Aqueous Reactions Dr Ron Rusay Aqueous Reactions There


































- Slides: 51

Aqueous Reactions Dr. Ron Rusay

Aqueous Reactions There are several general types: 1) Precipitation: An insoluble salt forms from the addition of solutions. (Refer to Solubility Rules) 2) Acid-Base Reactions (Neutralization) generally produces a salt plus water 3) Oxidation-Reduction (Redox) there is a change in oxidation numbers between reactants and products

Solution Test Apparatus for Electrolytes

Conductivity

Electrolytes Aqueous solutions can be categorized into 3 types: non-electrolytes, strong electrolytes or weak electrolytes based on their ability to conduct electricity. A solution must have ions to conduct. Pure Water does not conduct. Aqueous solutions can be tested for conductivity which will determine the degree of ionization of the solutes. It is possible to have full or partial ionization.

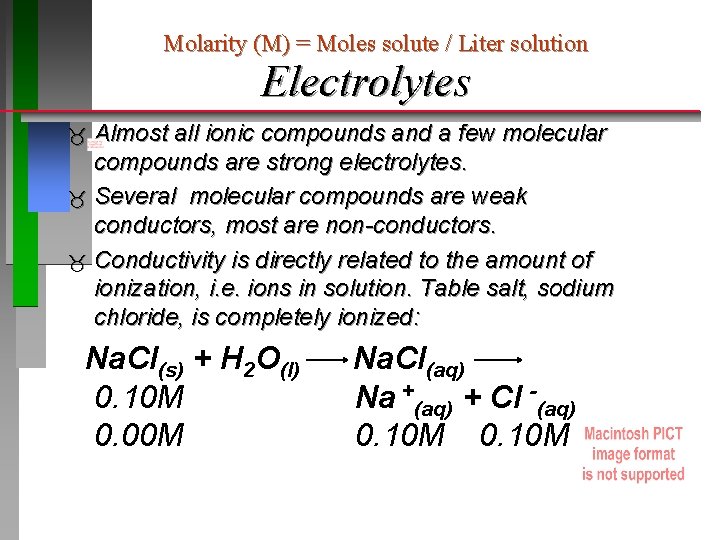
Molarity (M) = Moles solute / Liter solution Electrolytes Almost all ionic compounds and a few molecular compounds are strong electrolytes. Several molecular compounds are weak conductors, most are non-conductors. Conductivity is directly related to the amount of ionization, i. e. ions in solution. Table salt, sodium chloride, is completely ionized: Na. Cl(s) + H 2 O(l) 0. 10 M 0. 00 M Na. Cl(aq) Na +(aq) + Cl -(aq) 0. 10 M

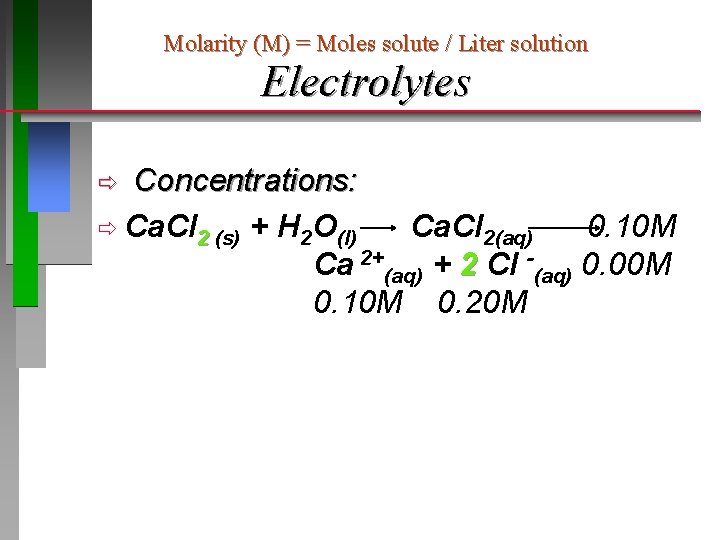
Molarity (M) = Moles solute / Liter solution Electrolytes Concentrations: ð Ca. Cl 2 (s) + H 2 O(l) Ca. Cl 2(aq) 0. 10 M Ca 2+(aq) + 2 Cl -(aq) 0. 00 M 0. 10 M 0. 20 M ð

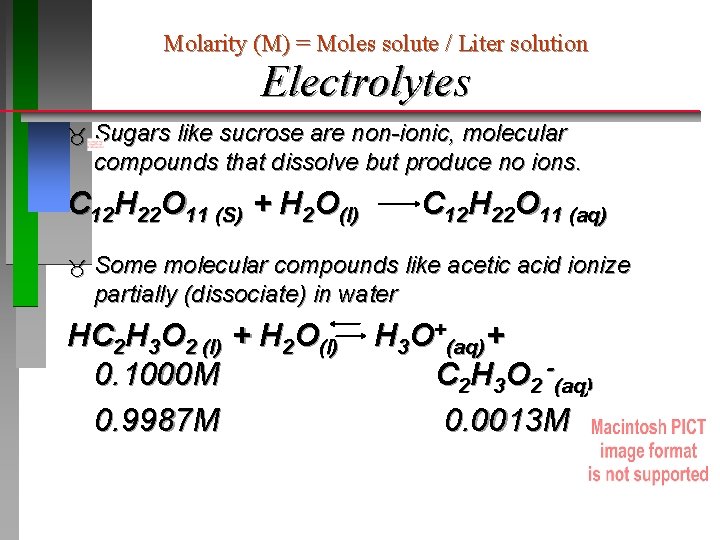
Molarity (M) = Moles solute / Liter solution Electrolytes Sugars like sucrose are non-ionic, molecular compounds that dissolve but produce no ions. C 12 H 22 O 11 (S) + H 2 O(l) C 12 H 22 O 11 (aq) Some molecular compounds like acetic acid ionize partially (dissociate) in water HC 2 H 3 O 2 (l) + H 2 O(l) 0. 1000 M 0. 9987 M H 3 O+(aq)+ C 2 H 3 O 2 -(aq) 0. 0013 M

Aqueous Acids ð Any compound that provides a proton can be considered an acid. Strong acids are sulfuric acid, nitric acid, perchloric acid, HI, HBr and HCl.

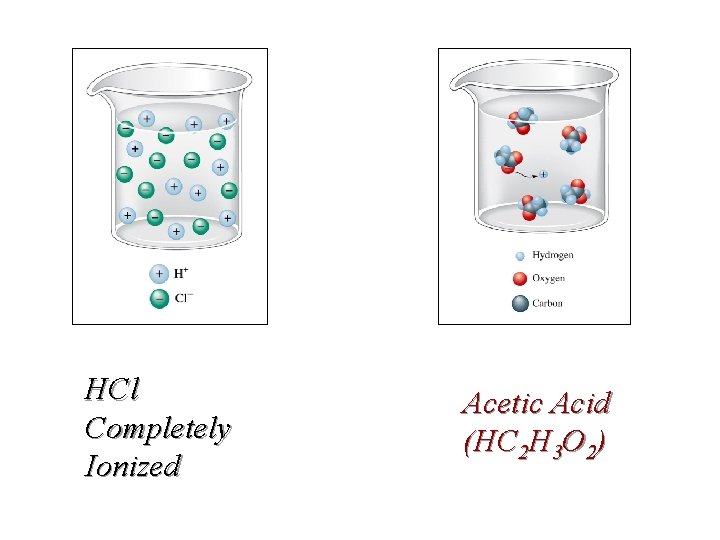
Electrolytes ð How would the conductivity of acetic acid compare to hydrochloric acid?

HCl Completely Ionized Acetic Acid (HC 2 H 3 O 2)

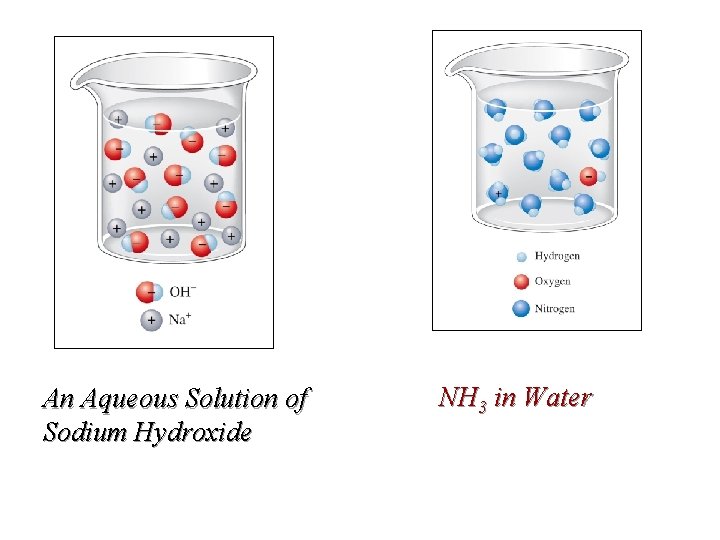
Aqueous Bases Any compound that accepts a proton is a base. The common bases are group IA & IIA metal hydroxide compounds. They are strong bases, dissociating completely in water. An example of a weak base is ammonia. NH 3 (g) + H 2 O(l) NH 3 (aq) NH 4+(aq)+ OH-(aq) Consider that aqueous ammonia is in equilibrium with ammonium hydroxide. The names have often been used interchangeably.

An Aqueous Solution of Sodium Hydroxide NH 3 in Water


QUESTION

ANSWER

Aqueous Reactions: Neutralization

Aqueous Reactions: Neutralization Net Ionic Equations HCl(aq) + Na. OH (aq) __________________________ Na. Cl (aq) + H 2 O(l) HCl(aq) H+(aq) + Cl -(aq) ð Na. OH (aq) Na+(aq)+ OH-(aq) ð Na. Cl (aq) Na+(aq)+ Cl-(aq) ð ________________________ Na+(aq)+ OH-(aq) + H+(aq) + Cl -(aq) Na+(aq)+ Cl-(aq) + H 2 O(l) ____________________________ H+(aq) + OH -(aq) H 2 O(l)

QUESTION An aqueous solution of H 2 SO 4 is added to aqueous Ba(OH)2. The reaction is monitored using a conductivity tester. Predict the correct statement(s). I) Both H 2 SO 4 and Ba(OH)2 are strong electrolytes. II) This is a neutralization reaction. III) This is a precipitation reaction. IV) The light bulb will glow at the neutralization point. A) II B) I and II C) I, II and III D) I, III and IV

ANSWER An aqueous solution of H 2 SO 4 is added to aqueous Ba(OH)2. The reaction is monitored using a conductivity tester. Predict the correct statement(s). I) Both H 2 SO 4 and Ba(OH)2 are strong electrolytes. II) This is a neutralization reaction. III) This is a precipitation reaction. IV) The light bulb will glow at the neutralization point. A) II B) I and II C) I, II and III D) I, III and IV

Aqueous Reactions: Acid-Base

QUESTION If an antacid contains Al(OH)3 it will form Al. Cl 3 upon neutralization of stomach acid. How many moles of Cl– ions are in 100. 0 m. L of 0. 010 M Al. Cl 3? A. 0. 0010 M B. 0. 010 M C. 0. 0030 M D. 0. 030 M Molarity (M) = Moles solute / Liter solution

ANSWER If an antacid contains Al(OH)3 it will form Al. Cl 3 upon neutralization of stomach acid. How many moles of Cl– ions are in 100. 0 m. L of 0. 010 M Al. Cl 3? A. 0. 0010 M B. 0. 010 M C. 0. 0030 M D. 0. 030 M Al. Cl 3 dissociates into 3 moles of Cl–. Molarity (M) = Moles solute / Liter solution

QUESTION

ANSWER

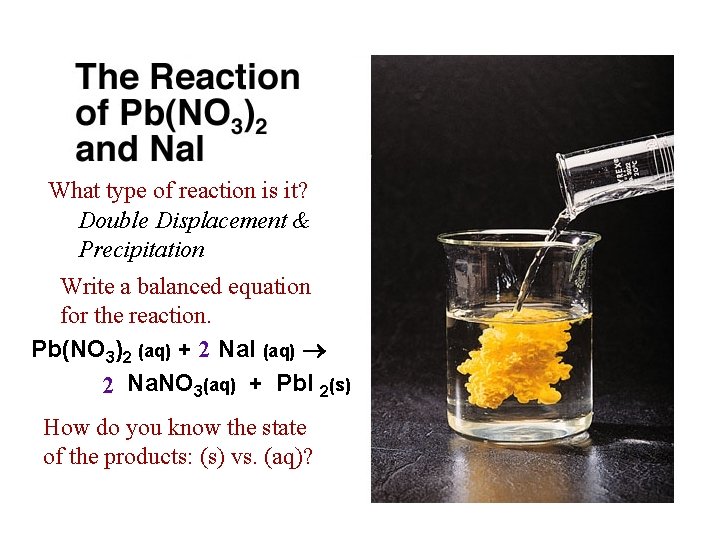
What type of reaction is it? Double Displacement & Precipitation Write a balanced equation for the reaction. Pb(NO 3)2 (aq) + 2 Na. I (aq) 2 Na. NO 3(aq) + Pb. I 2(s) How do you know the state of the products: (s) vs. (aq)?


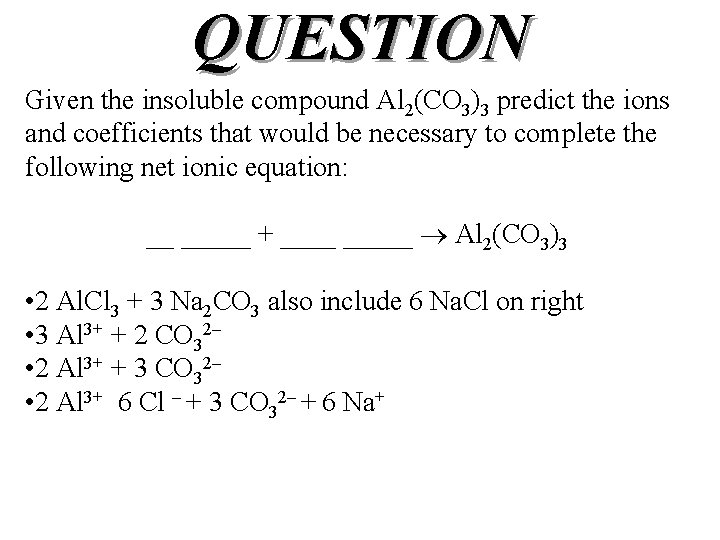
QUESTION Given the insoluble compound Al 2(CO 3)3 predict the ions and coefficients that would be necessary to complete the following net ionic equation: __ _____ + _____ Al 2(CO 3)3 • 2 Al. Cl 3 + 3 Na 2 CO 3 also include 6 Na. Cl on right • 3 Al 3+ + 2 CO 32– • 2 Al 3+ + 3 CO 32– • 2 Al 3+ 6 Cl – + 3 CO 32– + 6 Na+

ANSWER Given the insoluble compound Al 2(CO 3)3 predict the ions and coefficients that would be necessary to complete the following net ionic equation: __ _____ + _____ Al 2(CO 3)3 • 2 Al. Cl 3 + 3 Na 2 CO 3 also include 6 Na. Cl on right • 3 Al 3+ + 2 CO 32– • 2 Al 3+ + 3 CO 32– • 2 Al 3+ 6 Cl – + 3 CO 32– + 6 Na+

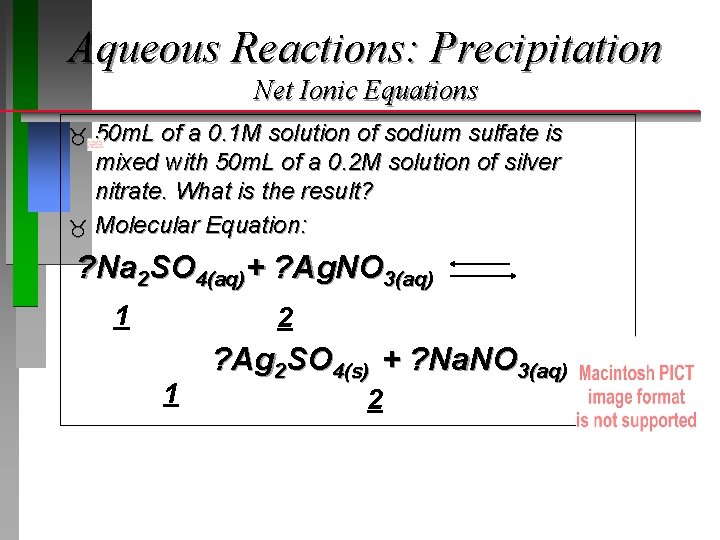
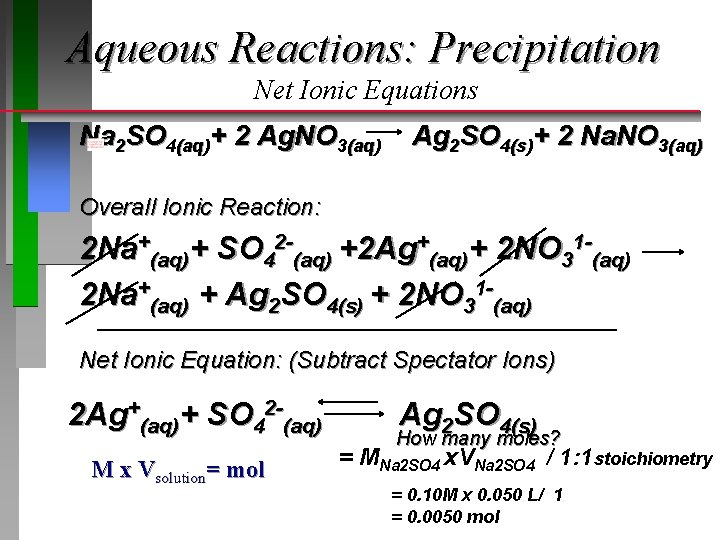
Aqueous Reactions: Precipitation Net Ionic Equations 50 m. L of a 0. 1 M solution of sodium sulfate is mixed with 50 m. L of a 0. 2 M solution of silver nitrate. What is the result? Molecular Equation: ? Na 2 SO 4(aq)+ ? Ag. NO 3(aq) 1 2 1 ? Ag 2 SO 4(s) + ? Na. NO 3(aq) 2

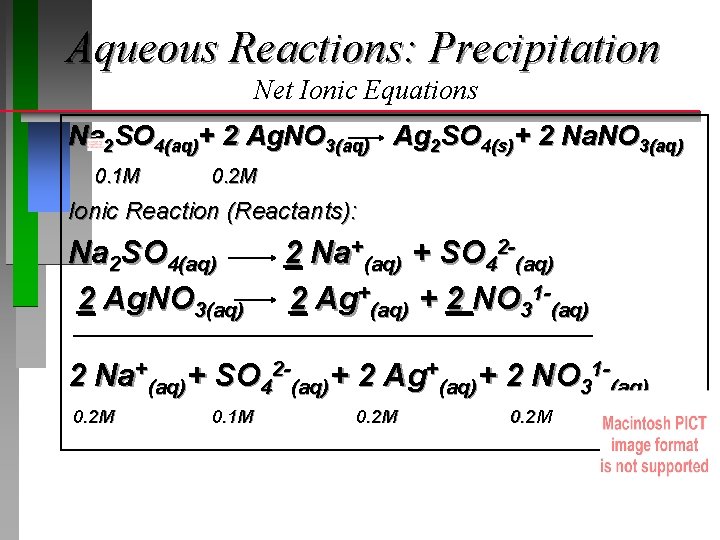
Aqueous Reactions: Precipitation Net Ionic Equations Na 2 SO 4(aq)+ 2 Ag. NO 3(aq) Ag 2 SO 4(s)+ 2 Na. NO 3(aq) 0. 1 M 0. 2 M Ionic Reaction (Reactants): Na 2 SO 4(aq) 2 Ag. NO 3(aq) 2 Na+(aq) + SO 42 -(aq) 2 Ag+(aq) + 2 NO 31 -(aq) 2 Na+(aq)+ SO 42 -(aq)+ 2 Ag+(aq)+ 2 NO 31 -(aq) 0. 2 M 0. 1 M 0. 2 M

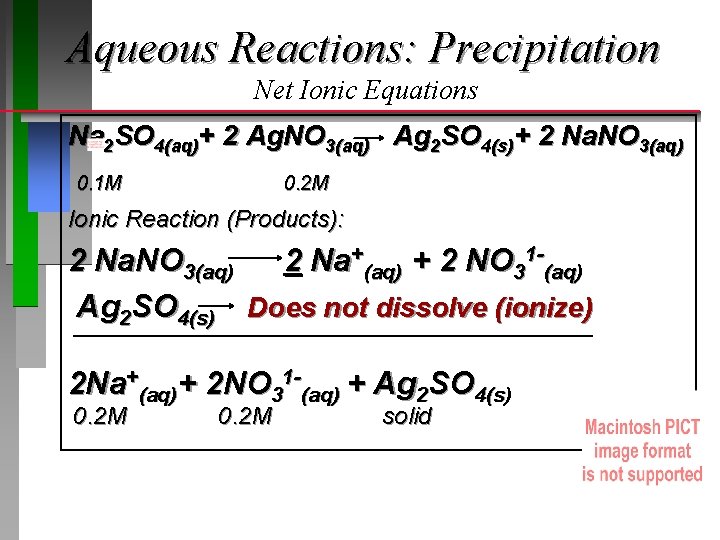
Aqueous Reactions: Precipitation Net Ionic Equations Na 2 SO 4(aq)+ 2 Ag. NO 3(aq) Ag 2 SO 4(s)+ 2 Na. NO 3(aq) 0. 1 M 0. 2 M Ionic Reaction (Products): 2 Na. NO 3(aq) 2 Na+(aq) + 2 NO 31 -(aq) Ag 2 SO 4(s) Does not dissolve (ionize) 2 Na+(aq)+ 2 NO 31 -(aq) + Ag 2 SO 4(s) 0. 2 M solid

Aqueous Reactions: Precipitation Net Ionic Equations Na 2 SO 4(aq)+ 2 Ag. NO 3(aq) Ag 2 SO 4(s)+ 2 Na. NO 3(aq) Overall Ionic Reaction: 2 Na+(aq)+ SO 42 -(aq) +2 Ag+(aq)+ 2 NO 31 -(aq) 2 Na+(aq) + Ag 2 SO 4(s) + 2 NO 31 -(aq) Net Ionic Equation: (Subtract Spectator Ions) 2 Ag+(aq)+ SO 42 -(aq) M x Vsolution= mol Ag 2 SO 4(s) How many moles? = MNa 2 SO 4 x. VNa 2 SO 4 / 1: 1 stoichiometry = 0. 10 M x 0. 050 L/ 1 = 0. 0050 mol

QUESTION

ANSWER –

Pb(NO 3)2 (aq) + 2 Na. I (aq) 2 Na. NO 3(aq) + Pb. I 2(s) Write a balanced Net Ionic equation for the reaction. Pb 2+ (aq) + 2 I 1 - (aq) Pb. I 2(s) What are the spectator ions in the reaction? 2 Na 1+ (aq); 2 NO 31 - (aq)

QUESTION

ANSWER E) All of these are soluble in water. According to the solubility rules for ionic compounds, compounds containing Group IA ions or nitrate ions will always be soluble. Compounds containing halides are generally soluble, aside from silver, lead and mercury(I) halides.

QUESTION If you began a reaction with the following ions in solution (all would be written with an (aq) subscript how would you represent the proper final net ionic equation? (Consult a solubility Table. ) 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– • • 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– No Reaction 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– Fe 3(PO 4)2 (s)+ 6 Na. NO 3 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– Fe 3(PO 4)2 (s)+ 6 Na+ + 6 NO 3– 2 PO 43– + 3 Fe 2+ Fe 3(PO 4)2 (s)

ANSWER If you began a reaction with the following ions in solution (all would be written with an (aq) subscript how would you represent the proper final net ionic equation? (Consult a solubility Table. ) 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– • • 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– No Reaction 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– Fe 3(PO 4)2 (s)+ 6 Na. NO 3 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– Fe 3(PO 4)2 (s)+ 6 Na+ + 6 NO 3– 2 PO 43– + 3 Fe 2+ Fe 3(PO 4)2 (s)

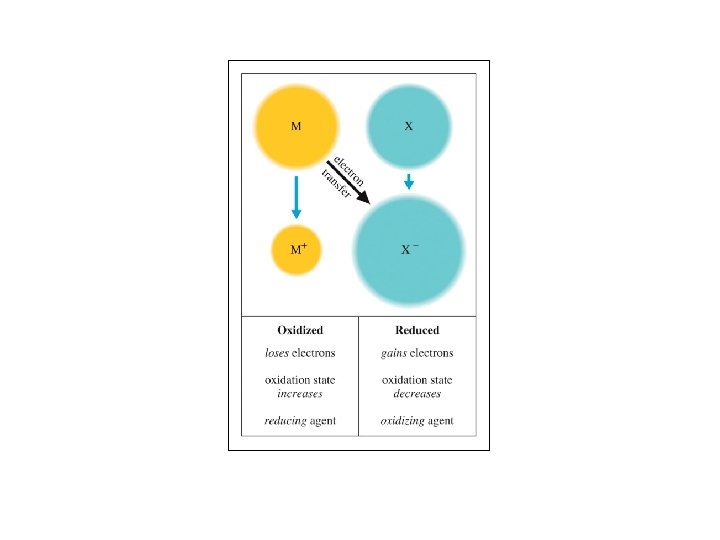
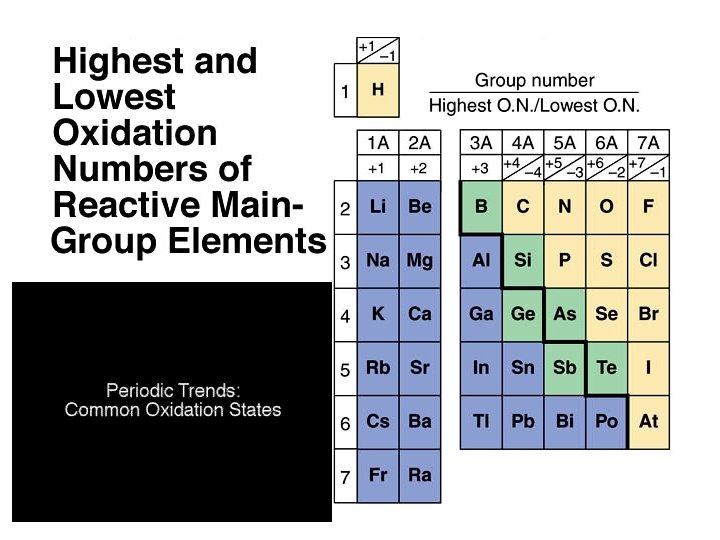
Oxidation-Reduction ð Oxidation is the loss of electrons. ð Reduction is the gain of electrons. ð The reactions occur together. One does not occur without the other. ð The terms are used relative to the change in the oxidation state or oxidation number of the reactant(s).

Aqueous Reactions: Oxidation - Reduction ð In the following reaction, identify what is being oxidized and what is being reduced. What is the total number of electrons involved in the process?


Oxidation Reduction Reactions

QUESTION In a redox reaction, oxidation and reduction must both occur. Which statement provides an accurate premise of redox chemistry? A. The substance that is oxidized must be the oxidizing agent. B. The substance that is oxidized must gain electrons. C. The substance that is oxidized must have a higher oxidation number afterwards. D. The substance that is oxidized must combine with oxygen.

ANSWER In a redox reaction, oxidation and reduction must both occur. Which statement provides an accurate premise of redox chemistry? A. The substance that is oxidized must be the oxidizing agent. B. The substance that is oxidized must gain electrons. C. The substance that is oxidized must have a higher oxidation number afterwards. D. The substance that is oxidized must combine with oxygen.



QUESTION

ANSWER B) NO 2 Oxygen almost always has an oxidation state of – 2 when part of a compound. The exception is when it is part of a peroxide. For example, hydrogen peroxide H 2 O 2. Then it has an oxidation state of – 1.

 Rusay
Rusay Types of reactions
Types of reactions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Reactions in aqueous solutions
Reactions in aqueous solutions Concentrated solution
Concentrated solution Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Aqueous solution practice problems
Aqueous solution practice problems Dilute solution
Dilute solution Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions 20 examples of redox reaction
20 examples of redox reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Ingilizce gramer zamanlar tablosu
Ingilizce gramer zamanlar tablosu Some or any bananas
Some or any bananas There's and there are
There's and there are A an some any
A an some any There is there are ejemplos
There is there are ejemplos Negetive sentences
Negetive sentences There is there are
There is there are If there is no struggle there is no progress explanation
If there is no struggle there is no progress explanation Pepper contable o incontable
Pepper contable o incontable There is there are negative form
There is there are negative form There is there are part of speech
There is there are part of speech There is there are
There is there are Eight dollars the price of a movie these days
Eight dollars the price of a movie these days There is and there
There is and there There is there are
There is there are Hay is
Hay is Demonstrativos
Demonstrativos Reactions of alkenes 1 chemsheets answers
Reactions of alkenes 1 chemsheets answers Do pickles conduct electricity
Do pickles conduct electricity Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Classify the non aqueous solvents
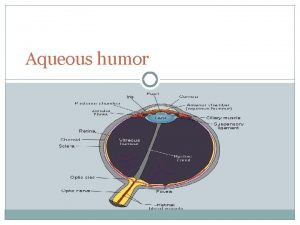
Classify the non aqueous solvents Aqueous humor
Aqueous humor General properties of aqueous solutions
General properties of aqueous solutions Classify non aqueous solvents
Classify non aqueous solvents Additional aspects of aqueous equilibria
Additional aspects of aqueous equilibria Clarity of aqueous extract test
Clarity of aqueous extract test Spiral organ of corti
Spiral organ of corti Non aqueous media
Non aqueous media Birdshot retinochoroidopathy
Birdshot retinochoroidopathy Aqueous equilibria
Aqueous equilibria Suspension vs solution
Suspension vs solution Additional aspects of aqueous equilibria
Additional aspects of aqueous equilibria Watery humour
Watery humour Modern chemistry chapter 13
Modern chemistry chapter 13 Polymorphs pour out to sink to bottom of anterior chamber
Polymorphs pour out to sink to bottom of anterior chamber Derive henderson equation
Derive henderson equation Mucostatic impression material examples
Mucostatic impression material examples Water and aqueous systems worksheet answers
Water and aqueous systems worksheet answers