Moles and Molarity Stoichiometry and Moles Stoichiometry and












- Slides: 51

Moles and Molarity

Stoichiometry and Moles

Stoichiometry and Moles

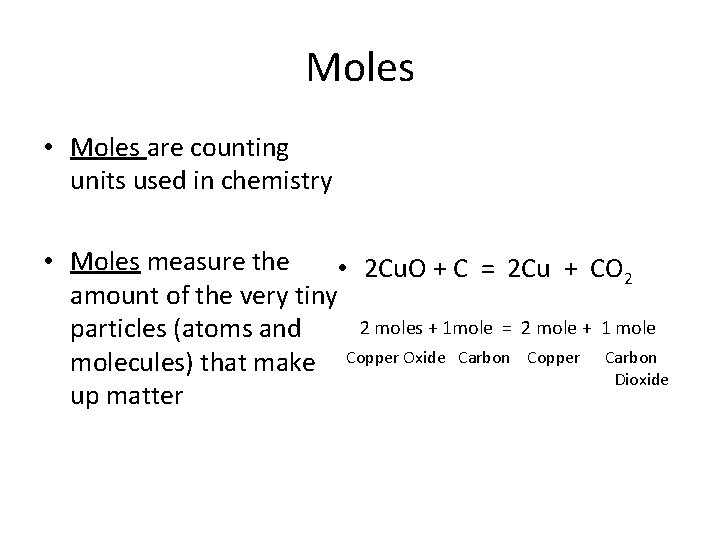
Moles • Moles are counting units used in chemistry • Moles measure the • 2 Cu. O + C = 2 Cu + CO 2 amount of the very tiny particles (atoms and 2 moles + 1 mole = 2 mole + 1 molecules) that make Copper Oxide Carbon Copper Carbon Dioxide up matter

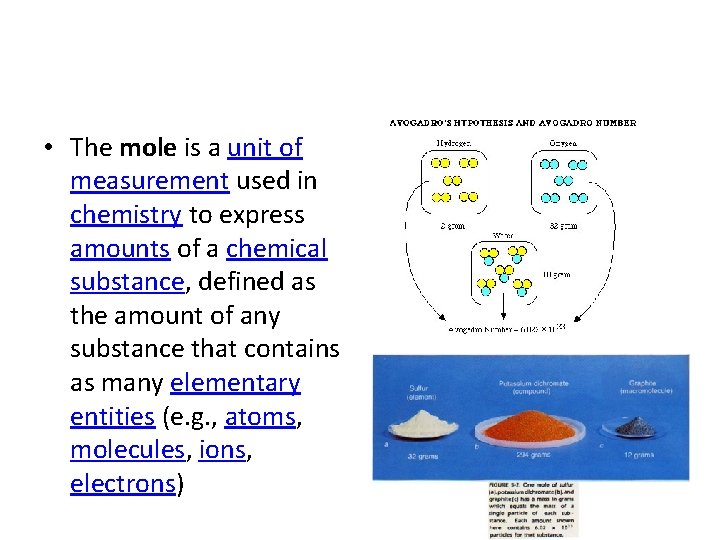
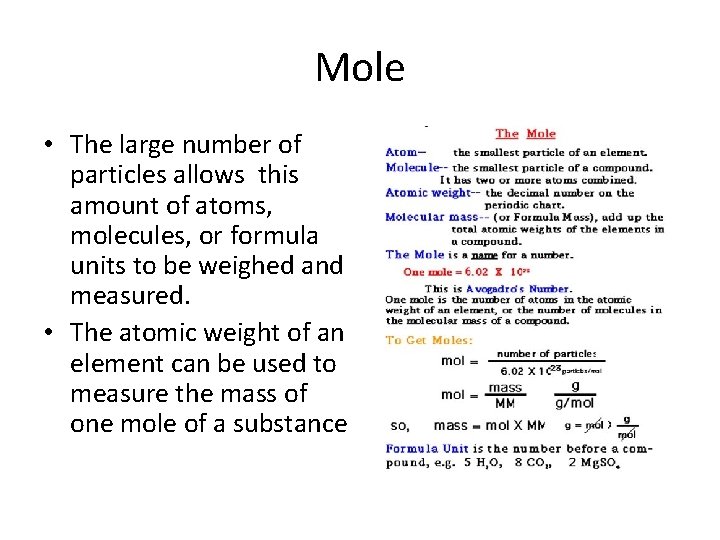
• The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as the amount of any substance that contains as many elementary entities (e. g. , atoms, molecules, ions, electrons)

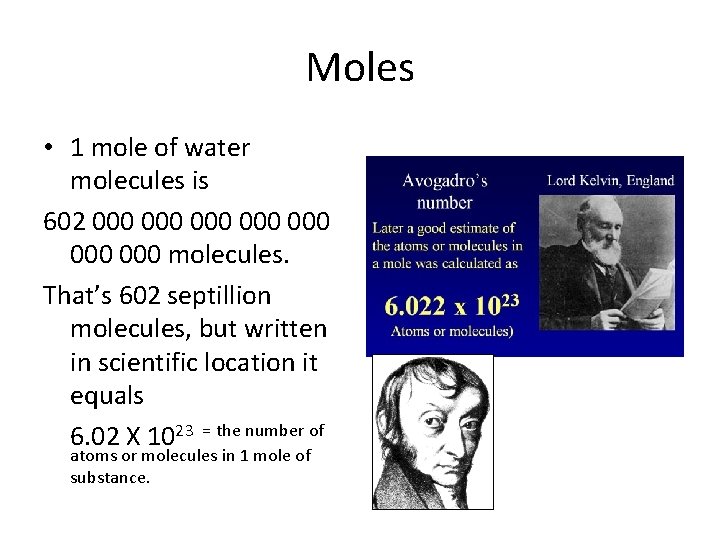
Moles • 1 mole of water molecules is 602 000 000 molecules. That’s 602 septillion molecules, but written in scientific notation it equals 6. 022 X 1023

Moles • 1 mole of water molecules is 602 000 000 molecules. That’s 602 septillion molecules, but written in scientific location it equals 6. 02 X 1023 = the number of atoms or molecules in 1 mole of substance.

Moles • 1 mole of water molecules is 602 000 000 molecules. That’s 602 septillion molecules, but written in scientific location it equals 6. 02 X 1023 = the number of atoms or molecules in 1 mole of substance. This amount is called: Avogadro’s number

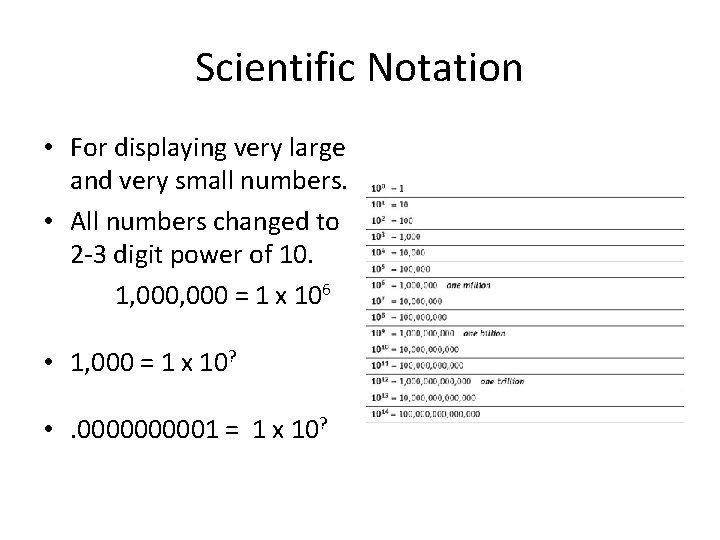
Scientific Notation • For displaying very large and very small numbers. • All numbers changed to 2 -3 digit power of 10. 1, 000 = 1 x 106 • 1, 000 = 1 x 10? • . 000001 = 1 x 10?

Scientific Notation • For displaying very large and very small numbers. • All numbers changed to 2 -3 digit power of 10. 1, 000 = 1 x 106 • 1, 000 = 1 x 103 • . 000001 = 1 x 10 -10

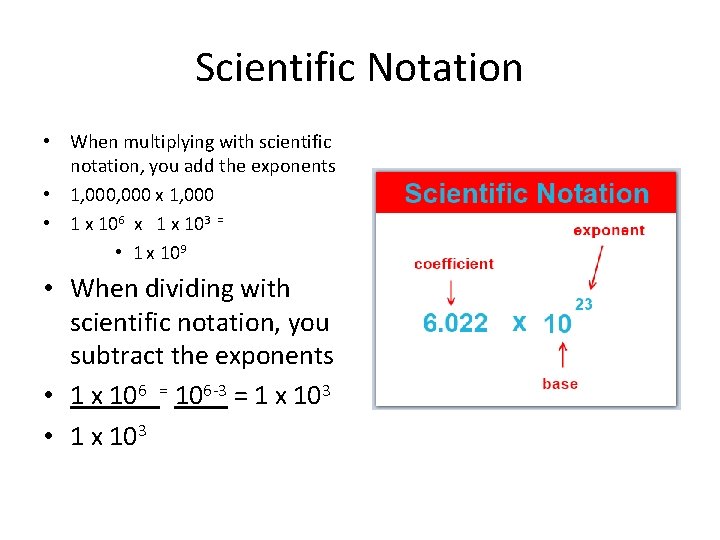
Scientific Notation • When multiplying with scientific notation, you add the exponents • 1, 000 x 1, 000 • 1 x 106 x 1 x 103 = • 1 x 109 • When dividing with scientific notation, you subtract the exponents

Scientific Notation • When multiplying with scientific notation, you add the exponents • 1, 000 x 1, 000 • 1 x 106 x 1 x 103 = • 1 x 109 • When dividing with scientific notation, you subtract the exponents • 1 x 106 = 106 -3 = 1 x 103 • 1 x 103

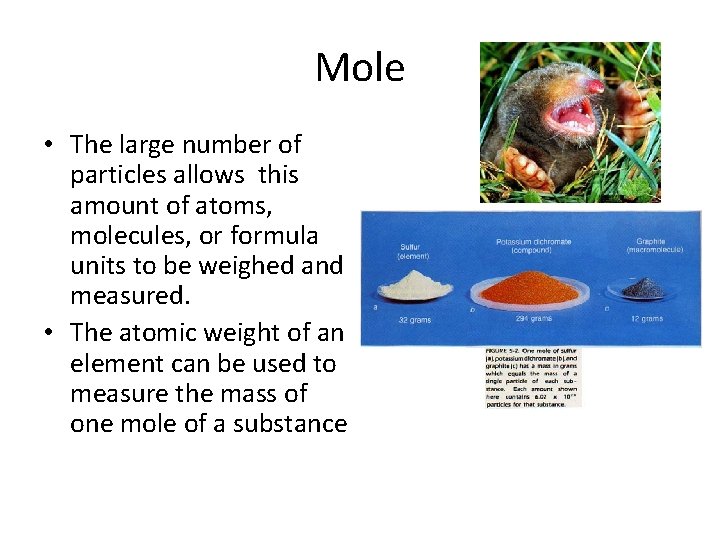
Mole • The large number of particles allows this amount of atoms, molecules, or formula units to be weighed and measured. • The atomic weight of an element can be used to measure the mass of one mole of a substance

Mole • The large number of particles allows this amount of atoms, molecules, or formula units to be weighed and measured. • The atomic weight of an element can be used to measure the mass of one mole of a substance

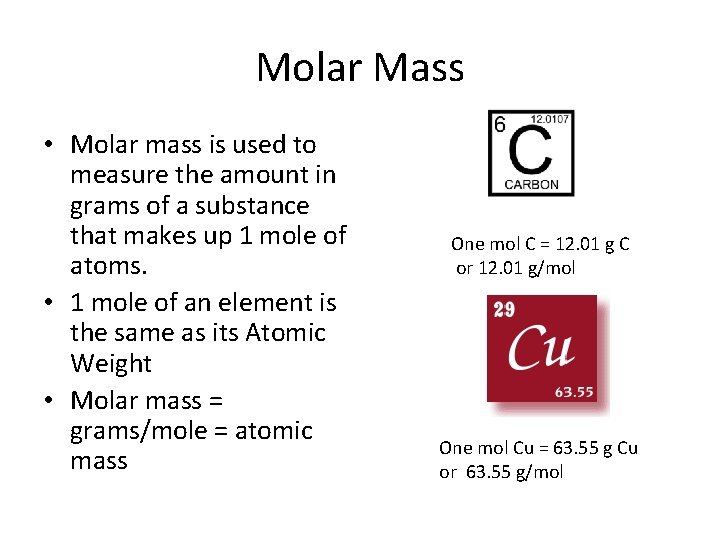
Molar Mass • Molar mass is used to measure the amount in grams of a substance that makes up 1 mole of atoms. • 1 mole of an element is the same as its Atomic Weight • Molar mass = grams/mole = atomic mass One mol C = 12. 01 g C or 12. 01 g/mol One mol Cu = 63. 55 g Cu or 63. 55 g/mol

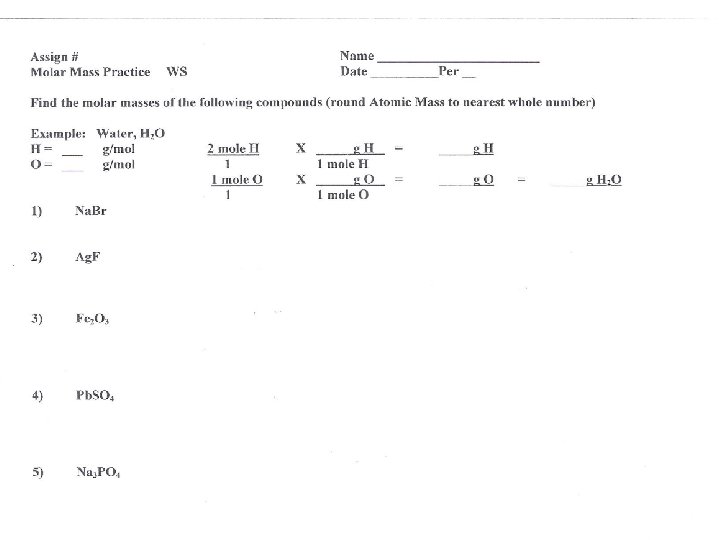
Molar mass of compounds • The molar mass of compounds or 1 mol C X 12. 01 g C = 12. 01 C molecules is the sum of 1 mol C the molar masses of its 2 mol O X 16. 00 g O = 32. 00 O atoms 1 mol O • The molar mass of CO 2 Molar mass of CO 2 = 12. 01 g + 32. 00 g contains one mole of C = 44. O 1 g CO 2 and 2 moles of Oxygen

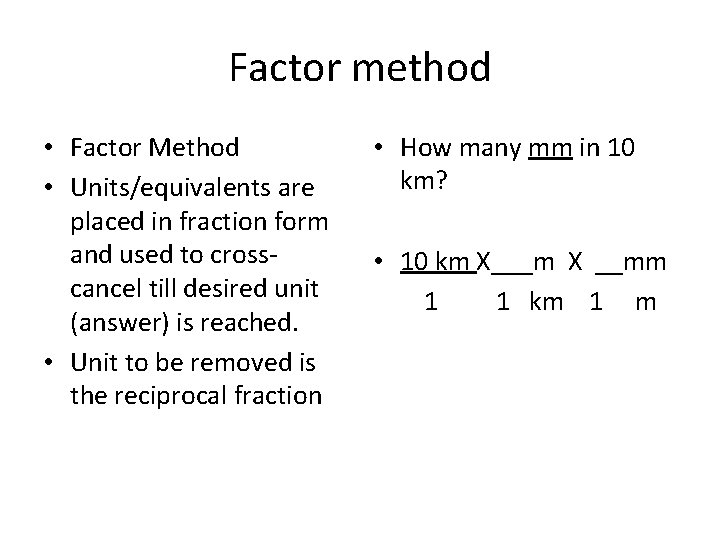
Factor method • Factor Method • Units/equivalents are placed in fraction form and used to crosscancel till desired unit (answer) is reached. • Unit to be removed is the reciprocal fraction • How many mm in 10 km? • 10 km X___m X __mm 1 km 1 m

Factor method • Factor Method • How many mm in 10 km? • Units/equivalents are placed in fraction form and used to crosscancel till desired unit • 10 km X 1000 mm (answer) is reached. • Unit to be removed is 1 km 1 m the reciprocal fraction

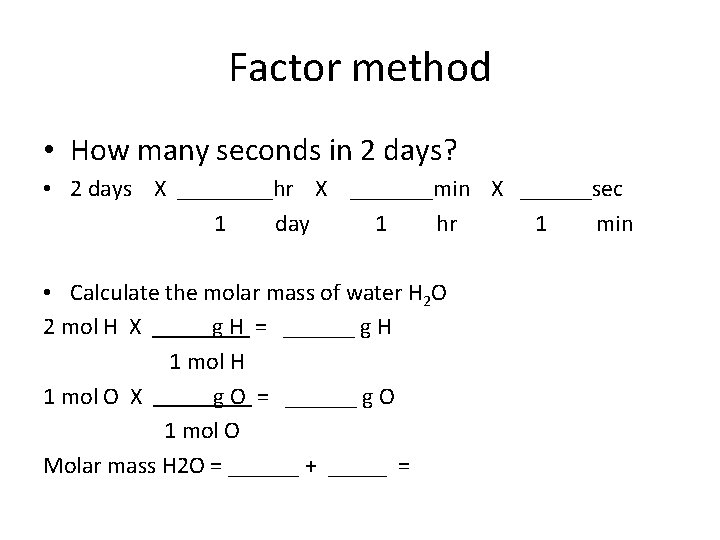
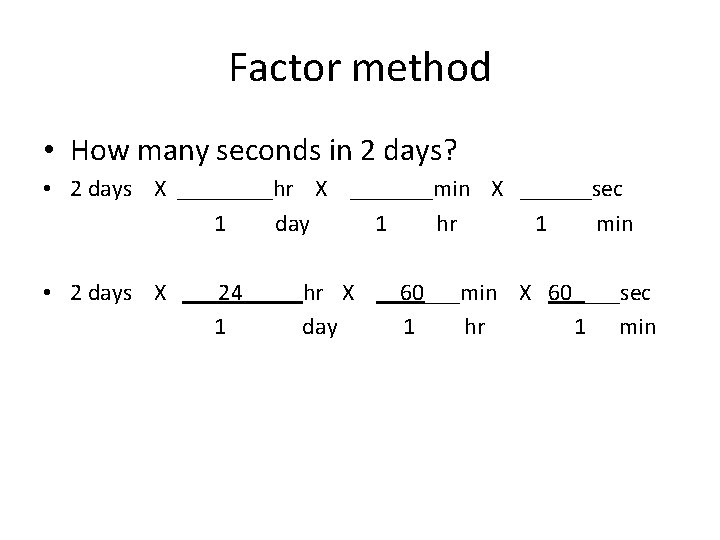
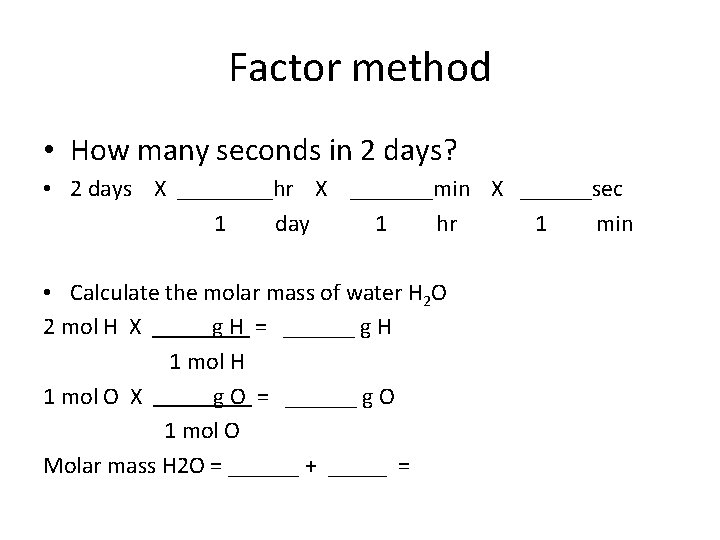
Factor method • How many seconds in 2 days? • 2 days X ____hr X _______min X ______sec 1 day 1 hr 1 min • Calculate the molar mass of water H 2 O 2 mol H X g H = ______ g H 1 mol H 1 mol O X g O = ______ g O 1 mol O Molar mass H 2 O = ______ + _____ =

Factor method • How many seconds in 2 days? • 2 days X ____hr X _______min X ______sec 1 day 1 hr 1 min • 2 days X ___24_____hr X __60___min X 60____sec 1 day 1 hr 1 min

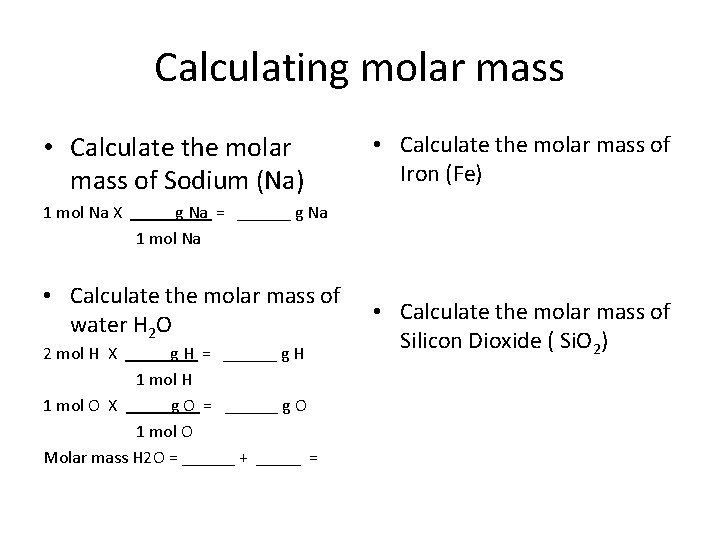
Calculating molar mass • Calculate the molar mass of Sodium (Na) • Calculate the molar mass of Iron (Fe) 1 mol Na X g Na = ______ g Na 1 mol Na • Calculate the molar mass of water H 2 O 2 mol H X g H = ______ g H 1 mol O X g O = ______ g O 1 mol O Molar mass H 2 O = ______ + _____ = • Calculate the molar mass of Silicon Dioxide ( Si. O 2)

Factor method • How many seconds in 2 days? • 2 days X ____hr X _______min X ______sec 1 day 1 hr 1 min • Calculate the molar mass of water H 2 O 2 mol H X g H = ______ g H 1 mol H 1 mol O X g O = ______ g O 1 mol O Molar mass H 2 O = ______ + _____ =

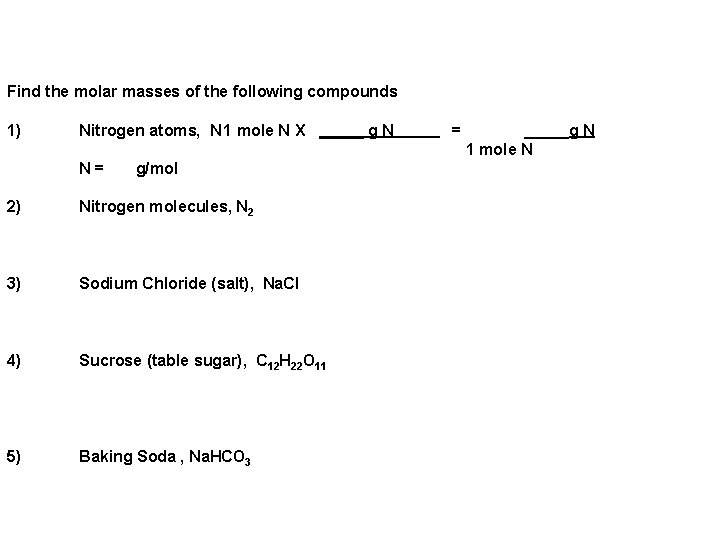
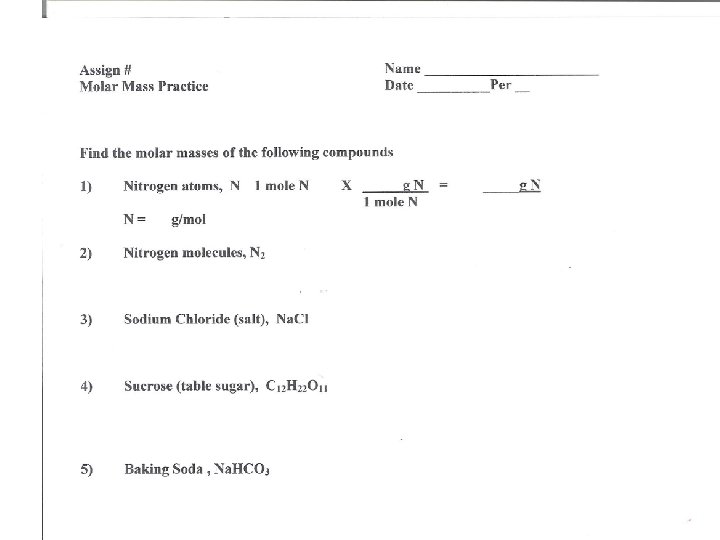
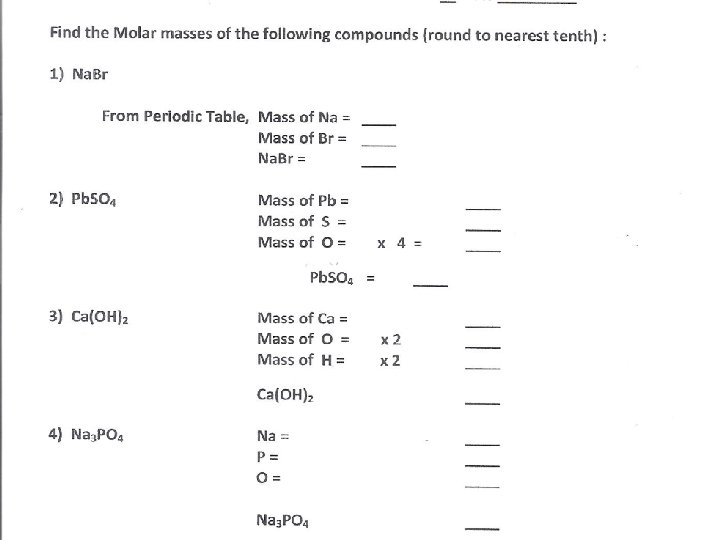
Find the molar masses of the following compounds 1) Nitrogen atoms, N 1 mole N X _____ g N N = g/mol 2) 3) 4) 5) Nitrogen molecules, N 2 Sodium Chloride (salt), Na. Cl Sucrose (table sugar), C 12 H 22 O 11 Baking Soda , Na. HCO 3 = _____g N 1 mole N






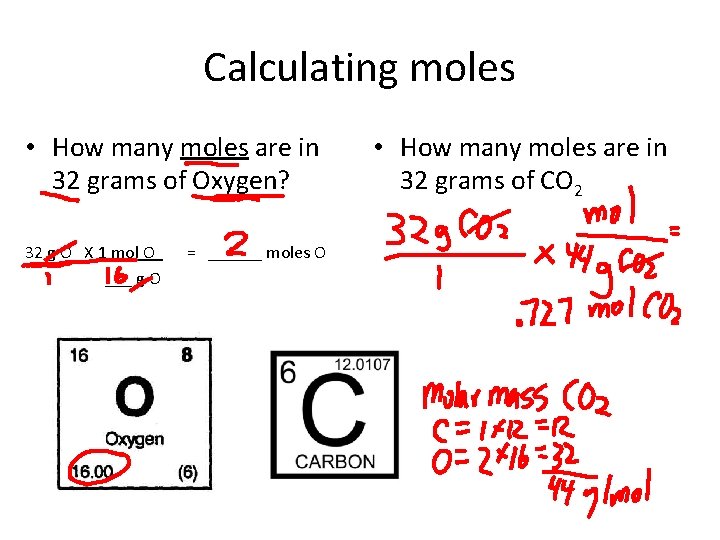
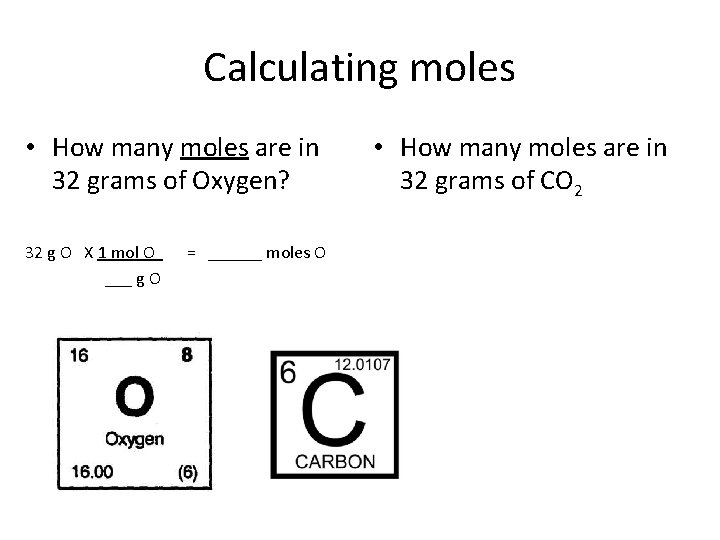
Calculating moles • How many moles are in 32 grams of Oxygen? 32 g O X 1 mol O = ______ moles O ___ g O • How many moles are in 32 grams of CO 2

Calculating moles • How many moles are in 32 grams of Oxygen? 32 g O X 1 mol O = ______ moles O ___ g O • How many moles are in 32 grams of CO 2

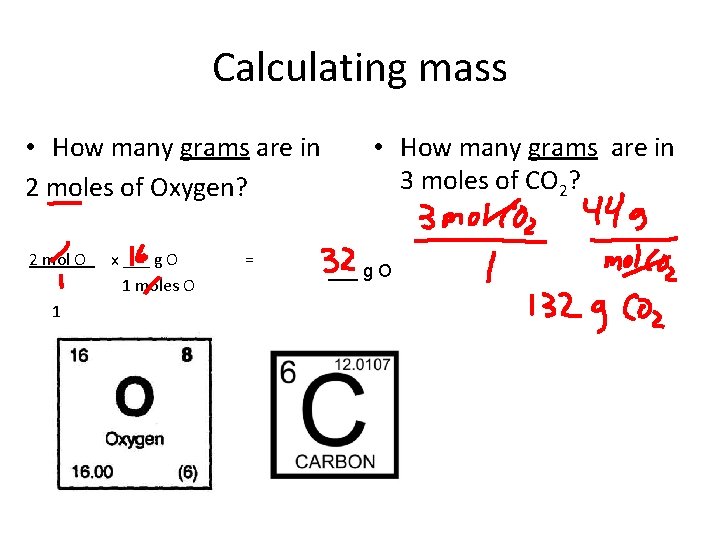
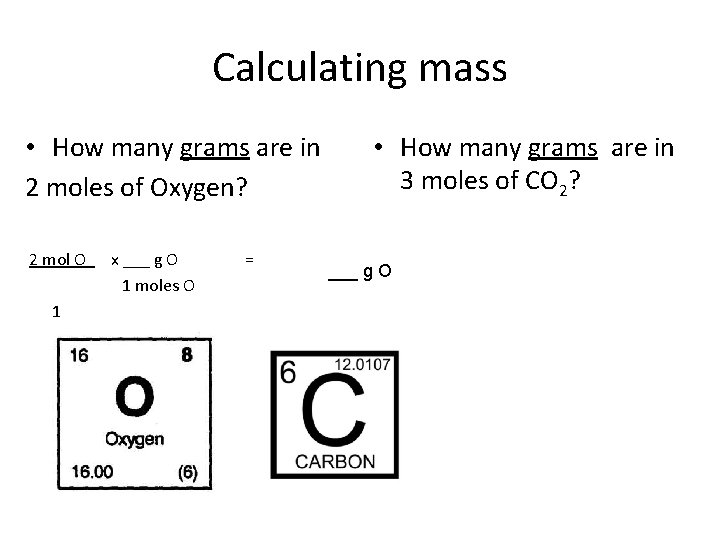
Calculating mass • How many grams are in 2 moles of Oxygen? 2 mol O x ___ g O 1 moles O 1 = • How many grams are in 3 moles of CO 2? ___ g O

Calculating mass • How many grams are in 2 moles of Oxygen? 2 mol O x ___ g O 1 moles O 1 = • How many grams are in 3 moles of CO 2? ___ g O

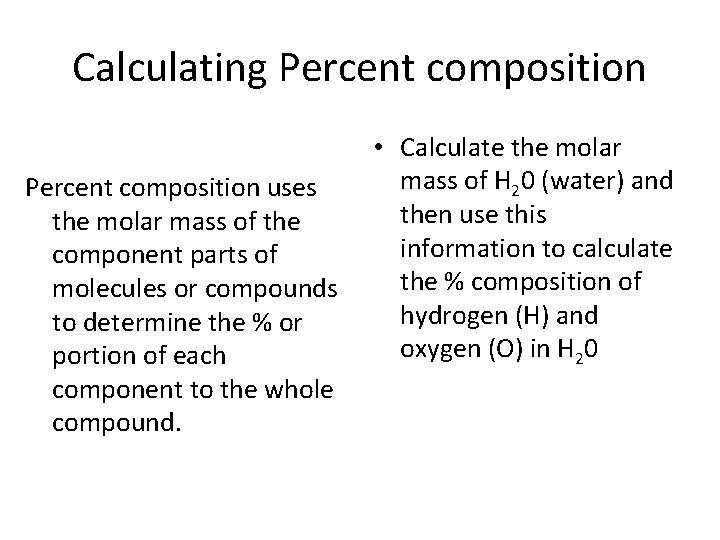
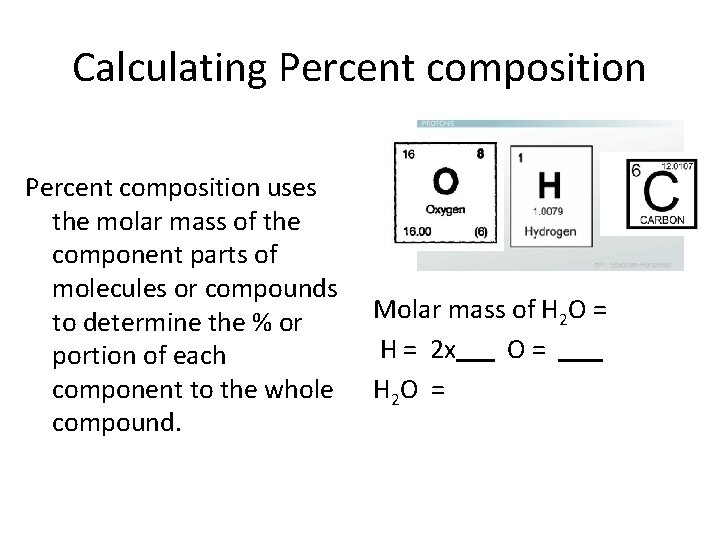
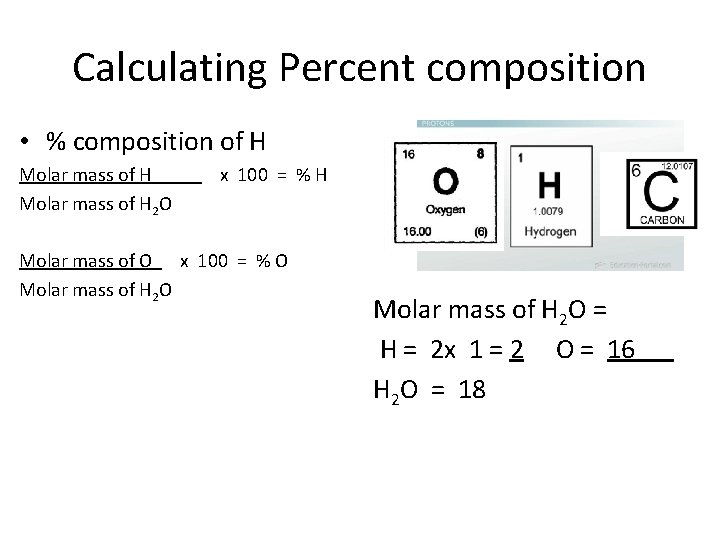
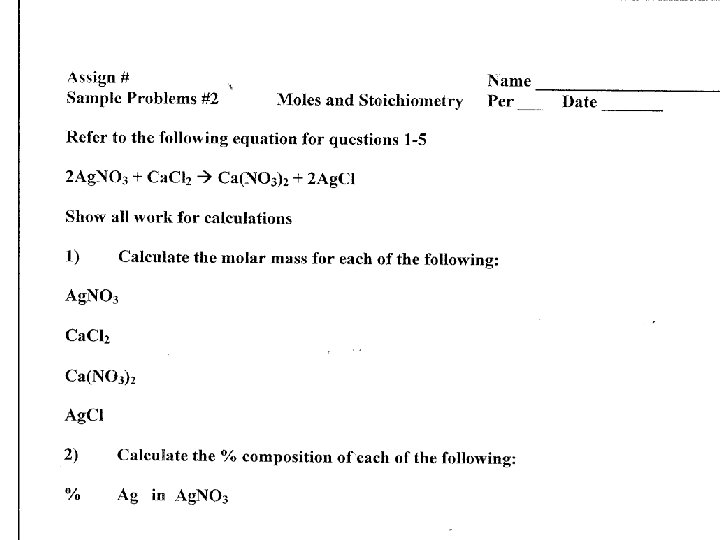
Calculating Percent composition uses the molar mass of the component parts of molecules or compounds to determine the % or portion of each component to the whole compound. • Calculate the molar mass of H 20 (water) and then use this information to calculate the % composition of hydrogen (H) and oxygen (O) in H 20

Calculating Percent composition uses the molar mass of the component parts of molecules or compounds to determine the % or portion of each component to the whole compound. Molar mass of H 2 O = H = 2 x O = H 2 O =

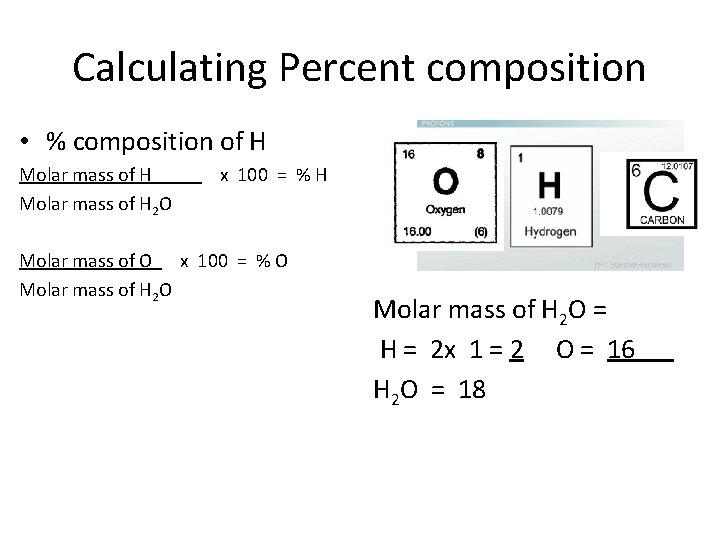
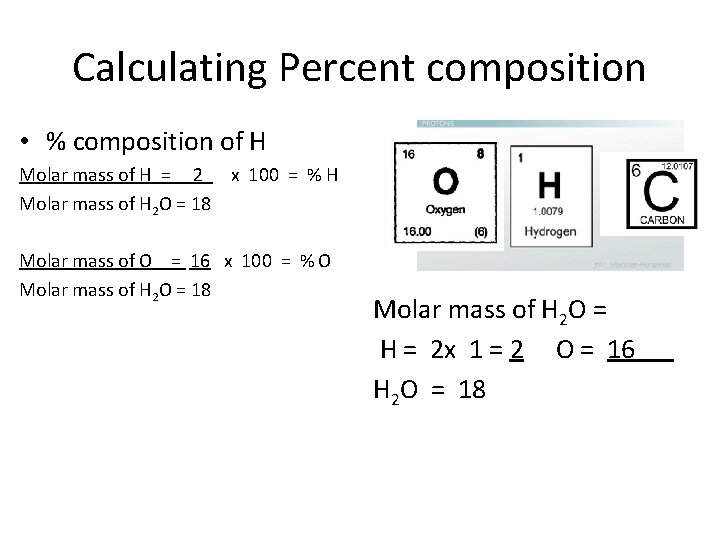
Calculating Percent composition • % composition of H Molar mass of H x 100 = % H Molar mass of H 2 O Molar mass of O x 100 = % O Molar mass of H 2 O = H = 2 x 1 = 2 O = 16 H 2 O = 18

Calculating Percent composition • % composition of H Molar mass of H x 100 = % H Molar mass of H 2 O Molar mass of O x 100 = % O Molar mass of H 2 O = H = 2 x 1 = 2 O = 16 H 2 O = 18

Calculating Percent composition • % composition of H Molar mass of H = 2 x 100 = % H Molar mass of H 2 O = 18 Molar mass of O = 16 x 100 = % O Molar mass of H 2 O = 18 Molar mass of H 2 O = H = 2 x 1 = 2 O = 16 H 2 O = 18

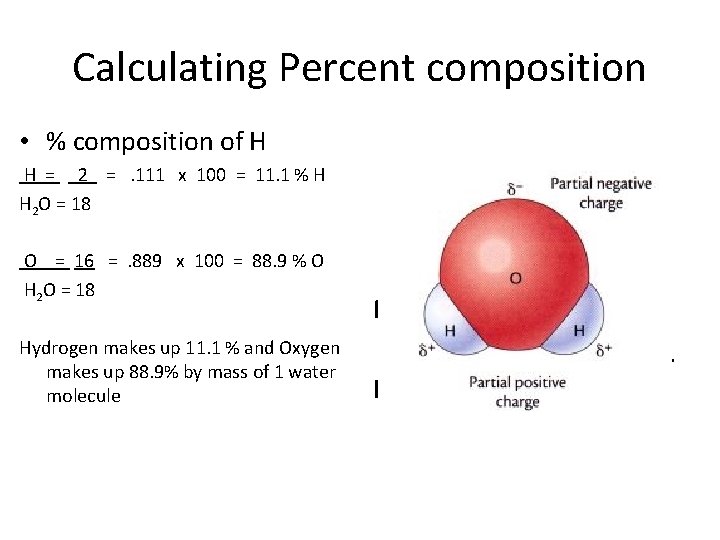
Calculating Percent composition • % composition of H H = 2 = . 111 x 100 = 11. 1 % H H 2 O = 18 O = 16 = . 889 x 100 = 88. 9 % O H 2 O = 18 Hydrogen makes up 11. 1 % and Oxygen makes up 88. 9% by mass of 1 water molecule Molar mass of H 2 O = H = 2 x 1 = 2 O = 16 H 2 O = 18

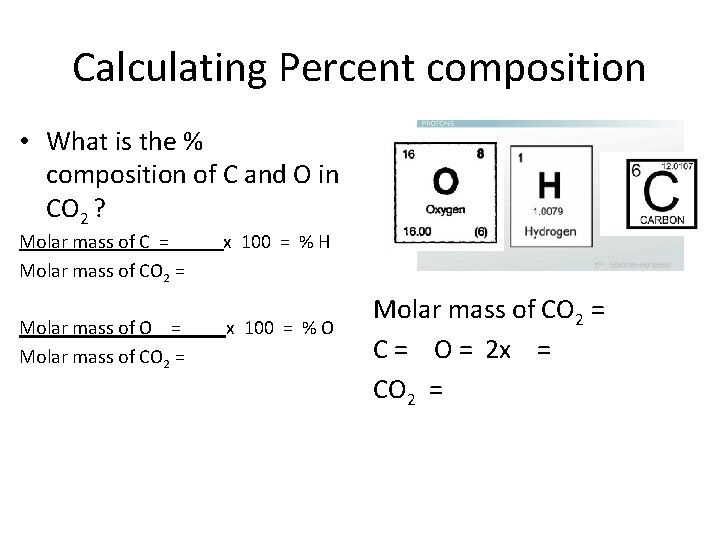
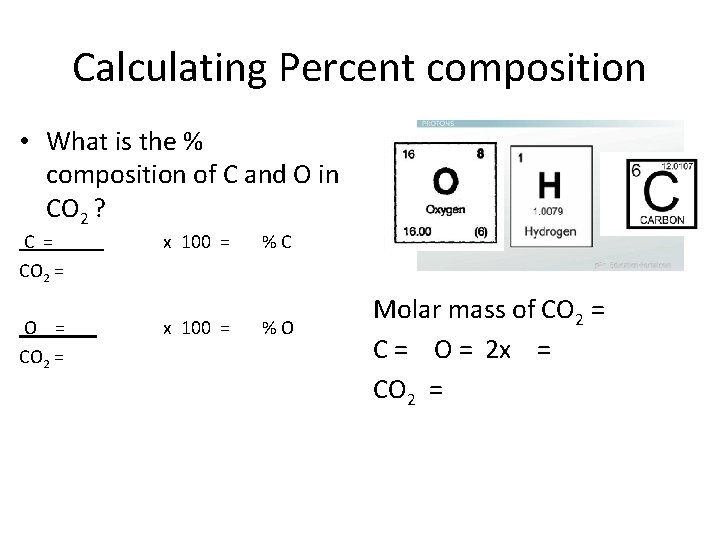
Calculating Percent composition • What is the % composition of C and O in CO 2 ? Molar mass of C = x 100 = % H Molar mass of CO 2 = Molar mass of O = x 100 = % O Molar mass of CO 2 = C = O = 2 x = CO 2 =

Calculating Percent composition • What is the % composition of C and O in CO 2 ? C = CO 2 = O = CO 2 = x 100 = % C x 100 = % O Molar mass of CO 2 = C = O = 2 x = CO 2 =

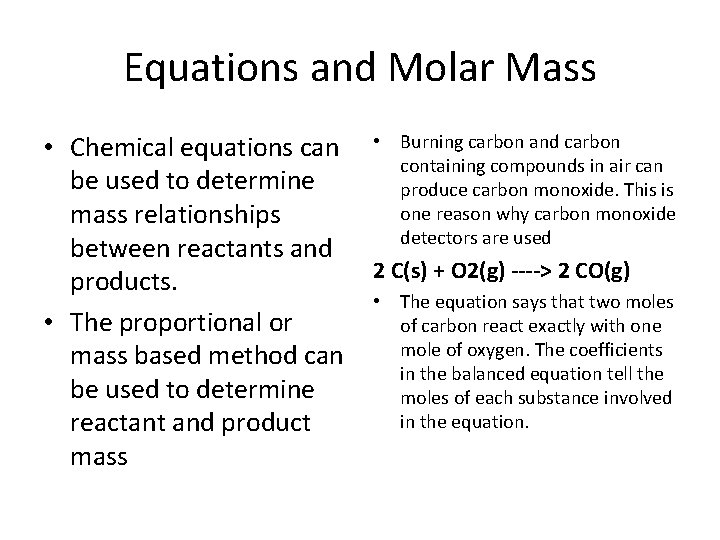
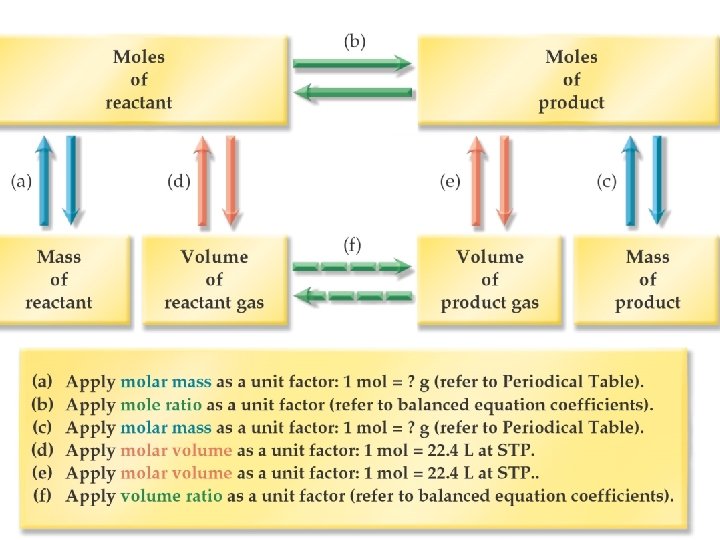
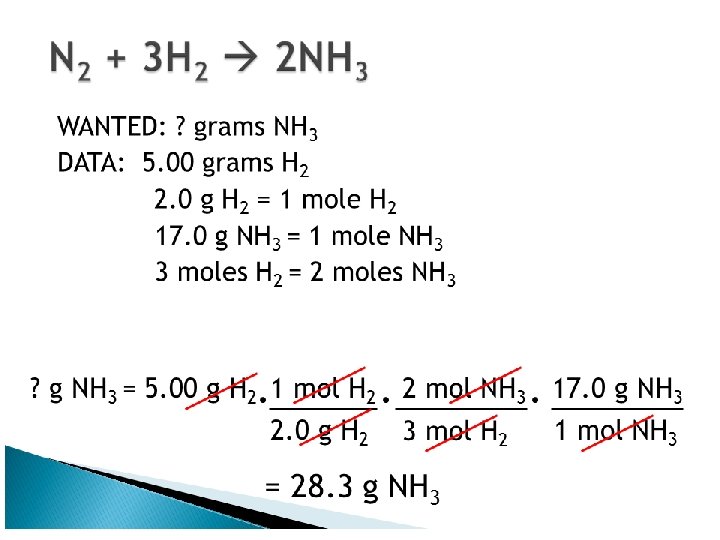
Equations and Molar Mass • Chemical equations can • Burning carbon and carbon containing compounds in air can be used to determine produce carbon monoxide. This is one reason why carbon monoxide mass relationships detectors are used between reactants and 2 C(s) + O 2(g) ----> 2 CO(g) products. • The equation says that two moles • The proportional or of carbon react exactly with one mole of oxygen. The coefficients mass based method can in the balanced equation tell the be used to determine moles of each substance involved in the equation. reactant and product mass









