Solutions Molarity What is a solution Solution homogeneous








- Slides: 15

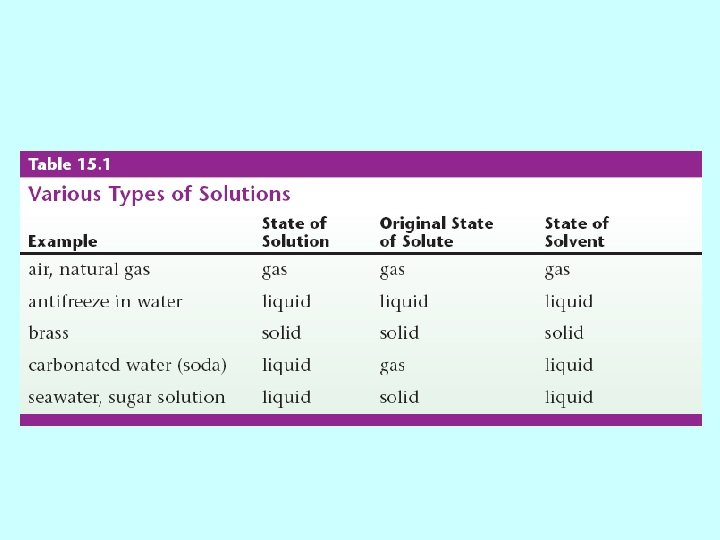
Solutions & Molarity What is a solution? • Solution – homogeneous mixture – Solvent – substance present in largest amount – Solutes – other substances in the solution – Aqueous solution – solution with water as the solvent


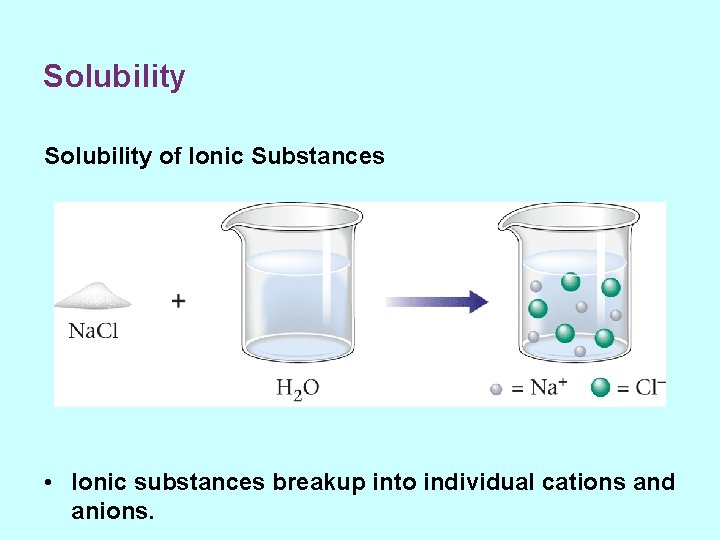
Solubility of Ionic Substances • Ionic substances breakup into individual cations and anions.

Solubility How Substances Dissolve • A “hole” must be made in the water structure for each solute particle. • The lost water-water interactions must be replaced by water-solute interactions. • “like dissolves like”

Solution Composition: An Introduction • The solubility of a solute is limited. – Saturated solution – contains as much solute as will dissolve at that temperature – Unsaturated solution – has not reached the limit of solute that will dissolve

Solution Composition: An Introduction – Supersaturated solution – occurs when a solution is saturated at an elevated temperature and then allowed to cool but all of the solid remains dissolved • Contains more dissolved solid than a saturated solution at that temperature • Unstable – adding a crystal causes precipitation

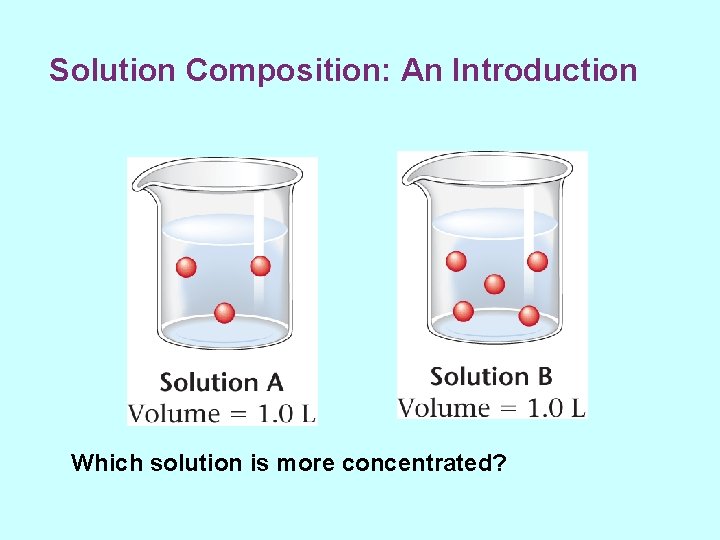
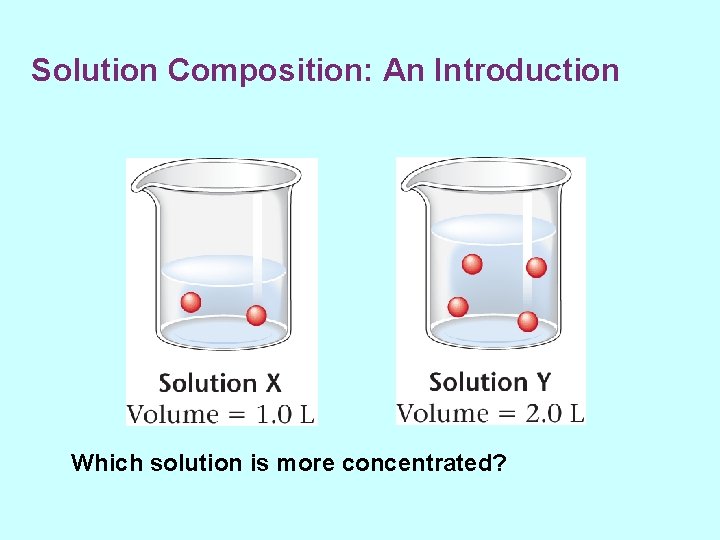
Solution Composition: An Introduction • Solutions are mixtures. • Amounts of substances can vary in different solutions. – Specify the amounts of solvent and solutes – Qualitative measures of concentration • concentrated – relatively large amount of solute • dilute – relatively small amount of solute

Solution Composition: An Introduction Which solution is more concentrated?

Solution Composition: An Introduction Which solution is more concentrated?

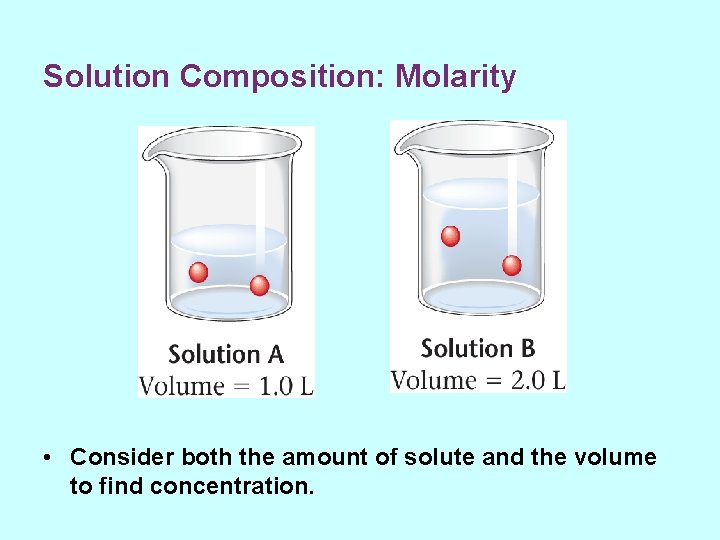
Solution Composition: Molarity • Concentration of a solution is the amount of solute in a given volume of solution.

Solution Composition: Molarity • Consider both the amount of solute and the volume to find concentration.

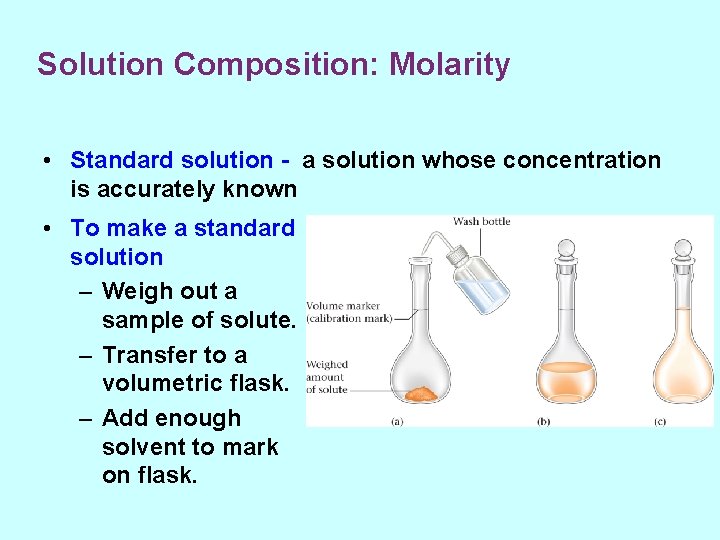
Solution Composition: Molarity • Standard solution - a solution whose concentration is accurately known • To make a standard solution – Weigh out a sample of solute. – Transfer to a volumetric flask. – Add enough solvent to mark on flask.

Boiling Point and Freezing Point • The presence of solute “particles” causes the liquid range to become wider. – Boiling point increases – Freezing point decreases

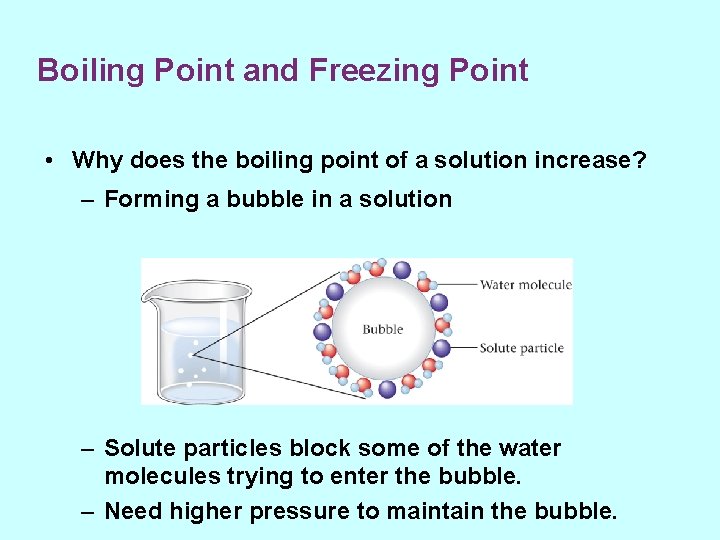
Boiling Point and Freezing Point • Why does the boiling point of a solution increase? – Forming a bubble in a solution – Solute particles block some of the water molecules trying to enter the bubble. – Need higher pressure to maintain the bubble.

Boiling Point and Freezing Point • Colligative property – a solution property that depends on the number of solute particles present