Solutions What are solutions Solution a homogeneous mixture






- Slides: 7

Solutions

What are solutions? • Solution – a homogeneous mixture of two or more substances in a single phase. • Solutions contain two components: a solute and a solvent. • Solute – the substance dissolved in a solution. • Solvent – the dissolving medium in a solution.

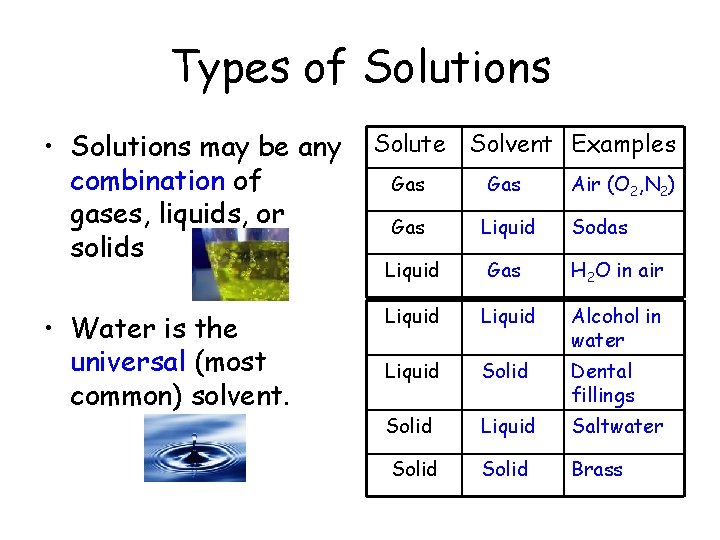
Types of Solutions • Solutions may be any combination of gases, liquids, or solids • Water is the universal (most common) solvent. Solute Solvent Examples Gas Air (O 2, N 2) Gas Liquid Sodas Liquid Gas H 2 O in air Liquid Alcohol in water Liquid Solid Dental fillings Solid Liquid Saltwater Solid Brass

Other Mixtures • Suspension – a heterogeneous mixture where the particles in the solvent are so large that they settle out unless the mixture is constantly stirred. • Colloid – a mixture in which particles of intermediate size do not settle in a mixture.

Electrolytes • Electrolyte – a substance that dissolves in water to give a solution that conducts electric current. • Nonelectrolyte – a substance that dissolves in water to give a solution that does not conduct an electric current.

Factors That Affect Solubility • Surface area – because the dissolving process happens at the surface area of a solute, crushing or grinding the solute speeds up the rate of dissolution. • Agitation – stirring or shaking helps spread out the solute particles which increases the rate of dissolution. • Heating – speeds up the particle motion therefore helping it dissolve the solute. • Pressure – changes how gases dissolve in liquids.

Saturation • Saturated solution – a solution that contains the maximum amount of dissolved solute. • Supersaturated solution – a solution that contains more dissolved solute than a saturated solution, eventually excess solute will precipitate out. • Unsaturated solution – a solution that does not have the maximum amount of dissolved solute.