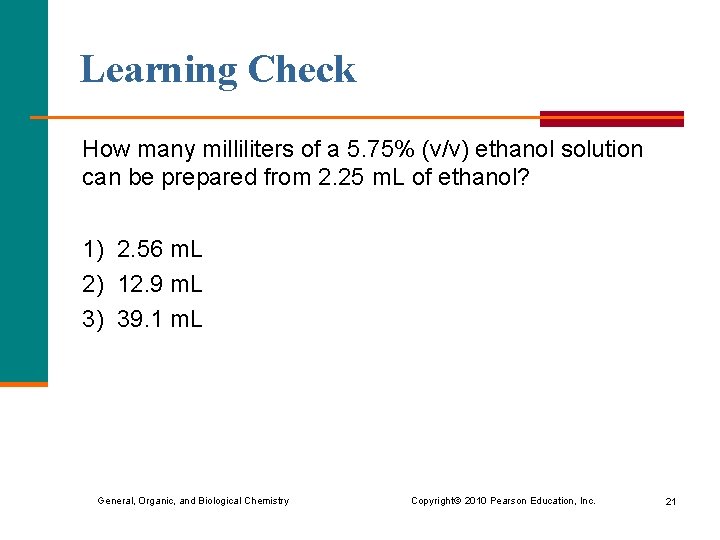
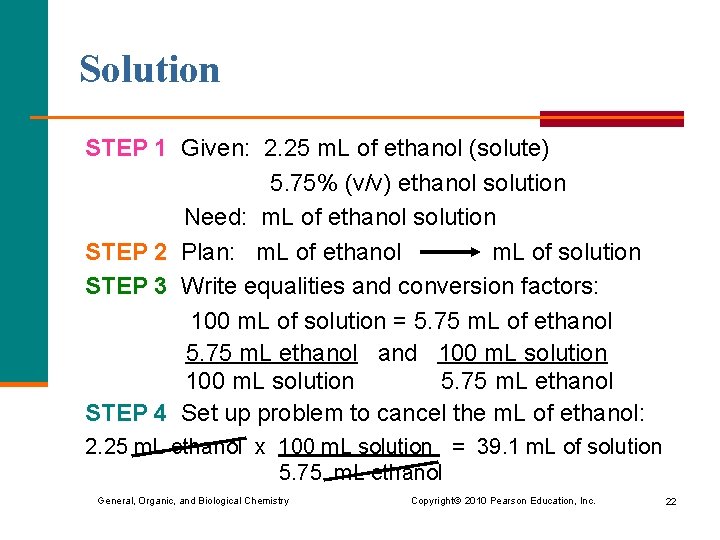
Learning Check How many milliliters of a 5. 75% (v/v) ethanol solution can be prepared from 2. 25 m. L of ethanol? 1) 2. 56 m. L 2) 12. 9 m. L 3) 39. 1 m. L General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 21

Solution STEP 1 Given: 2. 25 m. L of ethanol (solute) 5. 75% (v/v) ethanol solution Need: m. L of ethanol solution STEP 2 Plan: m. L of ethanol m. L of solution STEP 3 Write equalities and conversion factors: 100 m. L of solution = 5. 75 m. L of ethanol 5. 75 m. L ethanol and 100 m. L solution 5. 75 m. L ethanol STEP 4 Set up problem to cancel the m. L of ethanol: 2. 25 m. L ethanol x 100 m. L solution = 39. 1 m. L of solution 5. 75. m. L ethanol General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 22

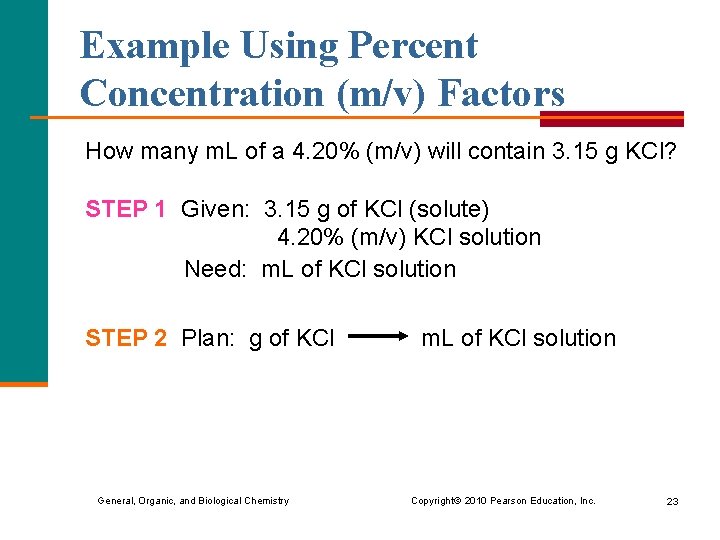
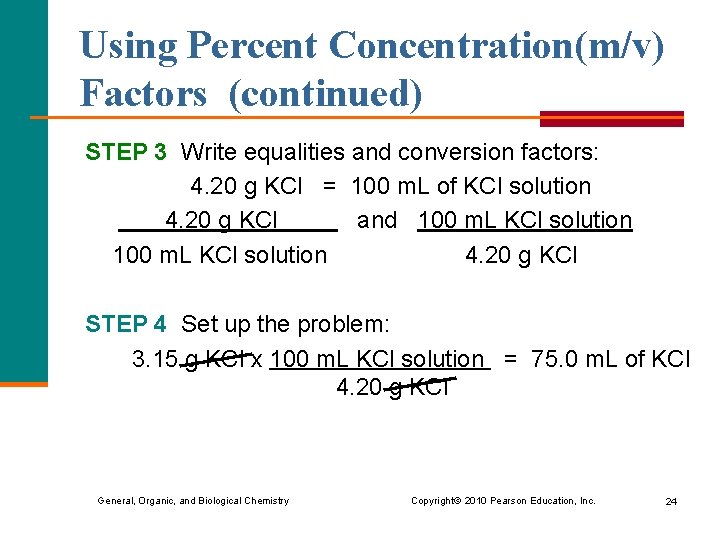
Example Using Percent Concentration (m/v) Factors How many m. L of a 4. 20% (m/v) will contain 3. 15 g KCl? STEP 1 Given: 3. 15 g of KCl (solute) 4. 20% (m/v) KCl solution Need: m. L of KCl solution STEP 2 Plan: g of KCl m. L of KCl solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 23

Using Percent Concentration(m/v) Factors (continued) STEP 3 Write equalities and conversion factors: 4. 20 g KCl = 100 m. L of KCl solution 4. 20 g KCl and 100 m. L KCl solution 4. 20 g KCl STEP 4 Set up the problem: 3. 15 g KCl x 100 m. L KCl solution = 75. 0 m. L of KCl 4. 20 g KCl General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 24

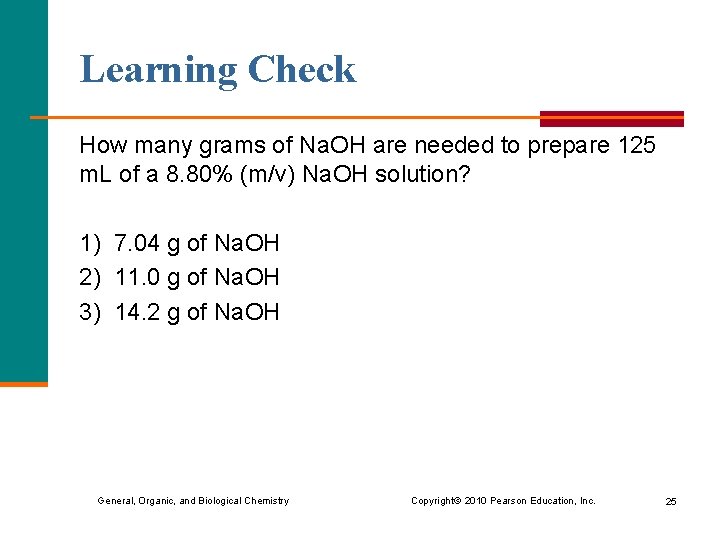
Learning Check How many grams of Na. OH are needed to prepare 125 m. L of a 8. 80% (m/v) Na. OH solution? 1) 7. 04 g of Na. OH 2) 11. 0 g of Na. OH 3) 14. 2 g of Na. OH General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 25

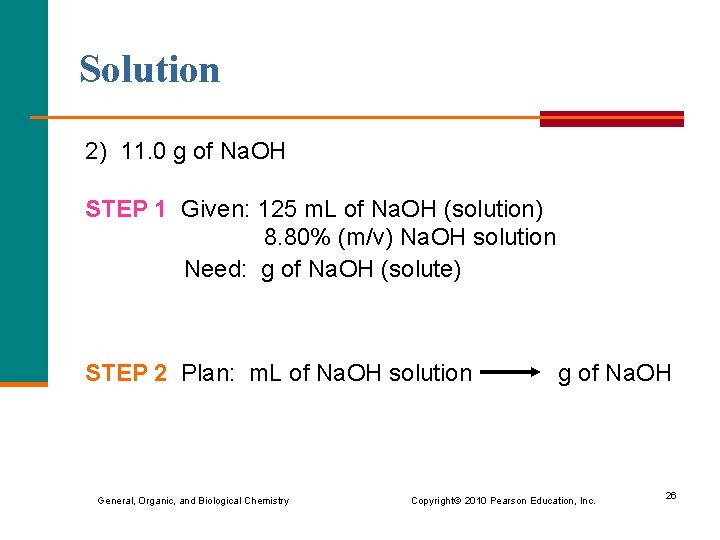
Solution 2) 11. 0 g of Na. OH STEP 1 Given: 125 m. L of Na. OH (solution) 8. 80% (m/v) Na. OH solution Need: g of Na. OH (solute) STEP 2 Plan: m. L of Na. OH solution g of Na. OH General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 26

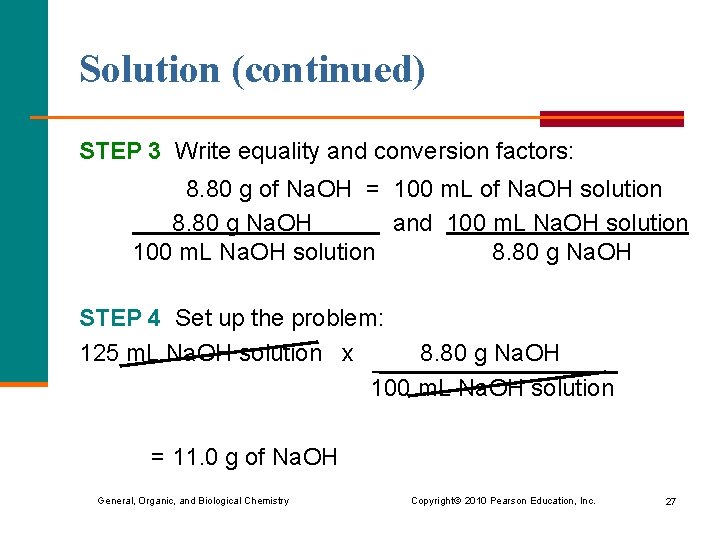
Solution (continued) STEP 3 Write equality and conversion factors: 8. 80 g of Na. OH = 100 m. L of Na. OH solution 8. 80 g Na. OH and 100 m. L Na. OH solution 8. 80 g Na. OH STEP 4 Set up the problem: 125 m. L Na. OH solution x 8. 80 g Na. OH 100 m. L Na. OH solution = 11. 0 g of Na. OH General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 27