Aqueous Reactions and Solution Stoichiometry CDO High School










- Slides: 21

Aqueous Reactions and Solution Stoichiometry CDO High School Aqueous Reactions

Solute and solvent § Solute: the substance that is dissolved in another substance § Solvent: the substance that something is dissolved into it Aqueous Reactions

Concentrated vs Dilute § Concentration: refers to how much solute is dissolved in the solvent § Concentrated: solutions with a large amount of solute dissolved in solvent § Dilute: solutions with a small amount of solute dissolved the solvent Aqueous Reactions

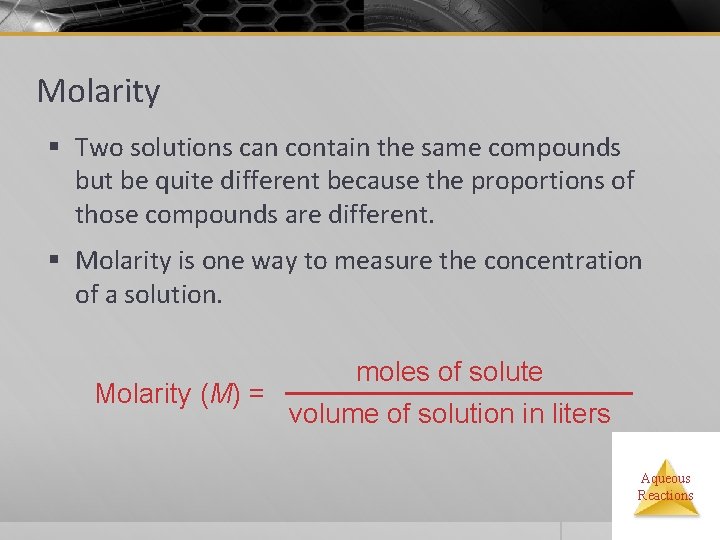
Molarity § Two solutions can contain the same compounds but be quite different because the proportions of those compounds are different. § Molarity is one way to measure the concentration of a solution. moles of solute Molarity (M) = volume of solution in liters Aqueous Reactions

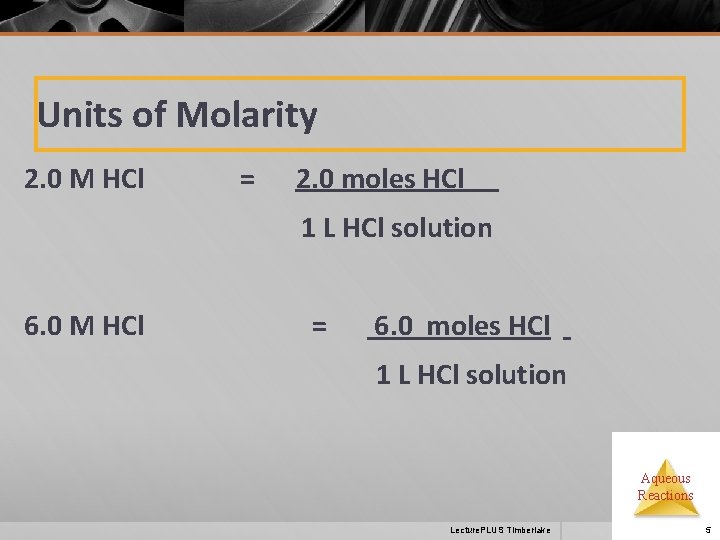
Units of Molarity 2. 0 M HCl = 2. 0 moles HCl 1 L HCl solution 6. 0 M HCl = 6. 0 moles HCl 1 L HCl solution Aqueous Reactions Lecture. PLUS Timberlake 5

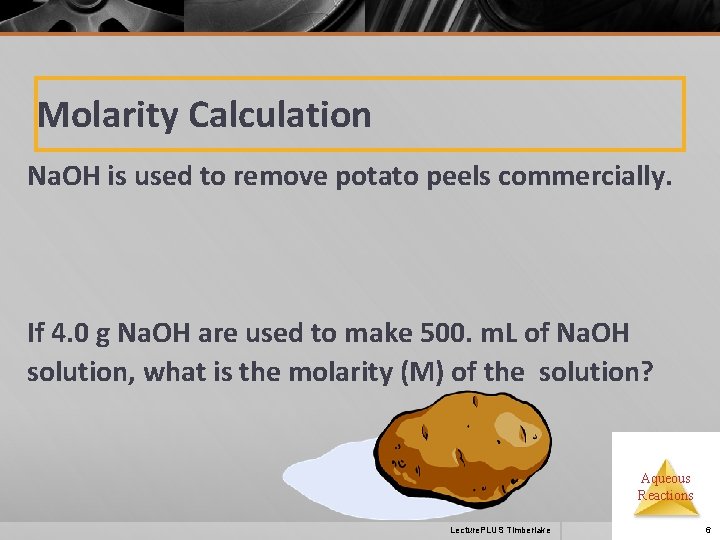
Molarity Calculation Na. OH is used to remove potato peels commercially. If 4. 0 g Na. OH are used to make 500. m. L of Na. OH solution, what is the molarity (M) of the solution? Aqueous Reactions Lecture. PLUS Timberlake 6

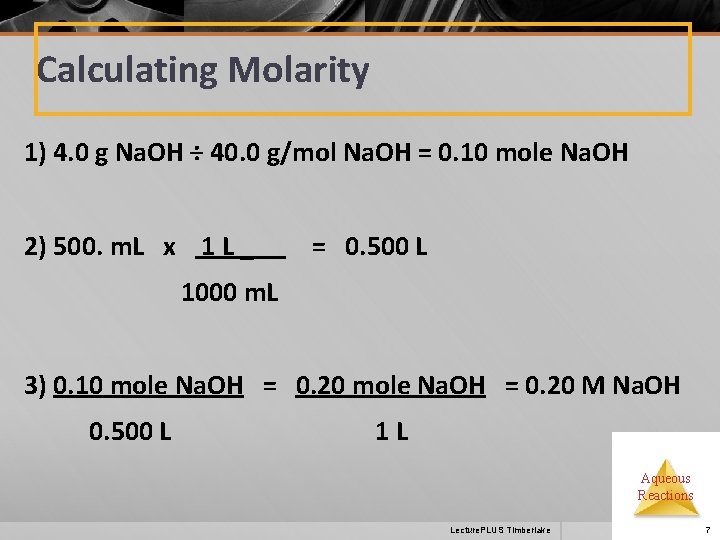
Calculating Molarity 1) 4. 0 g Na. OH ÷ 40. 0 g/mol Na. OH = 0. 10 mole Na. OH 2) 500. m. L x 1 L _ = 0. 500 L 1000 m. L 3) 0. 10 mole Na. OH = 0. 20 M Na. OH 0. 500 L 1 L Aqueous Reactions Lecture. PLUS Timberlake 7

Learning Check M 1 Dr an o A KOH solution with a volume of 400 m. L contains 2 mole KOH. What is the molarity of the solution? Aqueous Reactions Lecture. PLUS Timberlake 8

Learning Check M 2 A glucose solution with a volume of 2. 0 L contains 72 g glucose (C 6 H 12 O 6). If glucose has a molar mass of 180. g/mole, what is the molarity of the glucose solution? Aqueous Reactions Lecture. PLUS Timberlake 9

Learning Check M 3 Stomach acid is a 0. 10 M HCl solution. How many moles of HCl are in 1500 m. L of stomach acid solution? Aqueous Reactions Lecture. PLUS Timberlake 10

Learning Check M 4 How many grams of KCl are present in 2. 5 L of 0. 50 M KCl? Aqueous Reactions Lecture. PLUS Timberlake 11

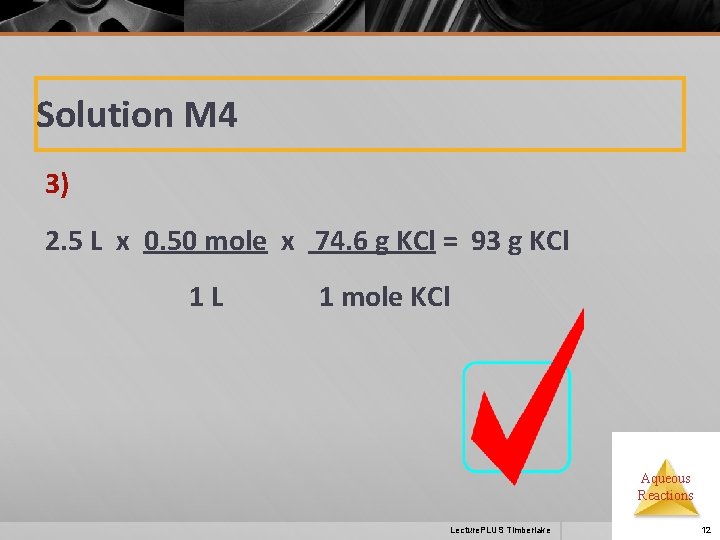
Solution M 4 3) 2. 5 L x 0. 50 mole x 74. 6 g KCl = 93 g KCl 1 L 1 mole KCl Aqueous Reactions Lecture. PLUS Timberlake 12

Learning Check M 5 How many milliliters of stomach acid, which is 0. 10 M HCl, contain 0. 15 mole HCl? Aqueous Reactions Lecture. PLUS Timberlake 13

Learning Check M 6 How many grams of Na. OH are required to prepare 400. m. L of 3. 0 M Na. OH solution? Aqueous Reactions Lecture. PLUS Timberlake 14

Solution Stoichiometry § The majority of work in research and industry involves solutions. Recall that solutions are easy to handle and are usually easier to control in reactions. § Solution stoichiometry – the procedure for calculating the molar concentration or volume of solution products or reactants Aqueous Reactions

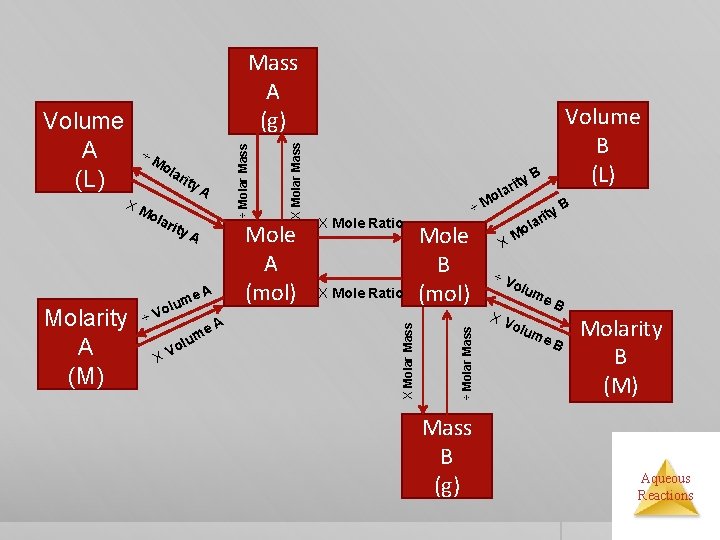
XM rit y. A ola Molarity A (M) rity A A me olu ÷V XV o e. A m lu Mole A (mol) X Mole Ratio ÷M Mole B (mol) Mass B (g) Volume B (L) B rity a l o ÷ Molar Mass ola X Molar Mass ÷M X Molar Mass Volume A (L) ÷ Molar Mass A (g) y. B it lar Mo X ÷V olu m XV e. B olu me B Molarity B (M) Aqueous Reactions

Example #1 § Ammonia and phosphoric acid solutions are used to produce ammonium hydrogen phosphate fertilizer. What volume of 14. 8 M NH 3(aq) is needed to react with 1000 L of 12. 9 M of H 3 PO 4(aq)? § 2 NH 3(aq) + 1000 L H 3 PO 4(aq) x 12. 9 mol 1 L (NH 4)2 HPO 4(aq) x 2 mol ÷ 14. 8 mol/L = 1740 L 1 mol Aqueous Reactions

Example #2 § In an experiment, . 01000 L sample of sulfuric acid solution reacts completely with 0. 0159 L of 0. 150 M potassium hydroxide. Calculate the concentration of the sulfuric acid. § H 2 SO 4(aq) + 2 KOH(aq) 0. 0159 L x 0. 150 mol x 1 L 1 mol 2 H 2 O(l) + K 2 SO 4(aq) x 1 = 0. 119 mol/L 0. 0100 L Aqueous Reactions

Example #3 § How many grams of silver chromate will form when 120 m. L of 0. 500 M silver nitrate are added to potassium chromate? 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq) Aqueous Reactions

Example #4 § If you mix 200 ml of 0. 100 M Pb(NO 3)2 and 300 ml of 0. 200 M Mg. Cl 2, how much Pb. Cl 2 precipitate will you form? Pb(NO 3)2(aq) + 2 Mg. Cl 2(aq) Pb. Cl 2(s) + Mg(NO 3)2(aq) Aqueous Reactions

Move to slide 7 of Molarity dilution ppt Aqueous Reactions