Chapter 4 Aqueous Reactions and Solution Stoichiometry Aqueous




















- Slides: 22

Chapter 4 Aqueous Reactions and Solution Stoichiometry

Aqueous Solutions in which water is the solvent Solution: homogeneous mixture of two or more substances in a single physical state Solvent: greater quantity; acts as the dissolving medium Solute: lesser quantity; is dissolved by solvent

Electrolytes A substance that conducts an electrical current when it is dissolved; contributes ions to the solution Soluble salts, acids and bases are electrolytes Nonelectrolyte: will not form ions in solution; will not conduct an electrical current

When in water… Ionic: ions dissociate and disperse thru sol’n Each ion is surrounded by water molecules Forms a stable complex and prevents recombination Polyatomic ions remain in groups

When in water… Molecular: bonding is strong enough to prevent dissociation with water molecules The entire molecular compound is surrounded by water molecules Become uniformly distributed thru water No ions formed = nonelectrolyte Acids, though covalent in nature, will ionize in aqueous solution

Strong & Weak Electrolytes Strong: produce many ions in sol’n; conducts current strongly Weak: produce few ions in sol’n; will conduct, but weakly Non: produces no ions in sol’n; will not conduct Weak electrolytes will establish an equilibrium between ions & molecule

Precipitation Rxns One insoluble product formed from aqueous reactants Precipitate: insoluble solid formed as the result of a rxn in sol’n Solubility: amount of a substance that may be dissolved in a given amount of solvent Solubility <0. 01 mol/L is insoluble Insoluble = attraction is too great to be broken by attraction for water molecules

Solubility Guidelines (Memorize These!!) Soluble Ions: Nitrate Acetate Halides Sulfate Hydrogen carbonate Ammonium Chlorate Perchlorate Group 1 ions Exceptions: None Ag, Hg, Pb Ag, Ca, Sr, Ba, Pb None None

Solubility Guidelines (Memorize These!!) Insoluble Ions: Carbonate Chromate Phosphate Sulfide Hydroxide Exceptions: Group 1 or ammonium Group 1, NH 4+, Ca, Mg Group 1 or ammonium Group 1, ammonium, Ca, Ba, Sr

Writing Ionic Equations Complete ionic equations show all electrolytes in their aqueous ionic form Spectator ions are those ions which are unchanged over the course of the reaction Net ionic equations remove spectator ions The AP exam will require you (as will I) to write all reactants and products in their ionic form!

Acids ionize (separate to form soluble ions) in aqueous solution All acids contribute hydrogen as the only positive ion in solution Monoprotic: donates 1 H+ Diprotic: donates 2 H+ Triprotic: donates 3 H+ Polyprotic acids donate in steps

Bases are substances that act to reduce the H+ ion concentration in a solution Hydroxide ions are the prototypical base Other compounds may also be classified as bases, including NH 3

Strength of Acids & Bases Determined by electrolyte strength Strong acid = strong electrolyte Memorize: HCl, HNO 3, HBr, HI, HCl. O 4, HCl. O 3, H 2 SO 4 Weak acid = weak electrolyte Strong Base = strong electrolyte Memorize: group 1 metal hydroxides and heavy group 2 metal hydroxides Weak base = weak electrolyte

Neutralization Reactions An acid and a base will react to produce water and a dissolved salt Net ionic reaction: H+ + OH- H 2 O

Oxidation-Reduction Rxns Transfer of electrons between reacting substances Oxidation: loss of electrons, increase in ox# Reduction: gain of electrons, decrease in ox# Ox Agent: gets reduced Red Agent: gets oxidized LEO GER-OAR

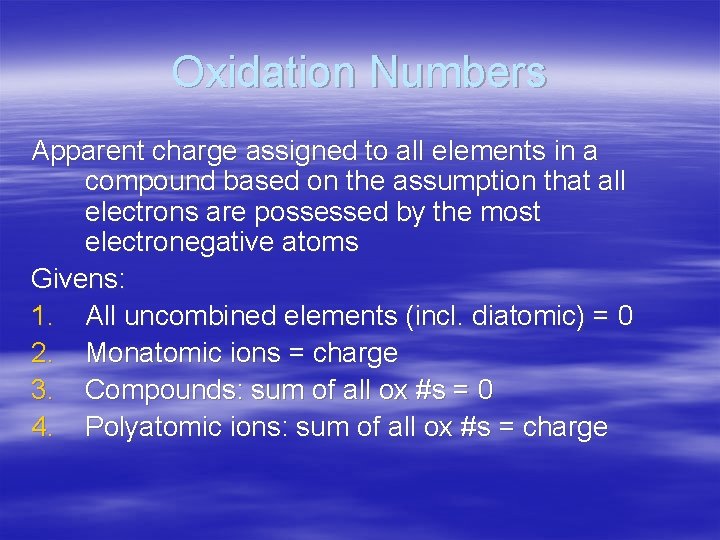
Oxidation Numbers Apparent charge assigned to all elements in a compound based on the assumption that all electrons are possessed by the most electronegative atoms Givens: 1. All uncombined elements (incl. diatomic) = 0 2. Monatomic ions = charge 3. Compounds: sum of all ox #s = 0 4. Polyatomic ions: sum of all ox #s = charge

Assigning Oxidation Numbers 1. 2. 3. 4. 5. 6. 7. Group 1 metals = +1 Group 2 = +2 Hydrogen = +1 (usually) Oxygen = -2 (usually) Halides = -1 (last resort) Constants: Al = +3 F = -1

Activity Series A list of half reactions ordered to show the ease of oxidation or reduction of a substance All metals above hydrogen will be oxidized by acid Group 1 and 2 metals will be oxidized by water

Concentration of Solutions Molarity: moles of solute per liter of solution Molality: moles of solute per kg of solvent Mole Fraction: moles component per total moles of solution Normality: equivalence per liter of solution Percent composition (pph): mass of component per total solution mass x 100 Parts per million: mass of component per total solution mass x 1, 000

Dilutions Proportionally weakening the concentration of a solution through the addition of solvent M 1 V 1 = M 2 V 2 Remember, electrolytes will increase the # moles in a solution Aliquot: a small sample of solution, usually used to form a more dilute solution

Solution Stoich We can interconvert between moles, mass, volume of solution, or concentration of solution depending upon our data How many moles of water form when 25. 0 m. L of 0. 100 M nitric acid is completely neutralized by sodium hydroxide?

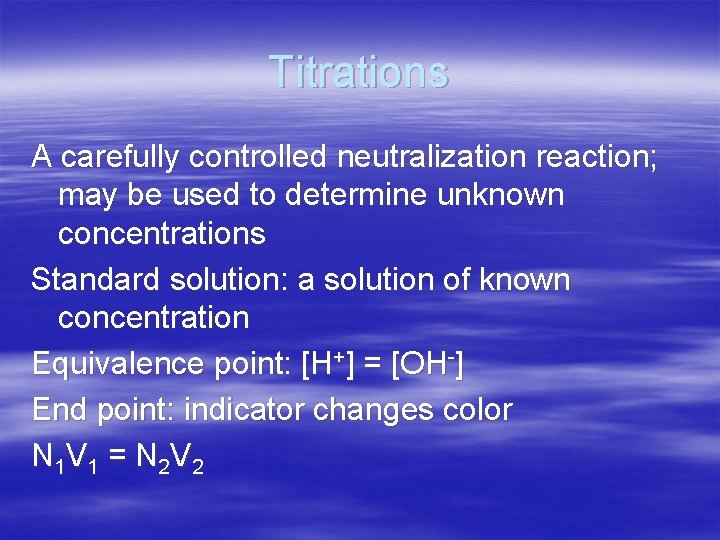
Titrations A carefully controlled neutralization reaction; may be used to determine unknown concentrations Standard solution: a solution of known concentration Equivalence point: [H+] = [OH-] End point: indicator changes color N 1 V 1 = N 2 V 2