Solutions Solutions Solutions are homogeneous mixtures of two

























- Slides: 47

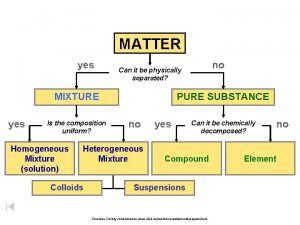
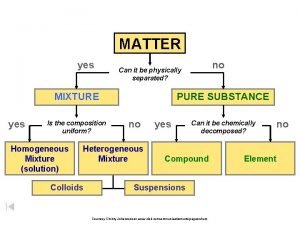
Solutions

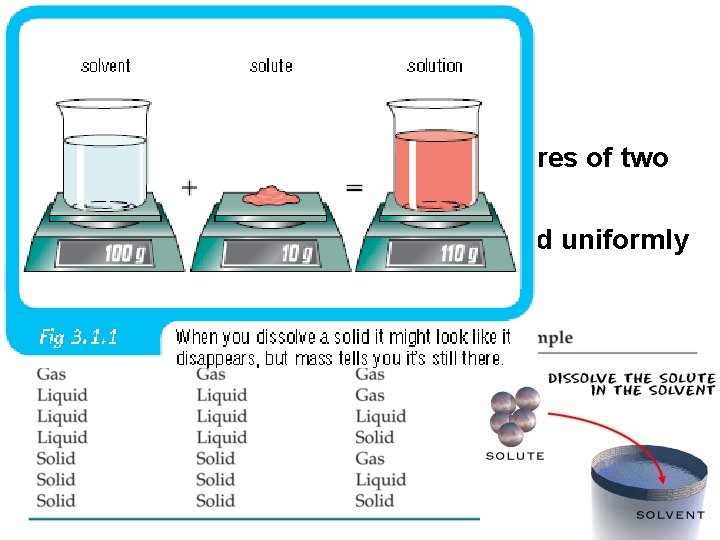
Solutions • Solutions are homogeneous mixtures of two or more pure substances. • In a solution, the solute is dispersed uniformly throughout the solvent

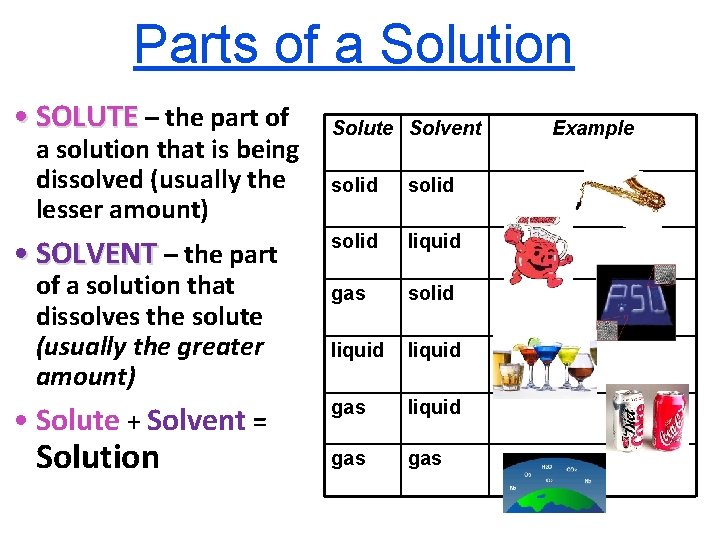
Parts of a Solution • SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) • SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) • Solute + Solvent = Solution Solute Solvent solid liquid gas solid liquid gas Example

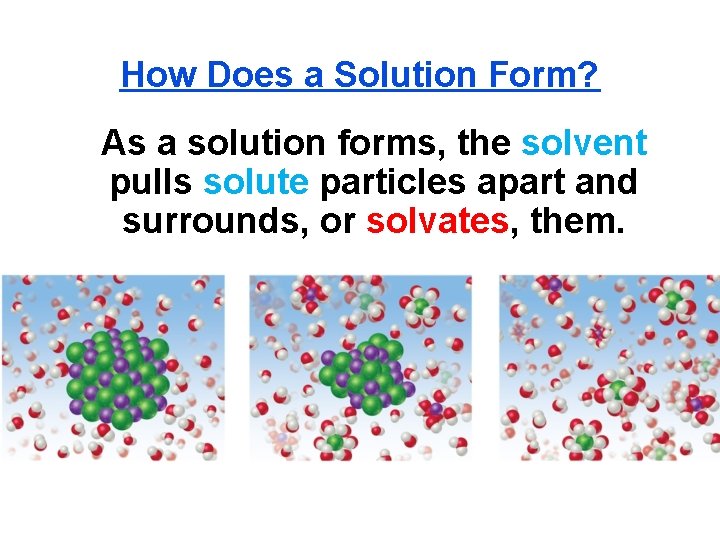
How Does a Solution Form? As a solution forms, the solvent pulls solute particles apart and surrounds, or solvates, them.


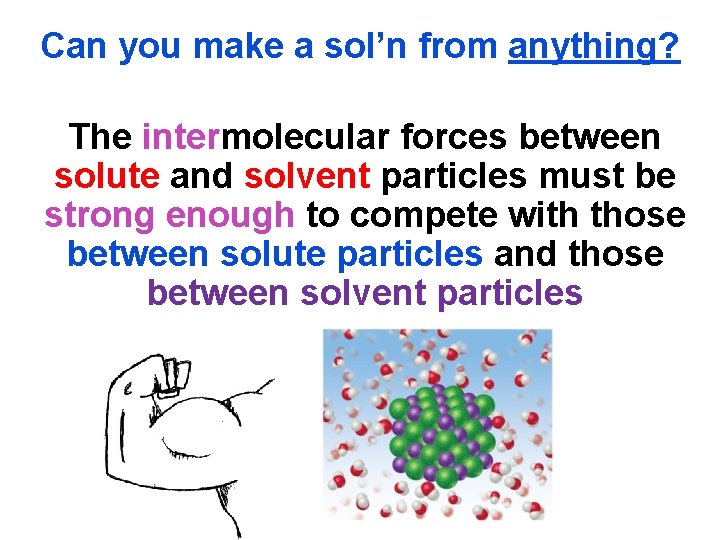
Can you make a sol’n from anything? The intermolecular forces between solute and solvent particles must be strong enough to compete with those between solute particles and those between solvent particles

“LIKE DISSOLVES LIKE” Substances with similar types of intermolecular forces dissolve in each other When a solute dissolves in a solvent, solute interactions and solvent-solvent interactions are partly replaced with solutesolvent interactions. The new forces created between solute & solvent must be comparable in strength to the forces destroyed within the solute& the solvent


Student, Beware! Just because a substance disappears when it comes in contact with a solvent, it doesn’t mean the substance dissolved

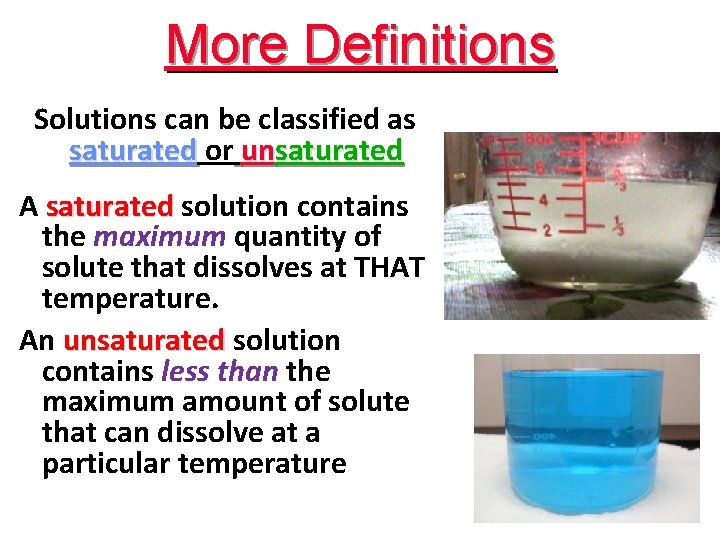
More Definitions Solutions can be classified as saturated or unsaturated A saturated solution contains the maximum quantity of solute that dissolves at THAT temperature. An unsaturated solution contains less than the maximum amount of solute that can dissolve at a particular temperature

Example: Saturated and Unsaturated Fats Saturated fats are called saturated because all of the bonds between the carbon atoms in a fat are single bonds. Thus, all the bonds on the carbon are occupied or “saturated” with hydrogen. These are stable and hard to decompose. The body can only use these for energy, and so the excess is stored. Thus, these should be avoided in diets. These are usually obtained from sheep and cattle fats. Butter and coconut oil are mostly saturated fats. Unsaturated fats have at least one double bond between carbon atoms; monounsaturated means there is one double bond, polysaturated means there are more than one double bond. Thus, there are some bonds that can be broken, chemically changed, and used for a variety of purposes. These are REQUIRED to carry out many functions in the body. Fish oils (fats) are usually unsaturated. Game animals (chicken, deer) are usually less saturated, but not as much as fish. Olive and canola oil are monounsaturated.

MORE Definitions SUPERSATURATED SOLUTIONS contain more solute than is possible to be dissolved Supersaturated solutions are unstable. The supersaturation is only temporary, and usually accomplished in one of two ways: 1. Warm the solvent so that it will dissolve more, then cool the solution 2. Evaporate some of the solvent carefully so that the solute does not solidify and come out of solution

Supersaturated ØSolvent holds more solute than is normally possible at that temperature. ØThese solutions are unstable; crystallization can usually be stimulated by adding a “seed crystal” or scratching the side of the flask.

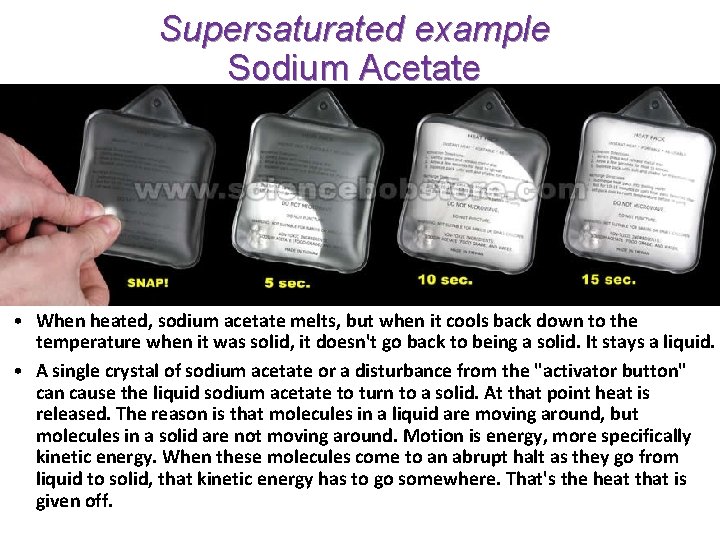
Supersaturated example Sodium Acetate • When heated, sodium acetate melts, but when it cools back down to the temperature when it was solid, it doesn't go back to being a solid. It stays a liquid. • A single crystal of sodium acetate or a disturbance from the "activator button" can cause the liquid sodium acetate to turn to a solid. At that point heat is released. The reason is that molecules in a liquid are moving around, but molecules in a solid are not moving around. Motion is energy, more specifically kinetic energy. When these molecules come to an abrupt halt as they go from liquid to solid, that kinetic energy has to go somewhere. That's the heat that is given off.


IONIC COMPOUNDS Compounds in Aqueous Solution Many reactions involve ionic compounds. When a substance is dissolved in water, it is called an aqueous sol’n (aq) KMn. O 4 in water K+(aq) + Mn. O 4 -(aq)

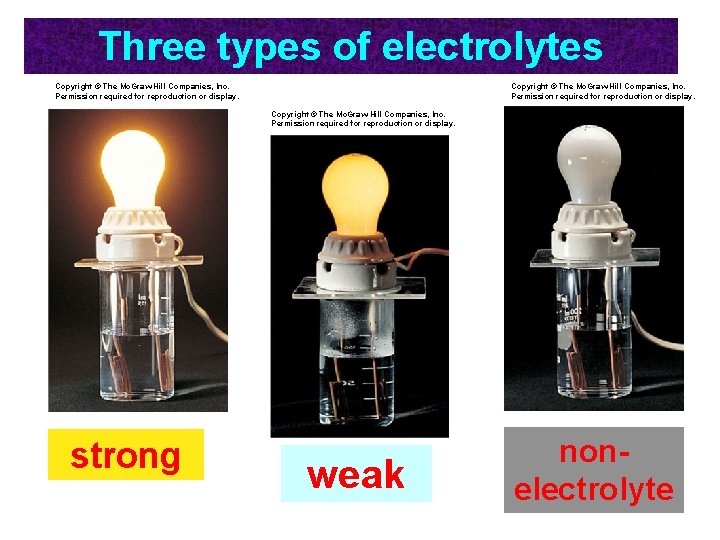
Aqueous Solutions How do we know ions are present in aqueous solutions? The solutions conduct electricity. They are called ELECTROLYTES HCl, Mg. Cl 2, and Na. Cl are strong electrolytes. They dissociate completely (or nearly so) into ions.

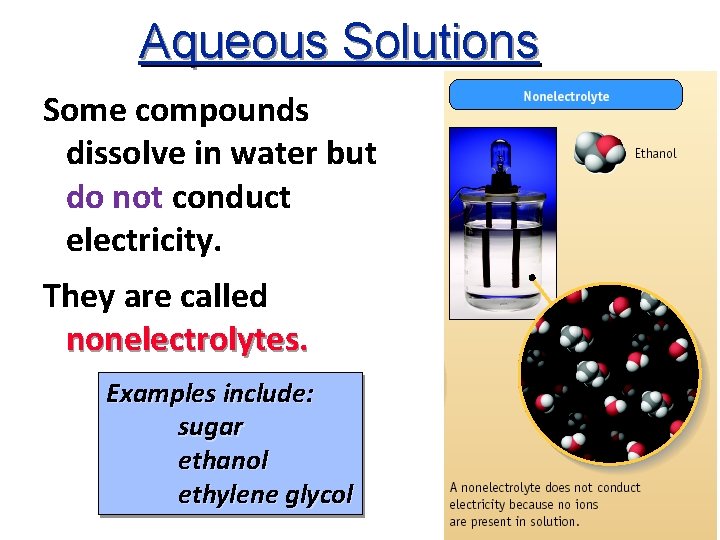
Aqueous Solutions Some compounds dissolve in water but do not conduct electricity. They are called nonelectrolytes. Examples include: sugar ethanol ethylene glycol

Three types of electrolytes Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. strong weak nonelectrolyte

Electrolytes in the Body Carry messages to and from the brain as electrical signals Maintain cellular function with the correct concentrations electrolytes Make your own Electrolyte 50 -70 g sugar One liter of warm water Pinch of salt 200 ml of sugar free fruit squash Mix, cool, and drink

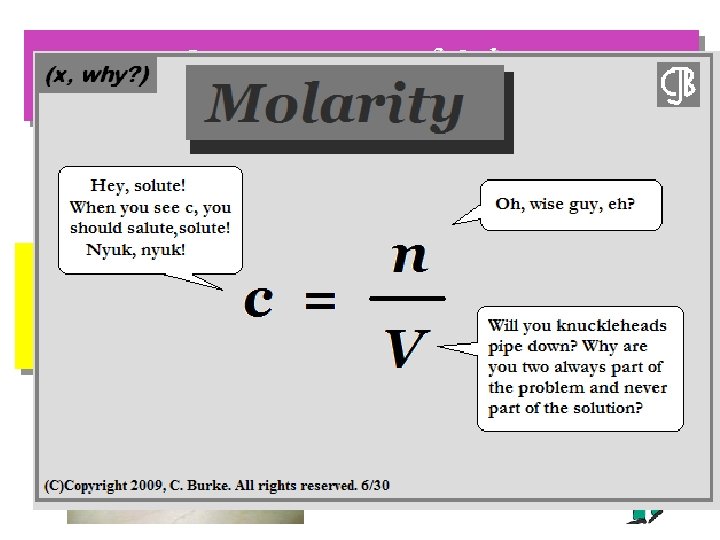
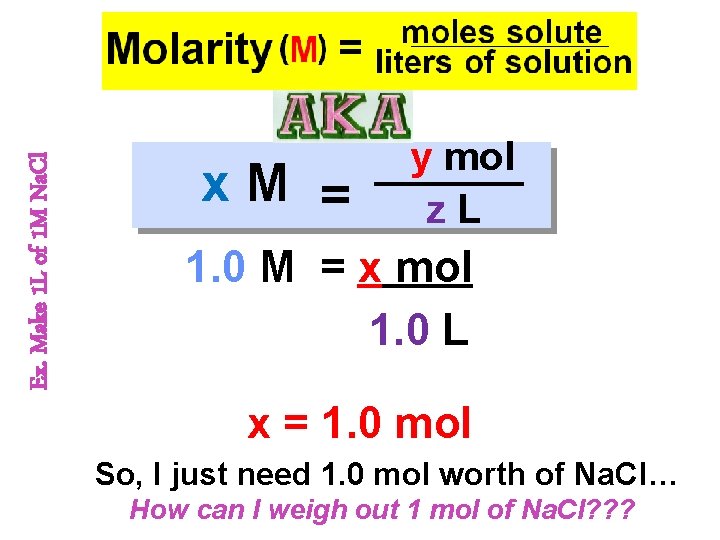
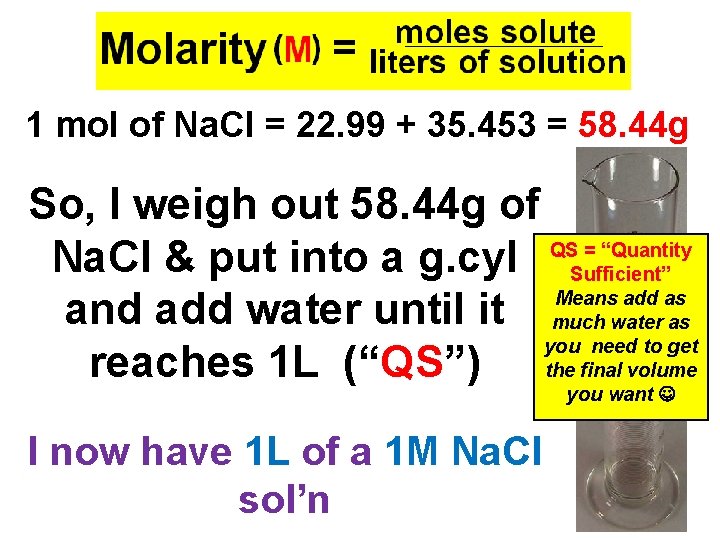
Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution


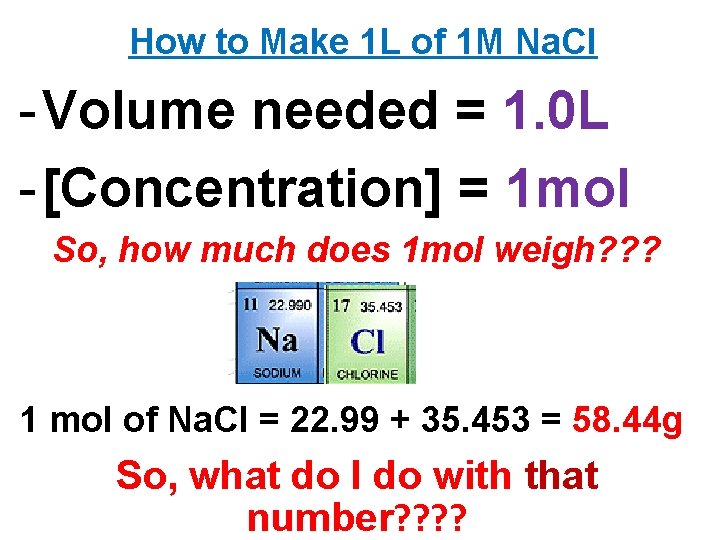
Making a Molar Solution So, you want to make a molar sol’n? INFORMATION YOU NEED TO MAKE SOL’N - Volume needed (in m. L or L) - Concentration needed (in mol) - Periodic Table & Calculator Ex. Make 1 L of 1 M Na. Cl

Ex. Make 1 L of 1 M Na. Cl x. M = y mol z. L 1. 0 M = x mol 1. 0 L x = 1. 0 mol So, I just need 1. 0 mol worth of Na. Cl… How can I weigh out 1 mol of Na. Cl? ? ?

How to Make 1 L of 1 M Na. Cl - Volume needed = 1. 0 L - [Concentration] = 1 mol So, how much does 1 mol weigh? ? ? 1 mol of Na. Cl = 22. 99 + 35. 453 = 58. 44 g So, what do I do with that number? ?

1 mol of Na. Cl = 22. 99 + 35. 453 = 58. 44 g So, I weigh out 58. 44 g of = “Quantity Na. Cl & put into a g. cyl QSSufficient” Means add as and add water until it much water as you need to get reaches 1 L (“QS”) the final volume you want I now have 1 L of a 1 M Na. Cl sol’n

1. 0 L of water was used to make 1. 0 L of solution. Notice the water left over.

What if I want 0. 5 L of 1 M Na. Cl? x. M = 1 M = y mol 0. 5 L y mol z. L y = 0. 5 mol (58. 44 g/mol) = 29. 22 g Na. Cl

x. M = y mol z. L So, I weigh out 29. 22 g of Na. Cl & put into a g. cyl and add water until it reaches 0. 5 L (“QS”) I now have 0. 5 L of a 1 M Na. Cl sol’n

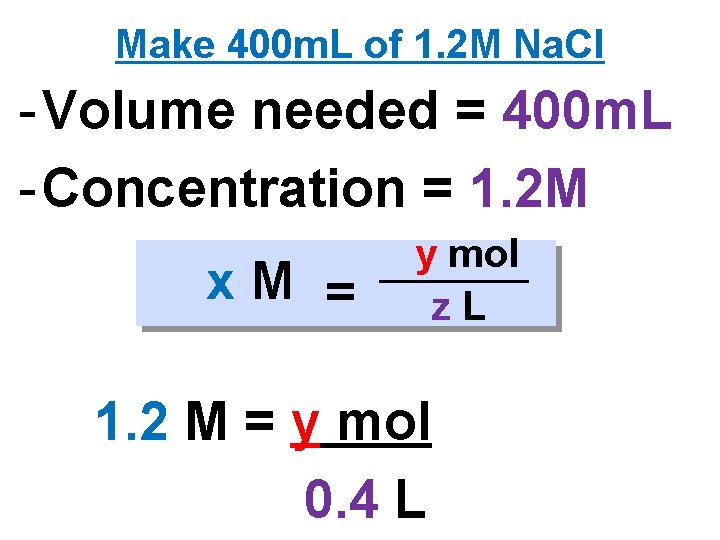
Make 400 m. L of 1. 2 M Na. Cl - Volume needed = 400 m. L - Concentration = 1. 2 M x. M = y mol z. L 1. 2 M = y mol 0. 4 L

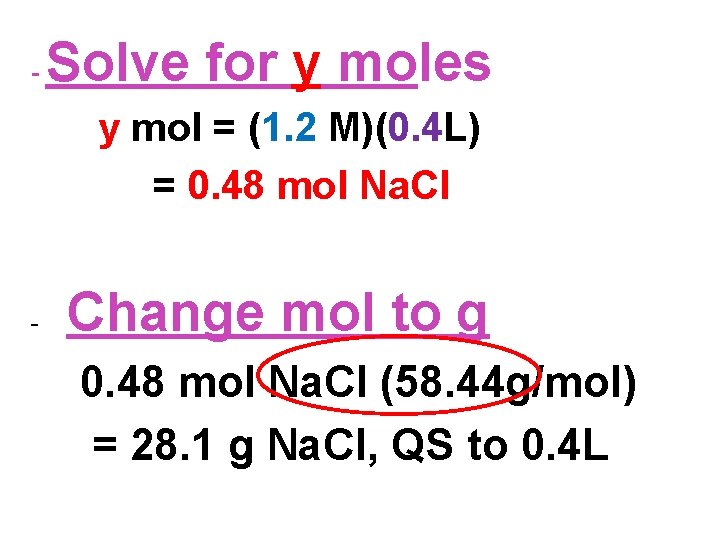
- Solve for y moles y mol = (1. 2 M)(0. 4 L) = 0. 48 mol Na. Cl - Change mol to g 0. 48 mol Na. Cl (58. 44 g/mol) = 28. 1 g Na. Cl, QS to 0. 4 L

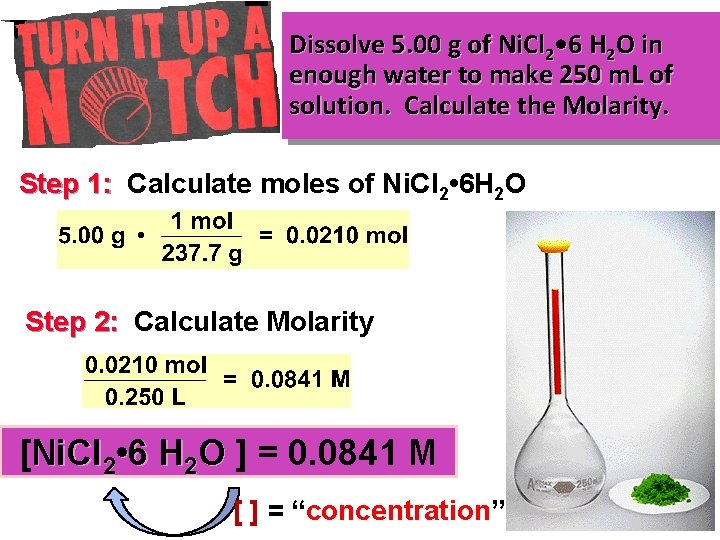
Dissolve 5. 00 g of Ni. Cl 2 • 6 H 2 O in enough water to make 250 m. L of solution. Calculate the Molarity. Step 1: Calculate moles of Ni. Cl 2 • 6 H 2 O Step 2: Calculate Molarity [Ni. Cl 2 • 6 H 2 O ] = 0. 0841 M [ ] = “concentration”

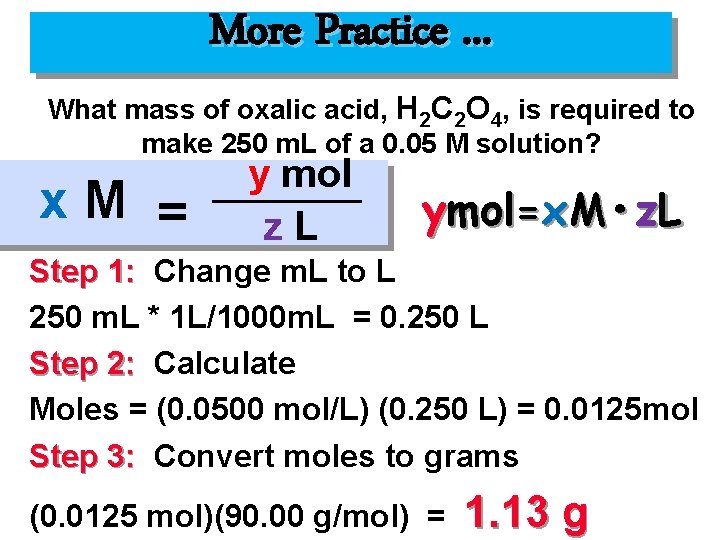
More Practice. . . What mass of oxalic acid, H 2 C 2 O 4, is required to make 250 m. L of a 0. 05 M solution? x. M = y mol z. L ymol=x. M • z. L Step 1: Change m. L to L 250 m. L * 1 L/1000 m. L = 0. 250 L Step 2: Calculate Moles = (0. 0500 mol/L) (0. 250 L) = 0. 0125 mol Step 3: Convert moles to grams (0. 0125 mol)(90. 00 g/mol) = 1. 13 g


Colligative Properties After you add a solute to a solvent, the properties of the solvent are changed: • Vapor pressure decreases • Melting point decreases • Boiling point increases These changes are called COLLIGATIVE PROPERTIES They depend only on the NUMBER of solute particles relative to solvent particles, not on the KIND of solute particles.


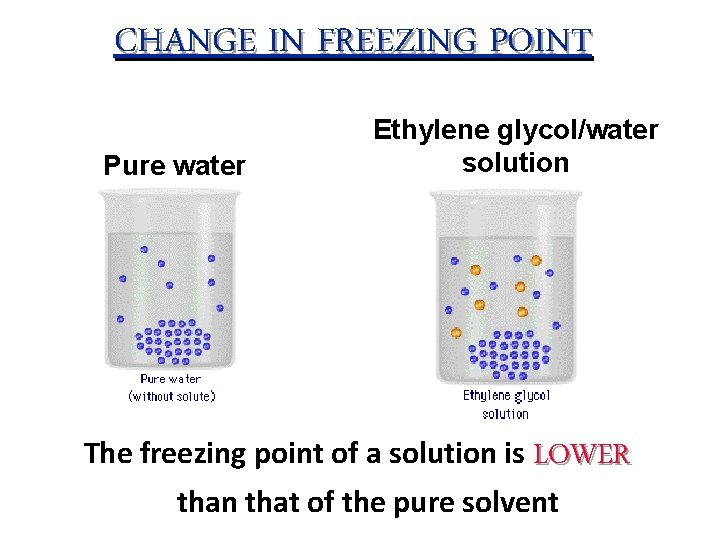
CHANGE IN FREEZING POINT Pure water Ethylene glycol/water solution The freezing point of a solution is LOWER than that of the pure solvent

CHANGE IN FREEZING POINT Common Applications of Freezing Point Depression Propylene glycol Ethylene glycol – deadly to small animals

Change in Boiling Point Common Applications of Boiling Point Elevation

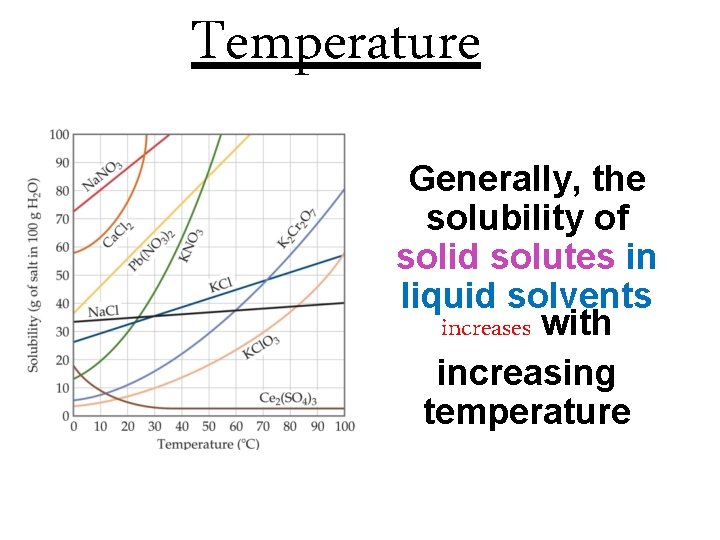
Temperature Generally, the solubility of solid solutes in liquid solvents increases with increasing temperature

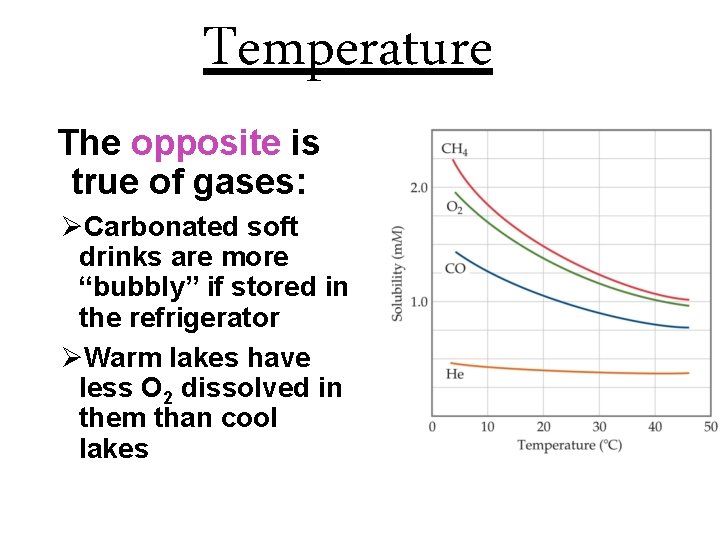
Temperature The opposite is true of gases: ØCarbonated soft drinks are more “bubbly” if stored in the refrigerator ØWarm lakes have less O 2 dissolved in them than cool lakes

SUSPENSIONS - Mixtures with large particles - NOT a solution (The solvent does NOT dissolve the solute) - Usually “murky” or “opaque” - Separate on standing

COLLOIDS - Mixtures that contain “clumps” of molecules (still too small to be seen by the naked eye) - Often look “cloudy” - Do NOT separate upon standing

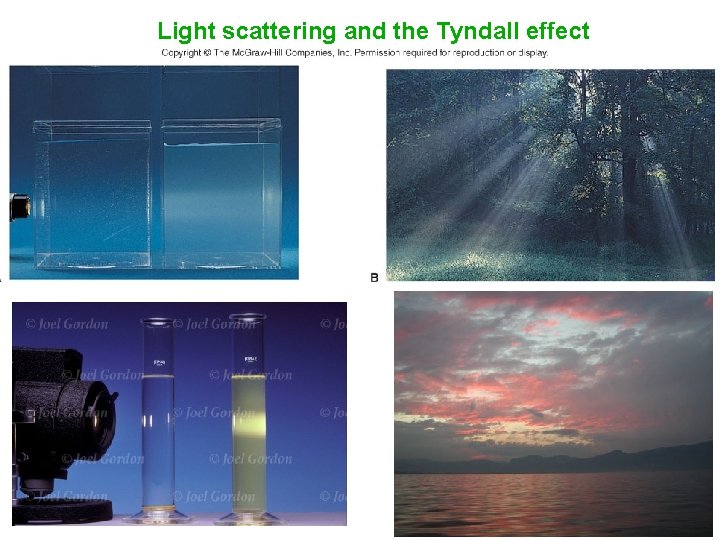
Tyndall Effect • Colloidal suspensions can scatter rays of light • This phenomenon is known as the Tyndall effect

Light scattering and the Tyndall effect

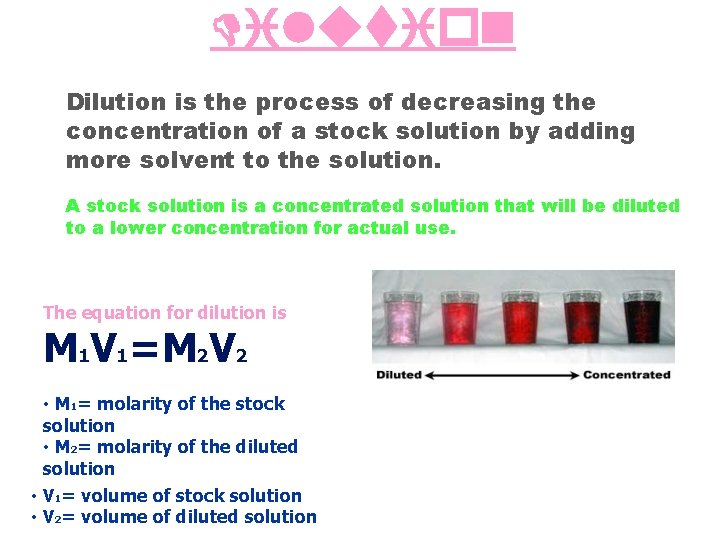
Dilution is the process of decreasing the concentration of a stock solution by adding more solvent to the solution. A stock solution is a concentrated solution that will be diluted to a lower concentration for actual use. The equation for dilution is M 1 V 1=M 2 V 2 • M 1= molarity of the stock solution • M 2= molarity of the diluted solution • V 1= volume of stock solution • V 2= volume of diluted solution

Dilution Example A stock solution of 1. 00 M of Na. Cl is available. How many milliliters are needed to make a 100. 0 m. L of 0. 750 M? What we know: the molarity of the stock solution which is 1. 00 M, and the two components of the diluted solution which are M 2= 0. 750 M and V 2= 100 m. L. M 1 V 1=M 2 V 2
 Insidan region jh
Insidan region jh Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Solutions are homogeneous mixtures
Solutions are homogeneous mixtures Homogeneous and non homogeneous differential equation
Homogeneous and non homogeneous differential equation Mixture
Mixture Mixture examples
Mixture examples Common homogeneous mixtures
Common homogeneous mixtures Facts about homogeneous mixtures
Facts about homogeneous mixtures Are solutions homogeneous
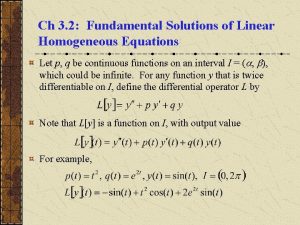
Are solutions homogeneous Fundamental solutions of linear homogeneous equations
Fundamental solutions of linear homogeneous equations Are solutions homogeneous
Are solutions homogeneous Homogeneous mixture is a solution
Homogeneous mixture is a solution Fundamental solutions of linear homogeneous equations
Fundamental solutions of linear homogeneous equations Mixture vs solution
Mixture vs solution Chapter 14 mixtures and solutions answer key
Chapter 14 mixtures and solutions answer key Chapter 13 mixtures and solutions answers
Chapter 13 mixtures and solutions answers Chapter 14 mixtures and solutions
Chapter 14 mixtures and solutions Mixtures solubility and acid/base solutions answer key
Mixtures solubility and acid/base solutions answer key Mixtures worksheet
Mixtures worksheet Fossweb mixtures and solutions
Fossweb mixtures and solutions Mixtures and solutions grade 7
Mixtures and solutions grade 7 Mixtures and solutions quiz
Mixtures and solutions quiz Solutions as special mixtures grade 6
Solutions as special mixtures grade 6 Solutions are heterogenous mixtures
Solutions are heterogenous mixtures Gently combine two mixtures
Gently combine two mixtures Orthographic projection system
Orthographic projection system Is chunky peanut butter homogeneous or heterogeneous
Is chunky peanut butter homogeneous or heterogeneous Recurrence relation
Recurrence relation Homogeneous solution
Homogeneous solution Homogeneous solution
Homogeneous solution A homogeneous mixture of a solute and solvent
A homogeneous mixture of a solute and solvent Homogeneous differential equation
Homogeneous differential equation How to separate heterogeneous mixtures
How to separate heterogeneous mixtures Type of differential equation
Type of differential equation Sampling techniques for qualitative research
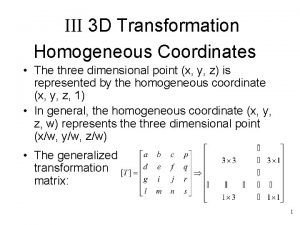
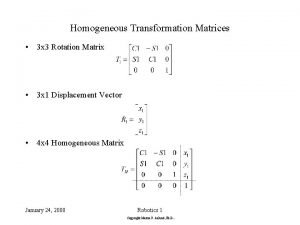
Sampling techniques for qualitative research Homogeneous transformation matrix
Homogeneous transformation matrix Homogeneous vs heterogeneous reactions
Homogeneous vs heterogeneous reactions Is mouthwash a heterogeneous mixture
Is mouthwash a heterogeneous mixture Homogenous countries
Homogenous countries Hetgnn
Hetgnn Uniform in composition
Uniform in composition The composition is uniform
The composition is uniform Volume solid liquid gas
Volume solid liquid gas Homogeneous transformation matrix
Homogeneous transformation matrix Homogeneous coordinates
Homogeneous coordinates Homogeneous transformation
Homogeneous transformation