Unit 7 Solution Chemistry Section 1 Molarity and








- Slides: 12

Unit 7: Solution Chemistry Section 1: Molarity and Molality

Solutions �Are a combination of at least two ingredients, such as water and salt �The substance in the larger quantity is the solvent �The substance in the smaller quantity is the solute �Are homogeneous mixtures �Don’t have to be liquids �Air is a solution composed of nitrogen (the solvent), oxygen, water vapor, carbon dioxide, and other gases �Brass is a solution composed of copper and zinc

Concentration �The higher the ratio of solute to solvent, the more concentrated the solution �Example: Kool-Aid can be as diluted or concentrated as you want it to be, just add more water to dilute the drink �The wrong concentration cause an object to work improperly or be harmful to living things �Example: Eye drops for humans can be harmful for a canine because of the different concentration requirements

Saturation �When the solute and solvent are put together, there is a limit as to how much solute can be dissolved �Saturated solution: no more solute will dissolve at a specific temperature �Unsaturated solution: more of the solute can be dissolved at the same temperature �Supersaturated solution: an unstable and temporary situation where the solution contains more dissolved solute than it normally would � Occurs when there is a change in temperature, volume, or pressure

Molarity (M) �The number of moles of solute per liter of solution �M = moles liter �Deals with concentration

Molarity Example 1 �What is the molarity of a solution made by dissolving 0. 3 mole of Ca(NO 3)2 in enough water to make 500 m. L of solution? M = moles liter = 0. 3 mol 0. 5 liter = 0. 6 M Ca(NO 3)2

Molarity Example 2 �How much 0. 5 M Na. Cl can be made from 0. 1 mole of Na. Cl? M = moles liter 0. 5 M = 0. 1 mole x liters 0. 5 x = 0. 1 x = 0. 2 L Na. Cl

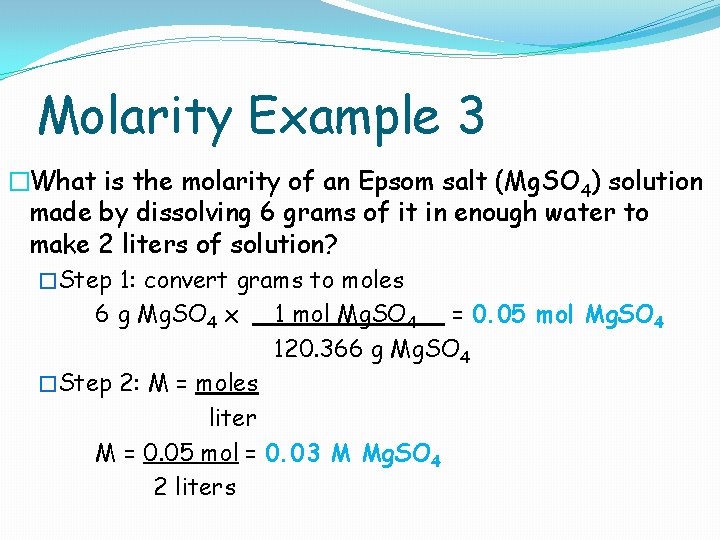
Molarity Example 3 �What is the molarity of an Epsom salt (Mg. SO 4) solution made by dissolving 6 grams of it in enough water to make 2 liters of solution? �Step 1: convert grams to moles 6 g Mg. SO 4 x 1 mol Mg. SO 4 = 0. 05 mol Mg. SO 4 120. 366 g Mg. SO 4 �Step 2: M = moles liter M = 0. 05 mol = 0. 03 M Mg. SO 4 2 liters

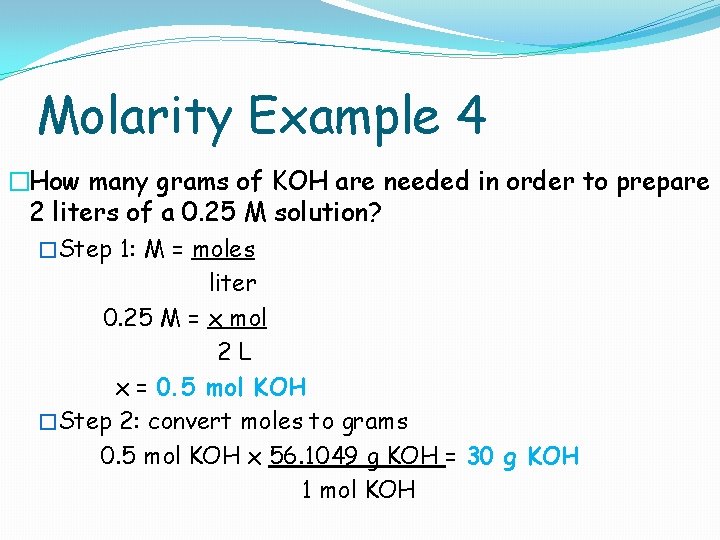
Molarity Example 4 �How many grams of KOH are needed in order to prepare 2 liters of a 0. 25 M solution? �Step 1: M = moles liter 0. 25 M = x mol 2 L x = 0. 5 mol KOH �Step 2: convert moles to grams 0. 5 mol KOH x 56. 1049 g KOH = 30 g KOH 1 mol KOH

Molality (m) �The number of moles of solute per kilogram of solvent �m = moles of solute kilogram of solvent �Also deals with concentration, but used less frequently

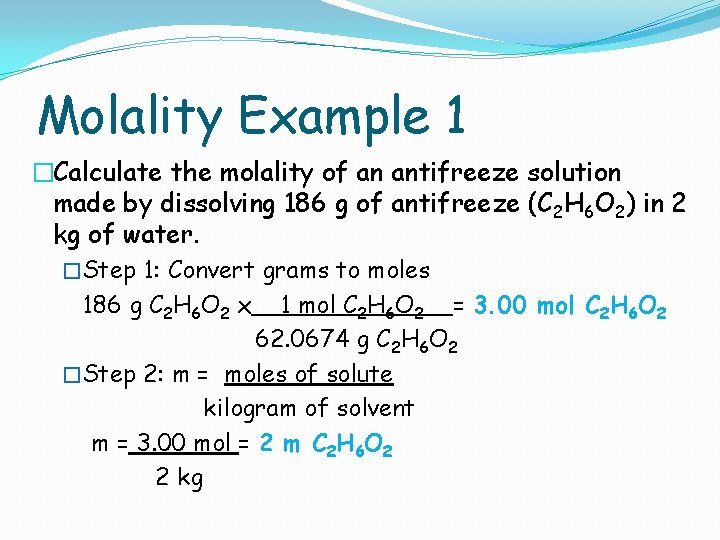
Molality Example 1 �Calculate the molality of an antifreeze solution made by dissolving 186 g of antifreeze (C 2 H 6 O 2) in 2 kg of water. �Step 1: Convert grams to moles 186 g C 2 H 6 O 2 x 1 mol C 2 H 6 O 2 = 3. 00 mol C 2 H 6 O 2 62. 0674 g C 2 H 6 O 2 �Step 2: m = moles of solute kilogram of solvent m = 3. 00 mol = 2 m C 2 H 6 O 2 2 kg

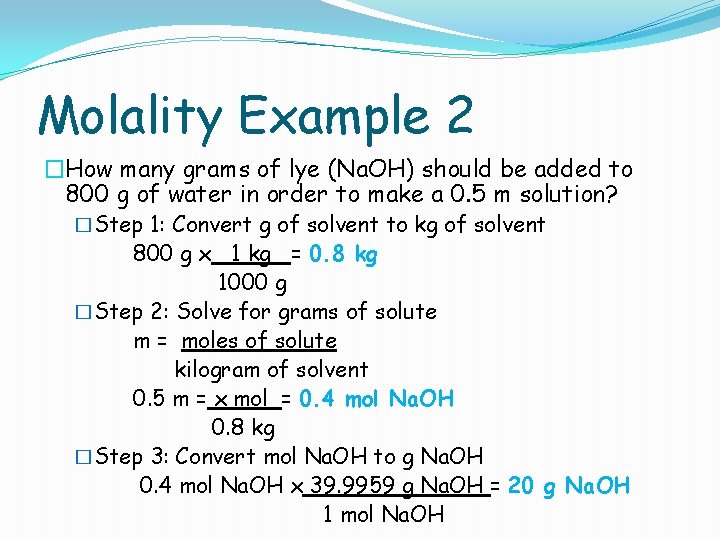
Molality Example 2 �How many grams of lye (Na. OH) should be added to 800 g of water in order to make a 0. 5 m solution? �Step 1: Convert g of solvent to kg of solvent 800 g x 1 kg = 0. 8 kg 1000 g �Step 2: Solve for grams of solute m = moles of solute kilogram of solvent 0. 5 m = x mol = 0. 4 mol Na. OH 0. 8 kg �Step 3: Convert mol Na. OH to g Na. OH 0. 4 mol Na. OH x 39. 9959 g Na. OH = 20 g Na. OH 1 mol Na. OH