Solutions Solutions Homogeneous mixtures evenly mixed Solutions Homogeneous


































- Slides: 47

Solutions

Solutions Homogeneous mixtures (evenly mixed)

Solutions Homogeneous mixtures (evenly mixed) Made of a solute dissolved in a solvent.

Solutions Homogeneous mixtures (evenly mixed) Made of a solute dissolved in a solvent. Can be liquid (salt water), gas (air), or solid (alloy)

Describing Concentration

Describing Concentration Qualitative ways of describing concentration:

Describing Concentration Qualitative ways of describing concentration: Very general descriptions:

Describing Concentration Qualitative ways of describing concentration: Very general descriptions: Dilute: a small amount of solute is dissolved

Describing Concentration Qualitative ways of describing concentration: Very general descriptions: Dilute: a small amount of solute is dissolved Concentrated: a large amount of solute is dissolved

Describing Concentration Qualitative ways of describing concentration: Very general descriptions: Dilute: a small amount of solute is dissolved Concentrated: a large amount of solute is dissolved More specific descriptions based on saturation:

Describing Concentration Qualitative ways of describing concentration: Very general descriptions: Dilute: a small amount of solute is dissolved Concentrated: a large amount of solute is dissolved More specific descriptions based on saturation: Unsaturated: more can dissolve

Describing Concentration Qualitative ways of describing concentration: Very general descriptions: Dilute: a small amount of solute is dissolved Concentrated: a large amount of solute is dissolved More specific descriptions based on saturation: Unsaturated: more can dissolve Saturated: no more can dissolve at that temperature

Describing Concentration Qualitative ways of describing concentration: Very general descriptions: Dilute: a small amount of solute is dissolved Concentrated: a large amount of solute is dissolved More specific descriptions based on saturation: Unsaturated: more can dissolve Saturated: no more can dissolve at that temperature Super-saturated: more is dissolve than normally possible at that temperature (very unstable condition).

Solution simulation. jar

Describing Concentration Quantitative way of describing concentration:

Describing Concentration Quantitative way of describing concentration: Molarity

Describing Concentration Quantitative way of describing concentration: Molarity Moles of solute per liter of solution

Describing Concentration Quantitative way of describing concentration: Molarity Moles of solute per liter of solution To calculate: # of moles divided by # of litter

Describing Concentration Quantitative way of describing concentration: Molarity Moles of solute per liter of solution To calculate: # of moles divided by # of litter Unit: M (for molar) -- upper case and in italics or underlined

Describing Concentration Quantitative way of describing concentration: Molarity Moles of solute per liter of solution To calculate: # of moles divided by # of litter Unit: M (for molar) -- upper case and in italics or underlined There are many other, less common ways



Solutions Vocabulary. pptx


Solubility: Factors affecting dissolving ability

Solubility: Factors affecting dissolving ability Polarity

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar Temperature

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar Temperature Solids are more soluble in water as temperature increases

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar Temperature Solids are more soluble in water as temperature increases Gases are less soluble in water as temperature increases

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar Temperature Solids are more soluble in water as temperature increases Gases are less soluble in water as temperature increases Pressure

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar Temperature Solids are more soluble in water as temperature increases Gases are less soluble in water as temperature increases Pressure Solids and liquids: solubilities aren't affected by pressure.

Solubility: Factors affecting dissolving ability Polarity polar dissolve in polar; non-polar in non-polar Temperature Solids are more soluble in water as temperature increases Gases are less soluble in water as temperature increases Pressure Solids and liquids: solubilities aren't affected by pressure. Gases: more soluble in water as pressure increases.

Solubility: How solutes dissolve

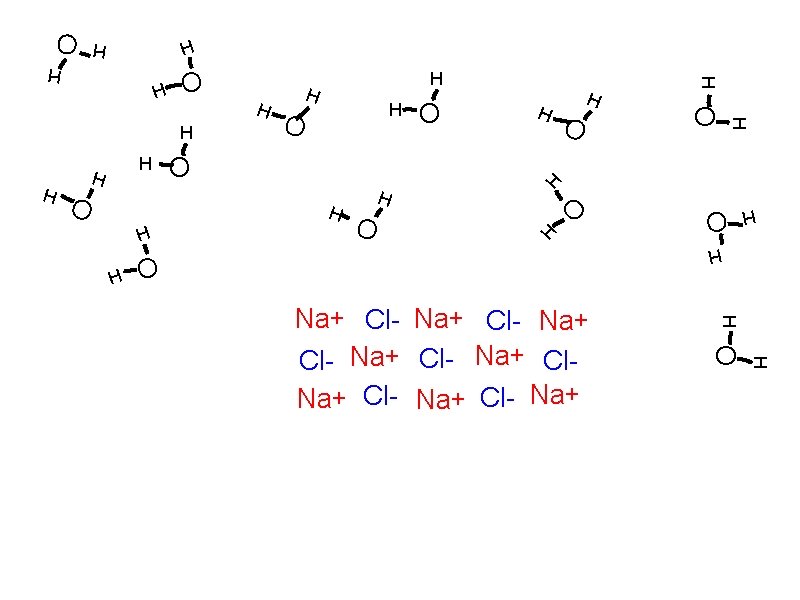
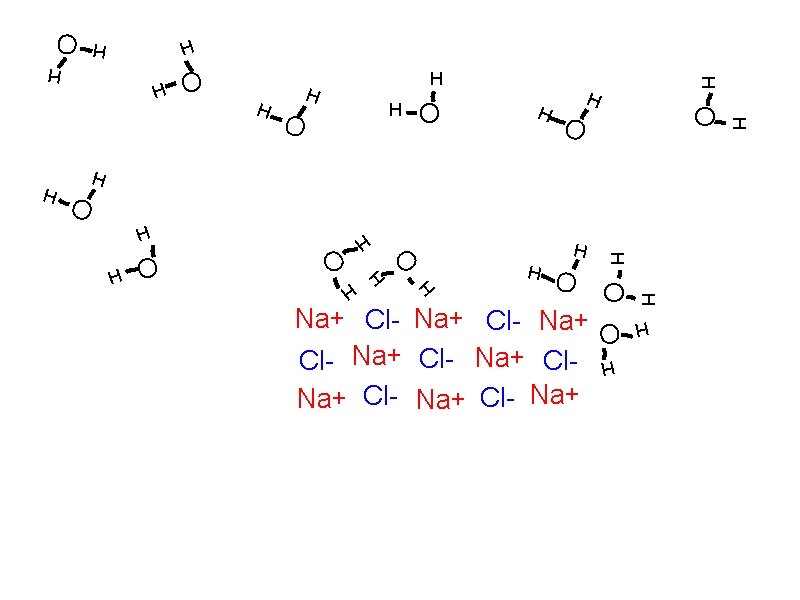
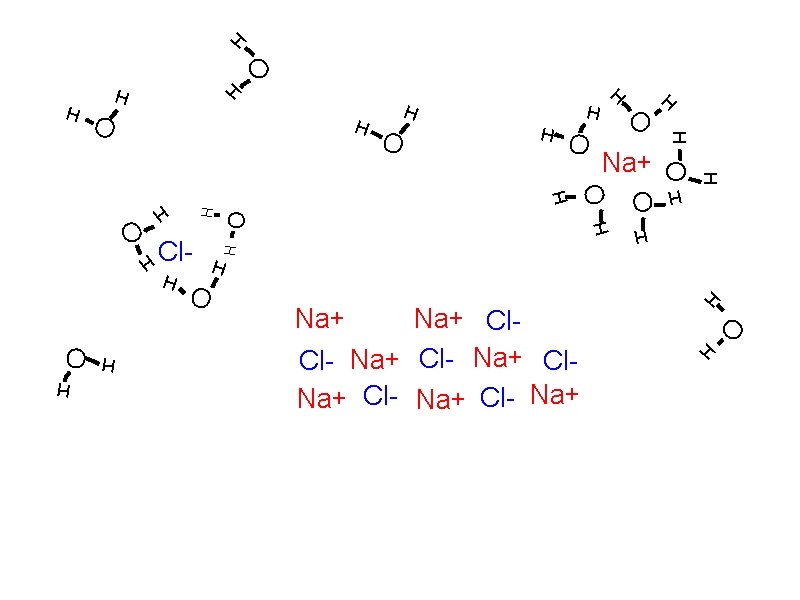
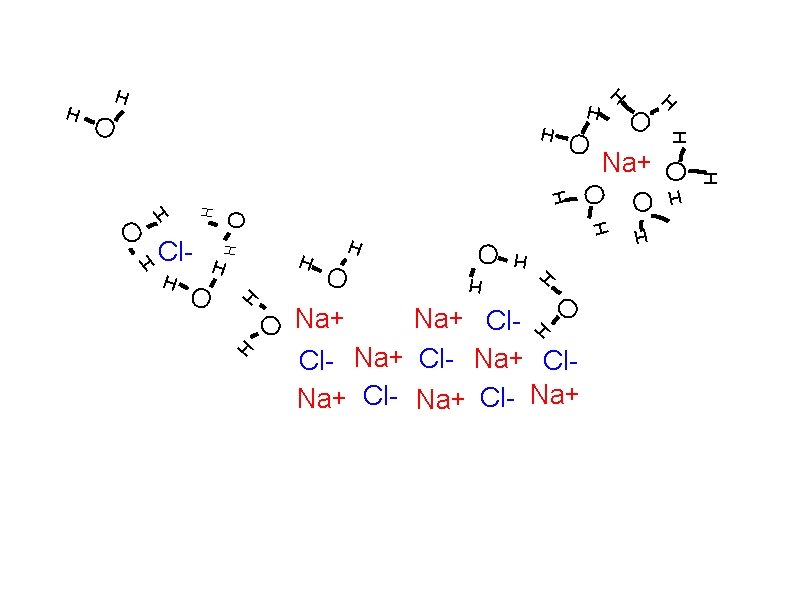
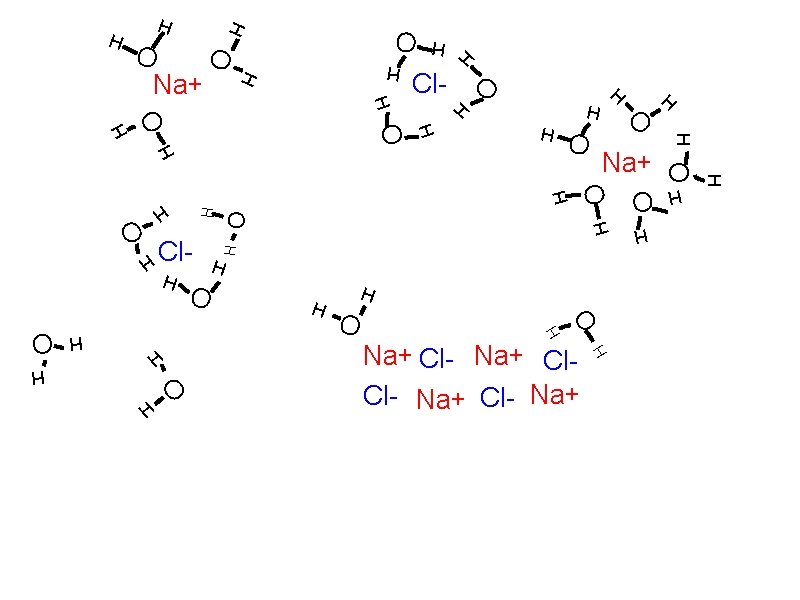
Solubility: How solutes dissolve Solvation

Solubility: How solutes dissolve Solvation Individual atoms, ions, or molecules are surrounded by solvent molecules and brought into solution.

Solubility: How solutes dissolve Solvation Individual atoms, ions, or molecules are surrounded by solvent molecules and brought into solution. When water is the solvent, the process is called hydration.

Solubility: How solutes dissolve Solvation Individual atoms, ions, or molecules are surrounded by solvent molecules and brought into solution. When water is the solvent, the process is called hydration. Dissociation

Solubility: How solutes dissolve Solvation Individual atoms, ions, or molecules are surrounded by solvent molecules and brought into solution. When water is the solvent, the process is called hydration. Dissociation The breaking up of an ionic compound into ions during solvation or hydration.

H H O O H O H H H O H H O Na+ Cl- Na+ Cl- Na+

H O O H H O O H H H O Na+ Cl- Na+ Cl- Na+ H H O H H H

O H O Na+ Cl. Cl- Na+ O Na+ H H H O Na+ H O H H O Cl- O H H O O H H H O H

H H O Na+ Cl. Cl- Na+ O H H H H O Na+ H O H Na+ O Cl. H O O H O H H H H

H O O O Na+ Cl- Na+ Cl. Cl- Na+ H H H H O H H H Na+ O H O H H H O Cl- H O H Cl- H H H O Na+ O H H H O H

Solutions In Class Review. pptx


Attachments Solution simulation. jar Solutions Vocabulary. pptx Solutions In Class Review. pptx