Chapters 12 13 Solutions Modern Chemistry Solution Solution




























- Slides: 44

Chapters 12 -13: Solutions Modern Chemistry

Solution � Solution – a homogeneous mixture of a solute and a solvent. � soluble - capable of being dissolved � Solute – the chemical that is dissolved � Solvent – the substance it is dissolved in ◦ Ex. ) Salt in water � Different types depend on state of solute/solvents: ◦ ◦ ◦ Solid-liquid Liquid-liquid Gas-liquid Liquid-gas Solid-solid Gas-gas


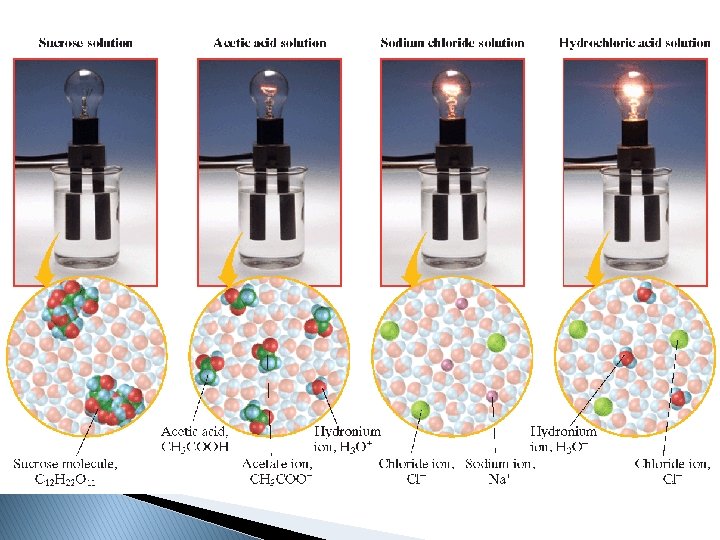
�A substance that dissolves in water to give a solution that conducts electric current is called an electrolyte. �Any soluble ionic compound, such as sodium chloride, Na. Cl, is an electrolyte.


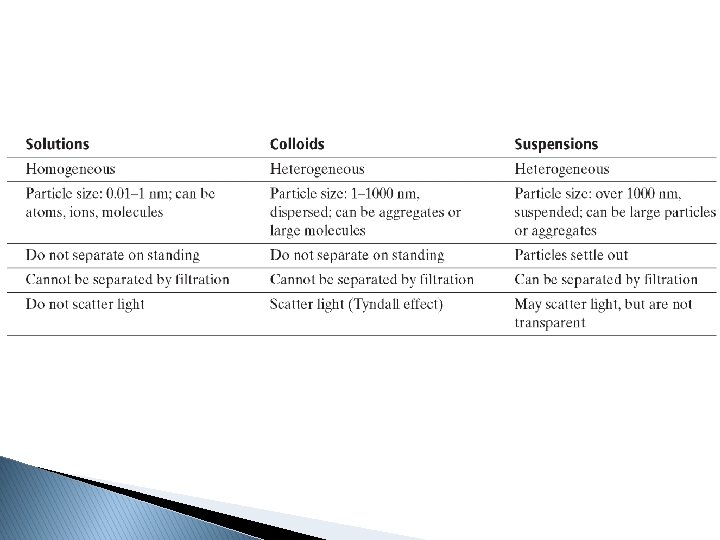
Do all compounds dissolve as well as others? � No � Solubility - Maximum amount of solute that can dissolve in a given amount of solvent at a given temperature

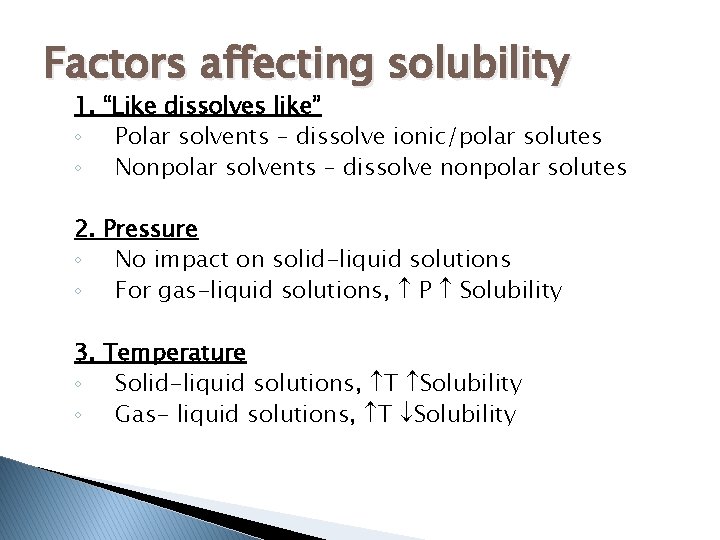
Factors affecting solubility 1. “Like dissolves like” ◦ Polar solvents – dissolve ionic/polar solutes ◦ Nonpolar solvents – dissolve nonpolar solutes 2. Pressure ◦ No impact on solid-liquid solutions ◦ For gas-liquid solutions, P Solubility 3. Temperature ◦ Solid-liquid solutions, T Solubility ◦ Gas- liquid solutions, T Solubility

Speed of solution formation Impacted by: 1. surface area 2. agitation 3. temperature

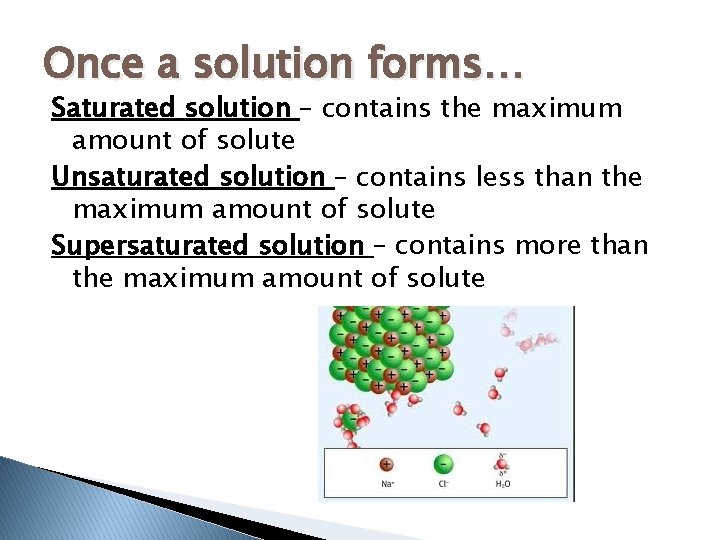
Once a solution forms… Saturated solution – contains the maximum amount of solute Unsaturated solution – contains less than the maximum amount of solute Supersaturated solution – contains more than the maximum amount of solute

Heat of solution � Energy change when a solution forms � Exothermic – releases energy (hot) � Endothermic – absorbs energy (cold)

Solutions calculations � 1. Molarity � 2. Dilutions � 3. Molality

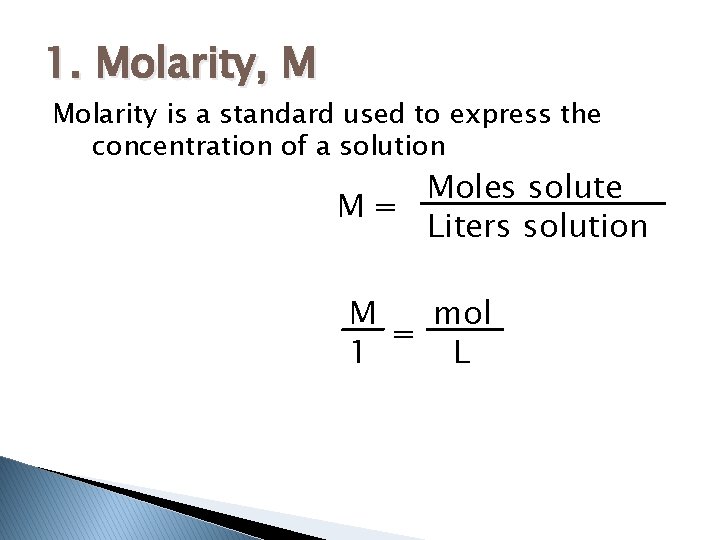
1. Molarity, M Molarity is a standard used to express the concentration of a solution Moles solute M= Liters solution M mol = 1 L

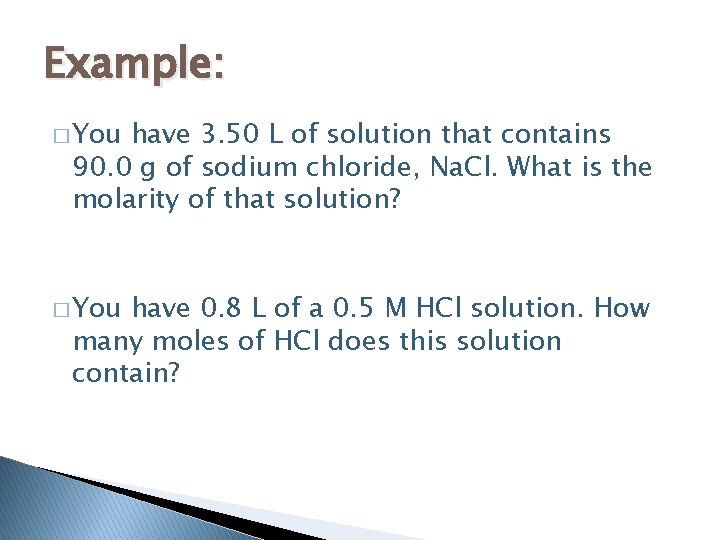
Example: � You have 3. 50 L of solution that contains 90. 0 g of sodium chloride, Na. Cl. What is the molarity of that solution? � You have 0. 8 L of a 0. 5 M HCl solution. How many moles of HCl does this solution contain?

� To produce 40. 0 g of silver chromate, you will need at least 23. 4 g of potassium chromate in solution as a reactant. All you have on hand is 5 L of a 6. 0 M K 2 Cr. O 4 solution. What volume of the solution is needed to give you the 23. 4 g K 2 Cr. O 4 needed for the reaction?

2. Dilutions � The dilution equation is used to accurately make weaker solutions from stronger stock solutions by adding water � M 1 V 1 = M 2 V 2 Stock solution � Weaker solution Initial Molarity x initial volume = final Molarity x final volume

Example � If you have 5 L of a 12 M HCl solution and you want a 3. 5 M solution, how much water should you add? � If you have 2 L of a 8. 5 M HCl solution and you accidentally added 0. 65 L of distilled water, what is the new molarity?

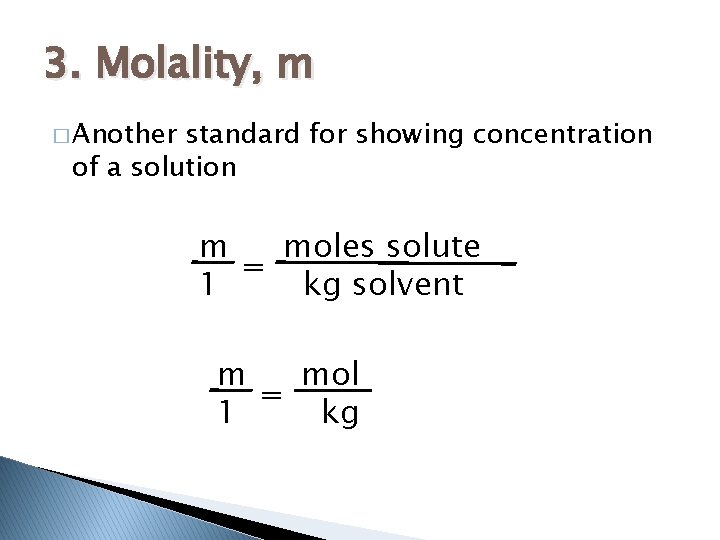
3. Molality, m � Another standard for showing concentration of a solution m moles solute = 1 kg solvent m mol = 1 kg

Examples �A solution was prepared by dissolving 17. 1 g of sucrose (table sugar, C 12 H 22 O 11) in 125 g of water. Find the molal concentration of this solution.

Examples cont. �A solution of iodine, I 2, in carbon tetrachloride, CCl 4, is used when iodine is needed for certain chemical tests. How much iodine must be added to prepare a 0. 480 m solution of iodine in CCl 4 if 100. 0 g of CCl 4 is used?

Chapter 12 Review �Page 426 �#’s 2 -5, 7 -9, 11 -12, 17, 19 -22, 26 -29

CHAPTER 13

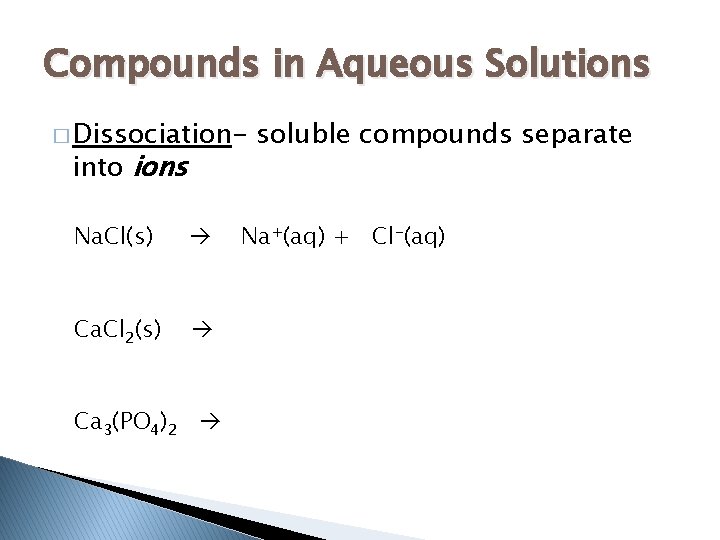
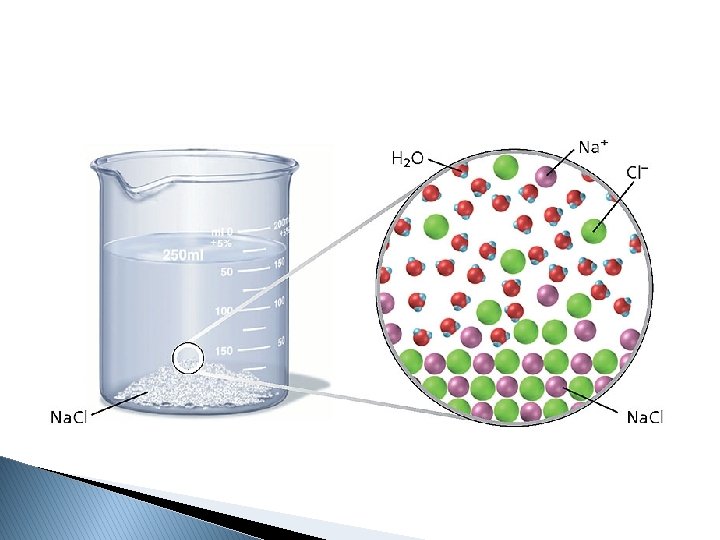
Compounds in Aqueous Solutions � Dissociation- into ions Na. Cl(s) Ca. Cl 2(s) Ca 3(PO 4)2 soluble compounds separate Na+(aq) + Cl-(aq)


� How many moles are produced when you dissociate Al 2(SO 4)3 ? � How many moles are produced when you dissolve 1 mole of sodium carbonate?

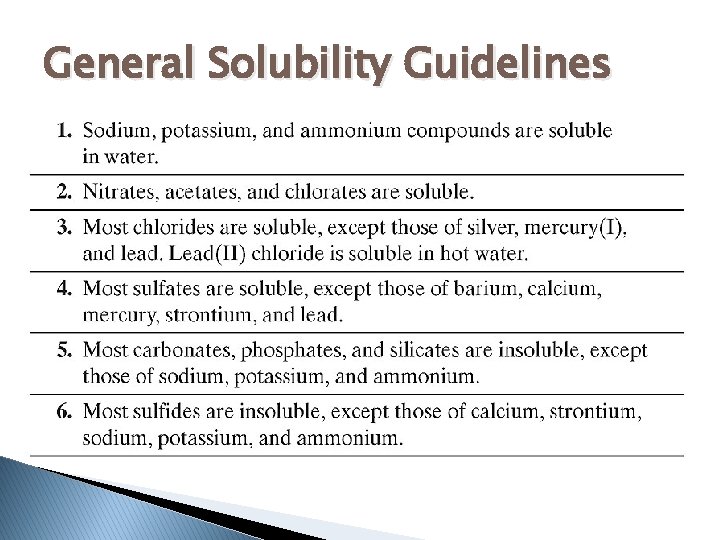
General Solubility Guidelines

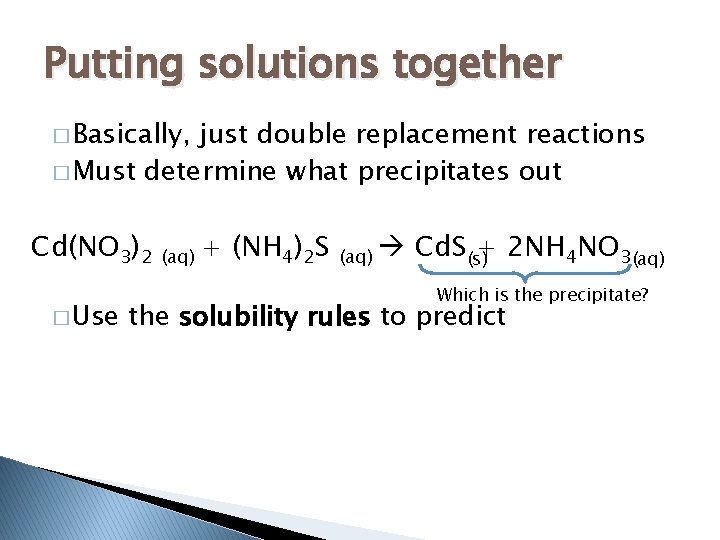
Putting solutions together � Basically, just double replacement reactions � Must determine what precipitates out Cd(NO 3)2 � Use (aq) + (NH 4)2 S (aq) Cd. S(s)+ 2 NH 4 NO 3(aq) Which is the precipitate? the solubility rules to predict

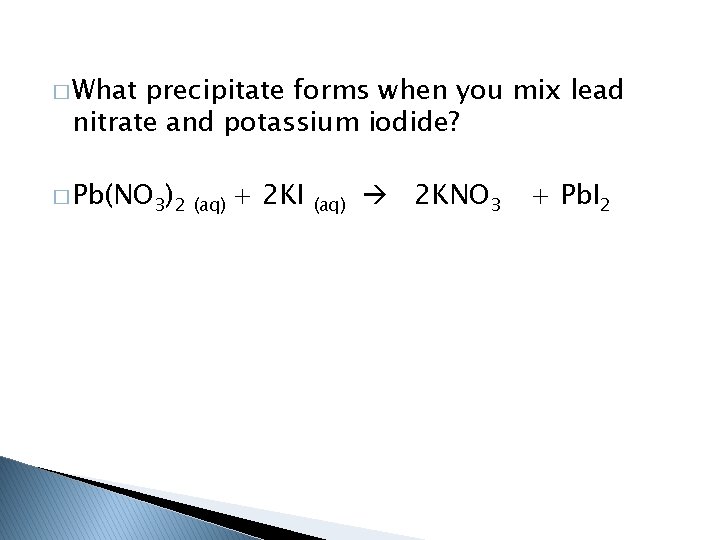
� What precipitate forms when you mix lead nitrate and potassium iodide? � Pb(NO 3)2 (aq) + 2 KI (aq) 2 KNO 3 + Pb. I 2

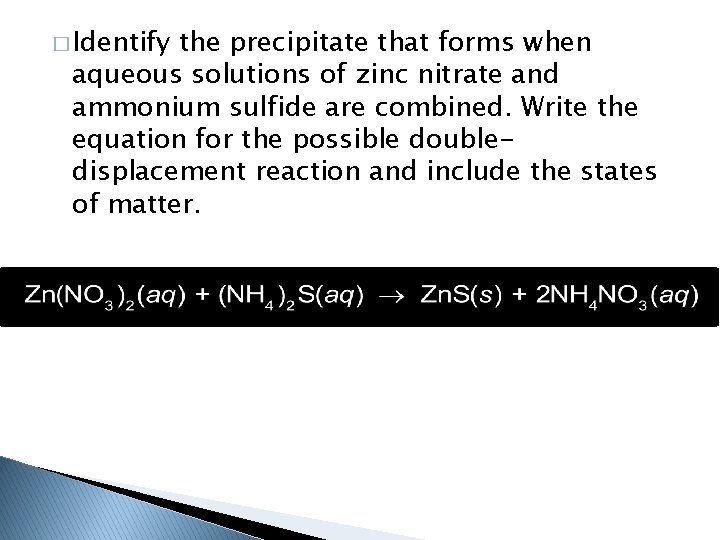
� Identify the precipitate that forms when aqueous solutions of zinc nitrate and ammonium sulfide are combined. Write the equation for the possible doubledisplacement reaction and include the states of matter.

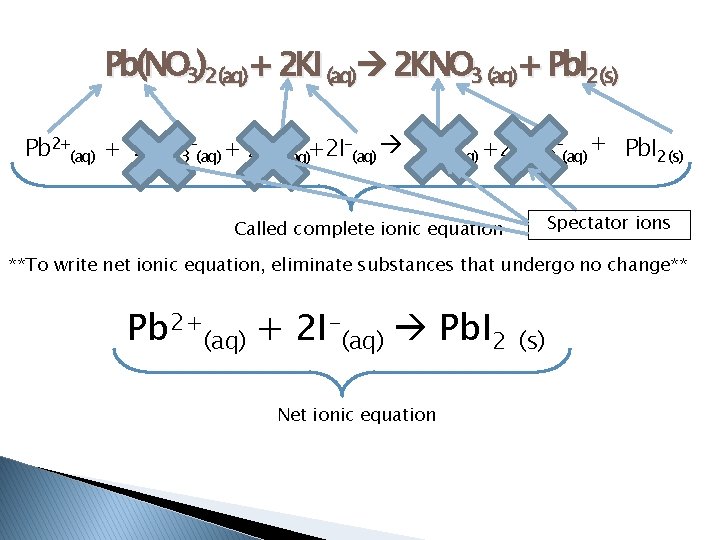
� Net Ionic Equation – includes only the compounds that undergo a chemical change in the reaction � Spectator ions – do not take part in a chemical reaction � Hint: Watch for a phase change! ◦ Solubility Table pg. 437 ◦ Common ion chart pg. 858

Pb(NO 3)2(aq)+ 2 KI (aq) 2 KNO 3 (aq)+ Pb. I 2(s) Pb 2+(aq) + 2 NO 3 -(aq) + 2 K+(aq)+2 I-(aq) 2 K+(aq) +2 NO 3 -(aq) + Pb. I 2 (s) Spectator ions Called complete ionic equation **To write net ionic equation, eliminate substances that undergo no change** Pb 2+(aq) + 2 I-(aq) Pb. I 2 Net ionic equation (s)

Write complete ionic and net ionic for: 1. Potassium sulfate and barium nitrate 2. Silver nitrate and sodium chloride

Net ionic equation � Strontium nitrate and sodium sulfate

Solution types � Electrolyte – conducts electricity ◦ Strong electrolytes vs. weak electrolytes ◦ HCl vs. HF � Nonelectrolyte – does not conduct electricity

Colligative Properties � Properties that depend on the concentration of solute particles ◦ Vapor Pressure ◦ Freezing Point ◦ Boiling Point

1. Vapor Pressure Lowering � Add solute, vapor pressure goes down (so boiling point goes up) � Non-volatile substance – is one that has little tendency to become a gas under existing conditions

2. Freezing Point Depression � Add solute, freezing point goes down � Kf= freezing point constant = -1. 86℃ � Tf = K f x m � Ex. ) Determine the freezing point of a water solution of fructose, C 6 H 12 O 6, made by dissolving 58. 0 g of fructose in 185 g of water.

� Ex 2. ) Calculate the molality of a solution of 39. 2 g of urea, H 4 N 2 CO, in 485 g of pure acetic acid. Determine the freezing point of this solution.

�A solution consists of 10. 3 g of glucose, C 6 H 12 O 6, dissolved in 250 g of water. What is the freezing point depression of the solution?

3. Boiling Point Elevation � Add solute, boiling point goes up � Kb = molal boiling point constant = 0. 51℃ � Tb = K b x m � Ex. A solution contains 50 g of sucrose, C 12 H 22 O 11, dissolved in 500 g of water. What is the boiling point elevation?

� Ex 2. If the boiling point elevation of an aqueous solution containing a nonvolatile electrolyte is 1. 02℃, what is the molality of the solution?

�A solution contains 450. 0 g of sucrose C 12 H 22 O 11, a nonelectrolyte, dissolved in 250. 0 g of acetic acid. What is the boiling point of the solution?

� In World War II, soldiers in the Sahara Desert needed a supply of antifreeze to protect the radiators of their vehicles. The temperature in the Sahara almost never drops to 0°C, so why was the antifreeze necessary?

Chapter 13 Review Pg. 458 #’s 1 -3, 5, 8, 9, 11, 12, 14, 19, 20, 21, 25, 26, 30
