Solutions Solution Solution A homogeneous mixture in which










![The p. H Scale p. H = log (1/ [H+]) = - log [H+] The p. H Scale p. H = log (1/ [H+]) = - log [H+]](https://slidetodoc.com/presentation_image_h/d517205590dc54fd56b8855defc8f534/image-31.jpg)



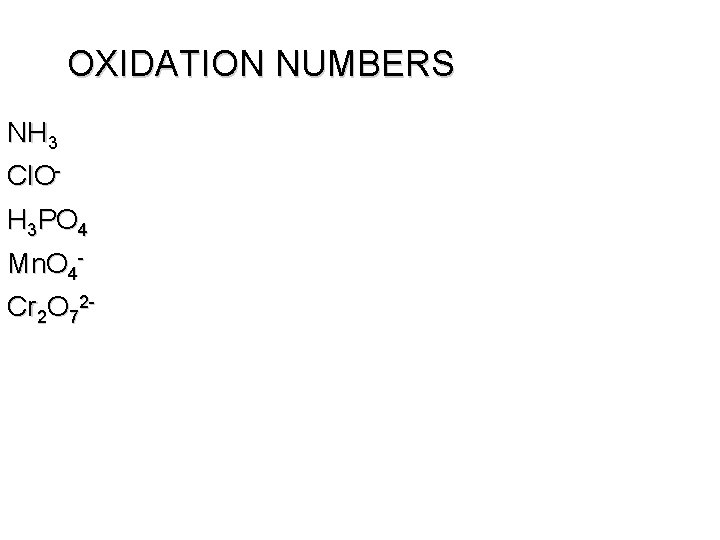


![Hess’s Law • • For example, we can calculate the Ho for reaction [1] Hess’s Law • • For example, we can calculate the Ho for reaction [1]](https://slidetodoc.com/presentation_image_h/d517205590dc54fd56b8855defc8f534/image-77.jpg)





- Slides: 111

Solutions – Solution, Solution A homogeneous mixture in which all of the material is in the same state. – Substances present in lesser amounts, called solutes, solutes are dispersed uniformly throughout the substance in the greater amount, the solvent – Aqueous solution — a solution in which the solvent is water – Nonaqueous solution — any substance other than water is the solvent – Many of the chemical reactions that are essential for life depend on the interaction of water molecules with dissolved compounds.

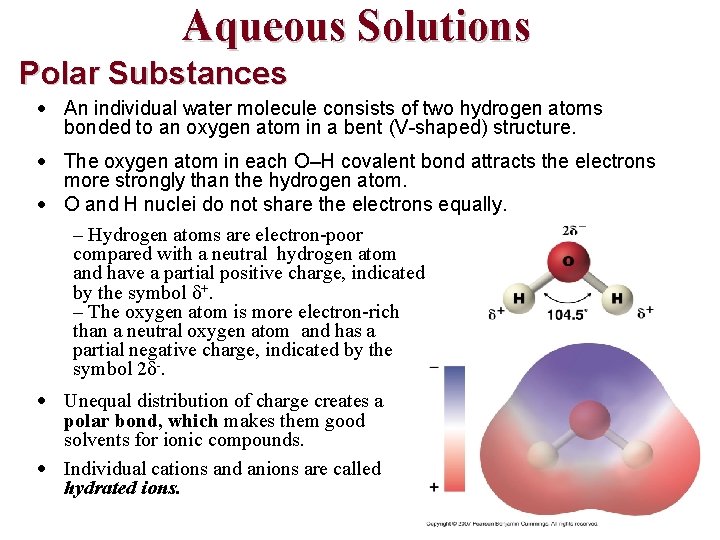
Aqueous Solutions Polar Substances An individual water molecule consists of two hydrogen atoms bonded to an oxygen atom in a bent (V-shaped) structure. The oxygen atom in each O–H covalent bond attracts the electrons more strongly than the hydrogen atom. O and H nuclei do not share the electrons equally. – Hydrogen atoms are electron-poor compared with a neutral hydrogen atom and have a partial positive charge, indicated by the symbol δ+. – The oxygen atom is more electron-rich than a neutral oxygen atom and has a partial negative charge, indicated by the symbol 2δ-. Unequal distribution of charge creates a polar bond, which makes them good solvents for ionic compounds. Individual cations and anions are called hydrated ions.

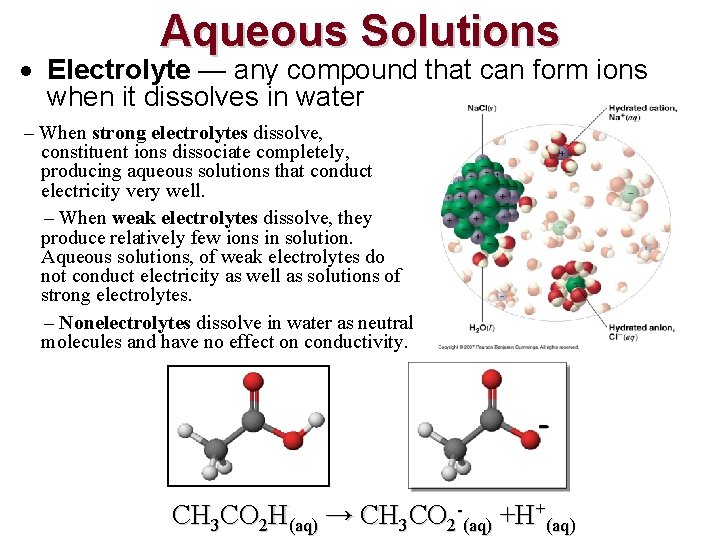
Aqueous Solutions Electrolyte — any compound that can form ions when it dissolves in water – When strong electrolytes dissolve, constituent ions dissociate completely, producing aqueous solutions that conduct electricity very well. – When weak electrolytes dissolve, they produce relatively few ions in solution. Aqueous solutions, of weak electrolytes do not conduct electricity as well as solutions of strong electrolytes. – Nonelectrolytes dissolve in water as neutral molecules and have no effect on conductivity. CH 3 CO 2 H(aq) → CH 3 CO 2 -(aq) +H+(aq)

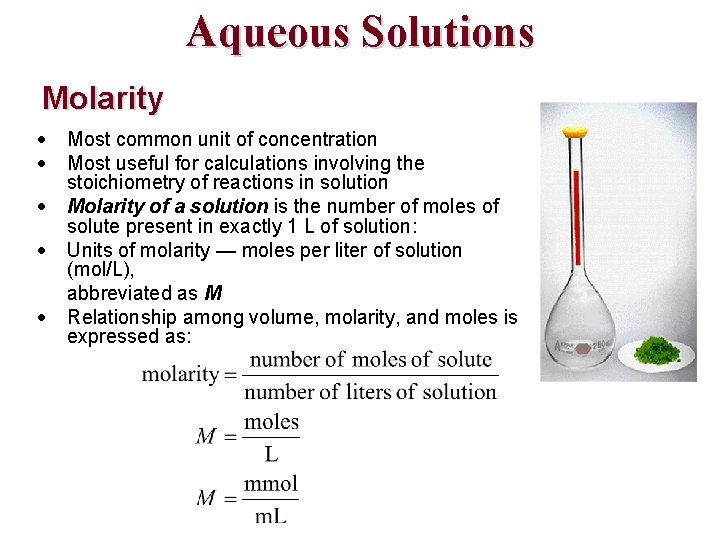
Aqueous Solutions Molarity Most common unit of concentration Most useful for calculations involving the stoichiometry of reactions in solution Molarity of a solution is the number of moles of solute present in exactly 1 L of solution: Units of molarity — moles per liter of solution (mol/L), abbreviated as M Relationship among volume, molarity, and moles is expressed as:

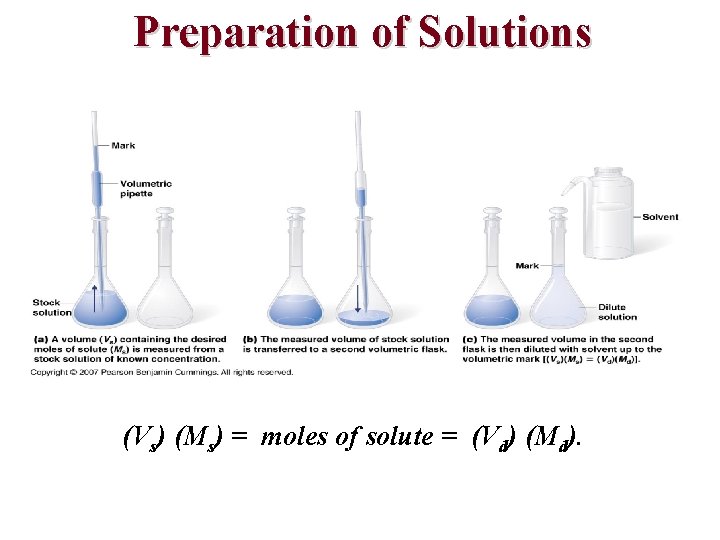
Preparation of Solutions

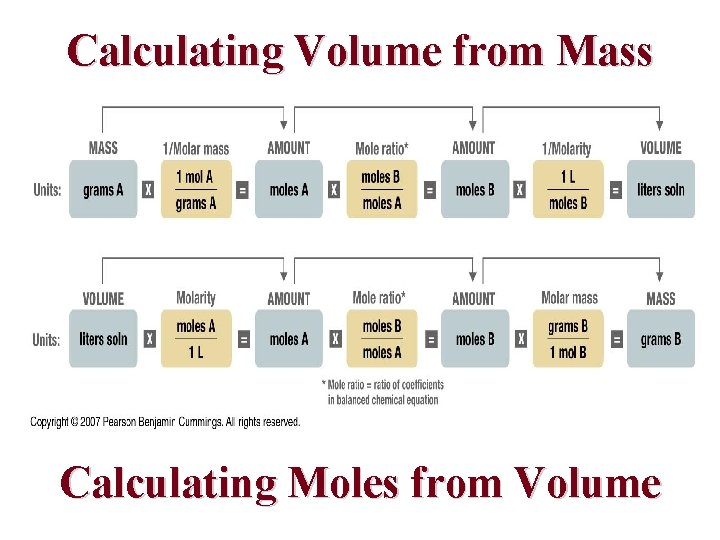
Calculating Volume from Mass Calculating Moles from Volume

Preparation of Solutions Problem: What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution?

Preparation of Solutions 0. 250 L of water was used to make 0. 250 L of solution. Notice the water left over.

Preparation of Solutions (Vs) (Ms) = moles of solute = (Vd) (Md).

Preparation of Solutions PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do?

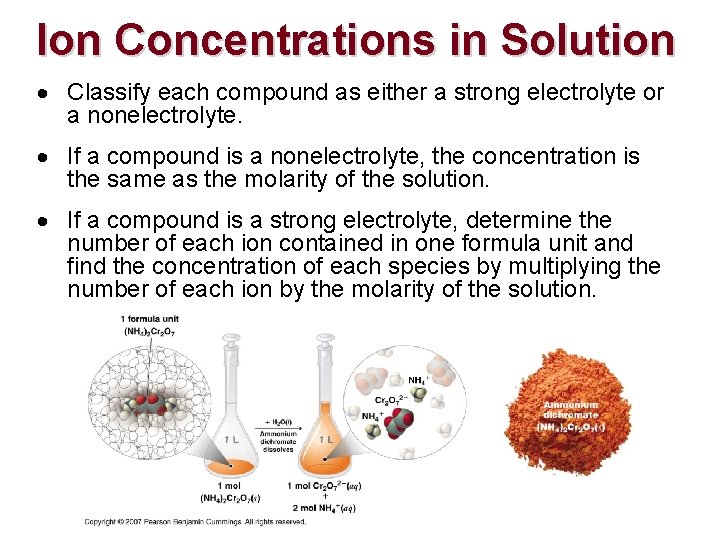
Ion Concentrations in Solution Classify each compound as either a strong electrolyte or a nonelectrolyte. If a compound is a nonelectrolyte, the concentration is the same as the molarity of the solution. If a compound is a strong electrolyte, determine the number of each ion contained in one formula unit and find the concentration of each species by multiplying the number of each ion by the molarity of the solution.

Preparation of Solutions PROBLEM: Dissolve 5. 00 g of Ni. Cl 2 • 6 H 2 O in enough water to make 250 m. L of solution. Calculate molarity of the solution and the concentration of each of the ions.

SOLUTION STOICHIOMETRY • Zinc reacts with acids to produce H 2 gas. • Have 10. 0 g of Zn • What volume of 2. 50 M HCl is needed to convert the Zn completely?

SOLUTION STOICHIOMETRY Zinc reacts with acids to produce H 2 gas. If you have 10. 0 g of Zn, what volume of 2. 50 M HCl is needed to convert the Zn completely?

Limiting Reactants in Solutions The concept of limiting reactants applies to reactions that are carried out in solution and reactions that involve pure substances. If all the reactants but one are present in excess, then the amount of the limiting reactant can be calculated. When the limiting reactant is not known, one can determine which reactant is limiting by comparing the molar amounts of the reactants with their coefficients in the balanced chemical equation. Use volumes and concentrations of solutions of reactants to calculate the number of moles of reactants.

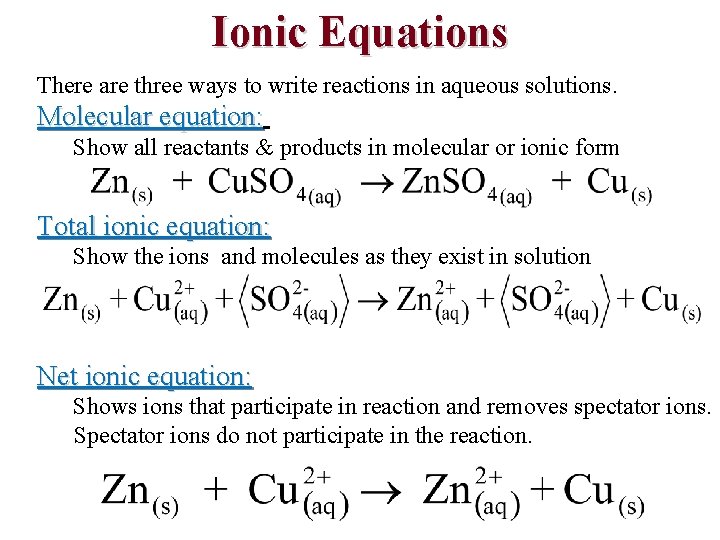
Ionic Equations • Chemical equation for a reaction in solution can be written in three ways: 1. Overall equation — shows all of the substances present in their undissociated form 2. Complete ionic equation — shows all of the substances present in the form in which they actually exist in solution 3. Net ionic equation – Derived from the complete ionic equation by omitting all spectator ions, ions that occur on both sides of the equation with the same coefficients – Demonstrate that many different combinations of reactants can give the same net chemical reaction

Ionic Equations There are three ways to write reactions in aqueous solutions. Molecular equation: Show all reactants & products in molecular or ionic form Total ionic equation: Show the ions and molecules as they exist in solution Net ionic equation: Shows ions that participate in reaction and removes spectator ions. Spectator ions do not participate in the reaction.

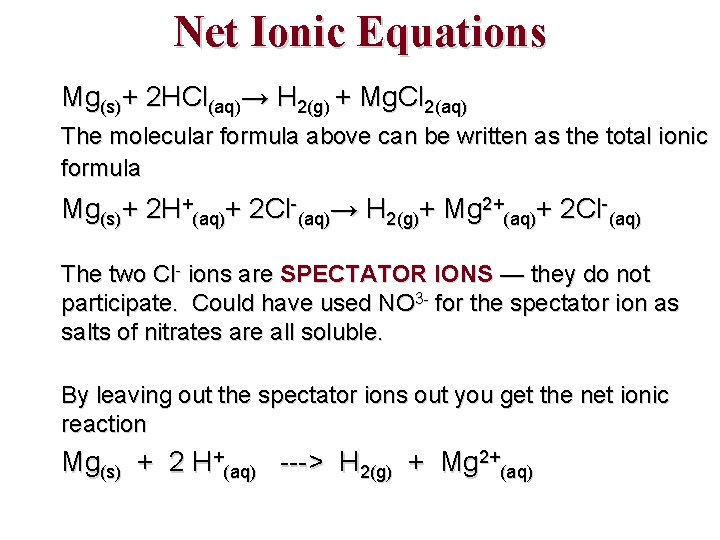
Net Ionic Equations Mg(s)+ 2 HCl(aq)→ H 2(g) + Mg. Cl 2(aq) The molecular formula above can be written as the total ionic formula Mg(s)+ 2 H+(aq)+ 2 Cl-(aq)→ H 2(g)+ Mg 2+(aq)+ 2 Cl-(aq) The two Cl- ions are SPECTATOR IONS — they do not participate. Could have used NO 3 - for the spectator ion as salts of nitrates are all soluble. By leaving out the spectator ions out you get the net ionic reaction Mg(s) + 2 H+(aq) ---> H 2(g) + Mg 2+(aq)

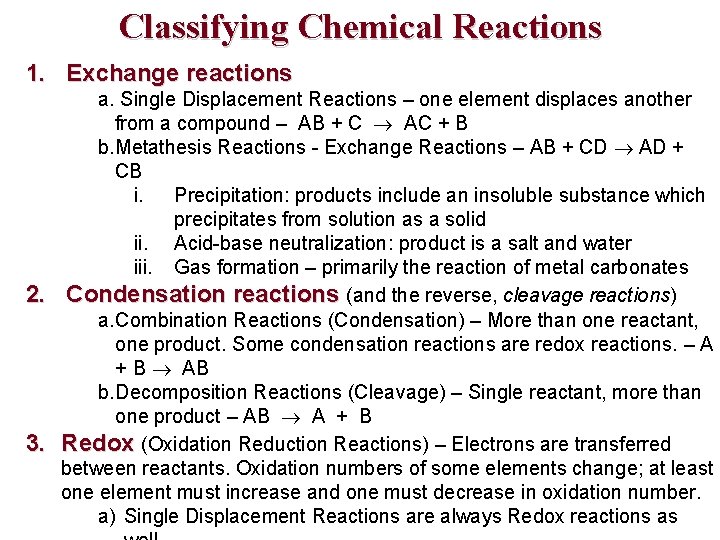
Classifying Chemical Reactions 1. Exchange reactions a. Single Displacement Reactions – one element displaces another from a compound – AB + C AC + B b. Metathesis Reactions - Exchange Reactions – AB + CD AD + CB i. Precipitation: products include an insoluble substance which precipitates from solution as a solid ii. Acid-base neutralization: product is a salt and water iii. Gas formation – primarily the reaction of metal carbonates 2. Condensation reactions (and the reverse, cleavage reactions) a. Combination Reactions (Condensation) – More than one reactant, one product. Some condensation reactions are redox reactions. – A + B AB b. Decomposition Reactions (Cleavage) – Single reactant, more than one product – AB A + B 3. Redox (Oxidation Reduction Reactions) – Electrons are transferred between reactants. Oxidation numbers of some elements change; at least one element must increase and one must decrease in oxidation number. a) Single Displacement Reactions are always Redox reactions as

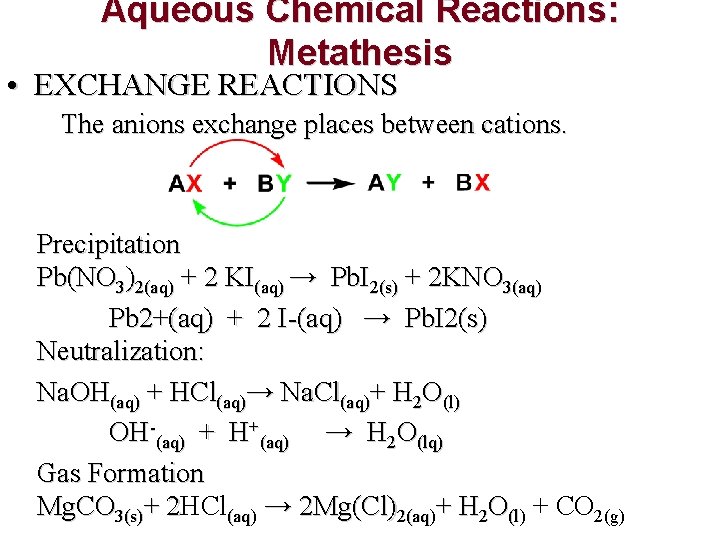
Aqueous Chemical Reactions: Metathesis • EXCHANGE REACTIONS The anions exchange places between cations. Precipitation Pb(NO 3)2(aq) + 2 KI(aq) → Pb. I 2(s) + 2 KNO 3(aq) Pb 2+(aq) + 2 I-(aq) → Pb. I 2(s) Neutralization: Na. OH(aq) + HCl(aq)→ Na. Cl(aq)+ H 2 O(l) OH-(aq) + H+(aq) → H 2 O(lq) Gas Formation Mg. CO 3(s)+ 2 HCl 2 (aq) → 2 Mg(Cl)2(aq)+ H 2 O(l) + CO 2(g)

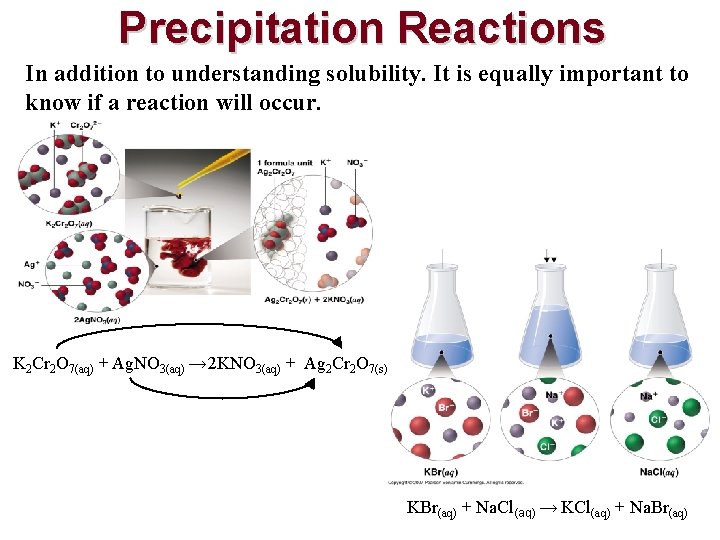
Precipitation Reactions • A reaction that yields an insoluble product, a precipitate, when two solutions are mixed • Are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble • Used to isolate metals that have been extracted from their ores and to recover precious metals for recycling

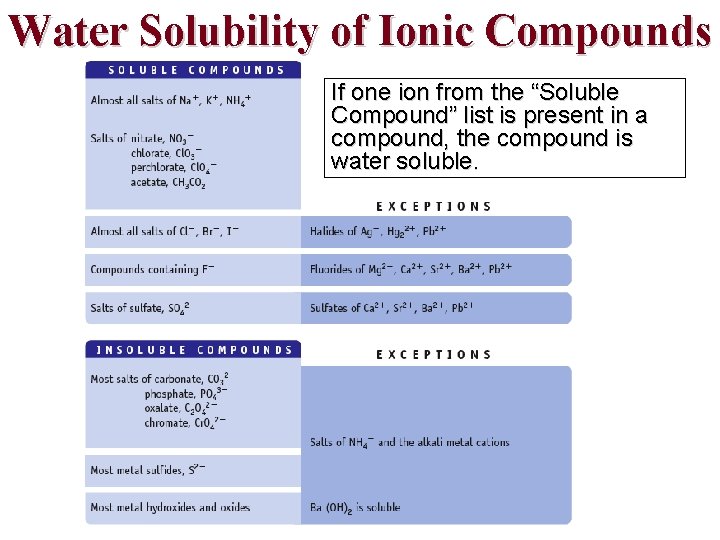
Water Solubility of Ionic Compounds If one ion from the “Soluble Compound” list is present in a compound, the compound is water soluble.

Precipitation Reactions In addition to understanding solubility. It is equally important to know if a reaction will occur. K 2 Cr 2 O 7(aq) + Ag. NO 3(aq) → 2 KNO 3(aq) + Ag 2 Cr 2 O 7(s) KBr(aq) + Na. Cl(aq) → KCl(aq) + Na. Br(aq)

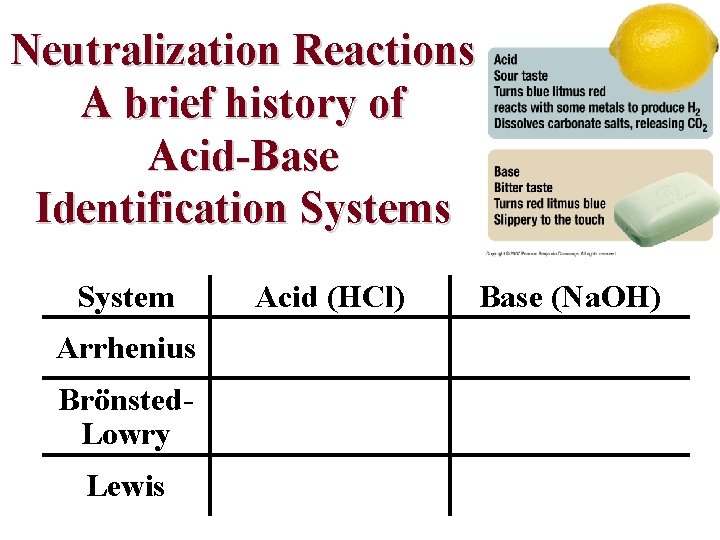
Neutralization Reactions A reaction in which an acid and a base react in stoichiometric amounts to produce water and a salt Strengths of the acid and base determine whether the reaction goes to completion 1. Reactions that go to completion a. Reaction of any strong acid with any strong base b. Reaction of a strong acid with a weak base c. Reaction of weak acid with a weak base 2. Reaction that does not go to completion is a reaction of a weak acid or a weak base with water

Neutralization Reactions A brief history of Acid-Base Identification Systems System Arrhenius Brönsted. Lowry Lewis Acid (HCl) Base (Na. OH)

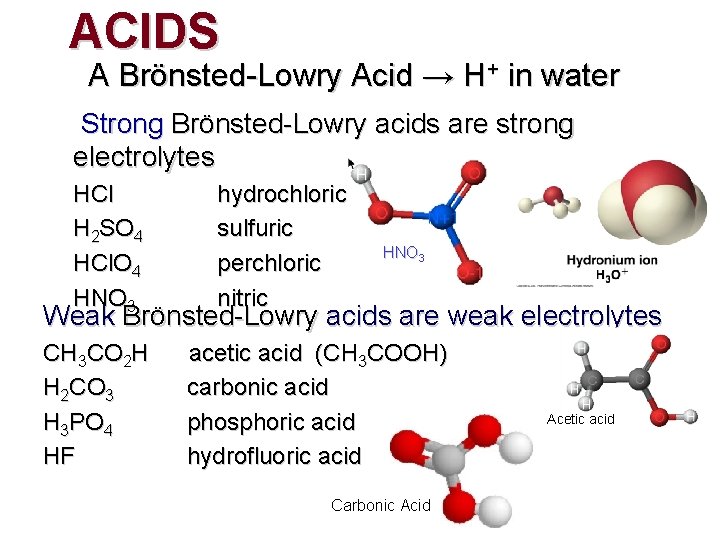
ACIDS A Brönsted-Lowry Acid → H+ in water Strong Brönsted-Lowry acids are strong electrolytes HCl H 2 SO 4 HCl. O 4 HNO 3 hydrochloric sulfuric perchloric nitric HNO 3 Weak Brönsted-Lowry acids are weak electrolytes CH 3 CO 2 H H 2 CO 3 H 3 PO 4 HF acetic acid (CH 3 COOH) carbonic acid phosphoric acid hydrofluoric acid Carbonic Acid Acetic acid

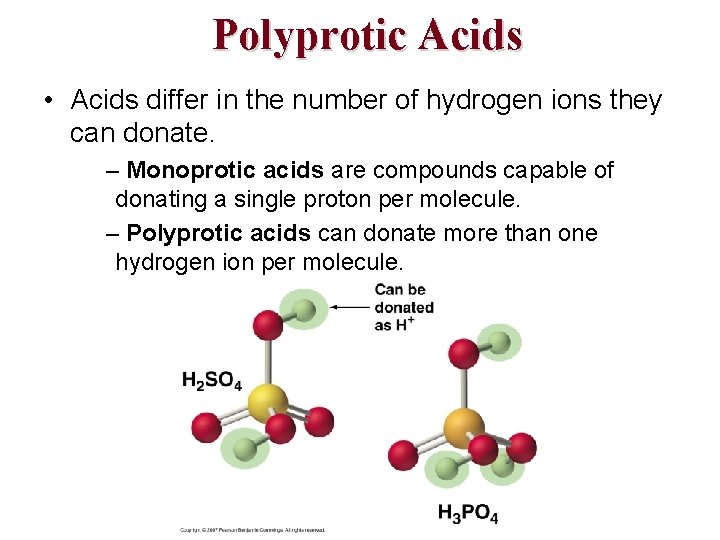
Polyprotic Acids • Acids differ in the number of hydrogen ions they can donate. – Monoprotic acids are compounds capable of donating a single proton per molecule. – Polyprotic acids can donate more than one hydrogen ion per molecule.

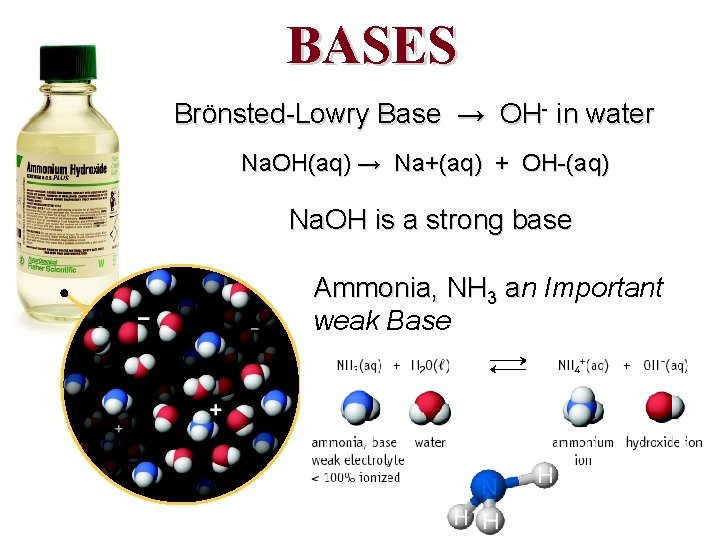
BASES Brönsted-Lowry Base → OH- in water Na. OH(aq) → Na+(aq) + OH-(aq) Na. OH is a strong base Ammonia, NH 3 an a Important weak Base

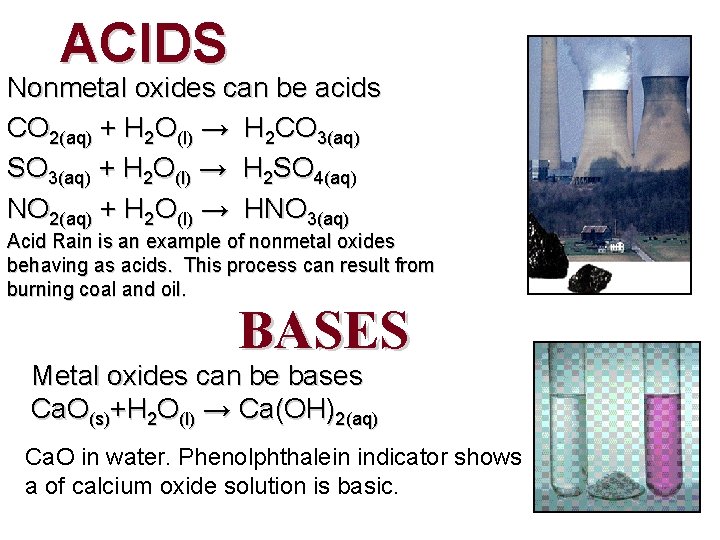
ACIDS Nonmetal oxides can be acids CO 2(aq) + H 2 O(l) → H 2 CO 3(aq) SO 3(aq) + H 2 O(l) → H 2 SO 4(aq) NO 2(aq) + H 2 O(l) → HNO 3(aq) Acid Rain is an example of nonmetal oxides behaving as acids. This process can result from burning coal and oil. BASES Metal oxides can be bases Ca. O(s)+H 2 O(l) → Ca(OH)2(aq) Ca. O in water. Phenolphthalein indicator shows a of calcium oxide solution is basic.

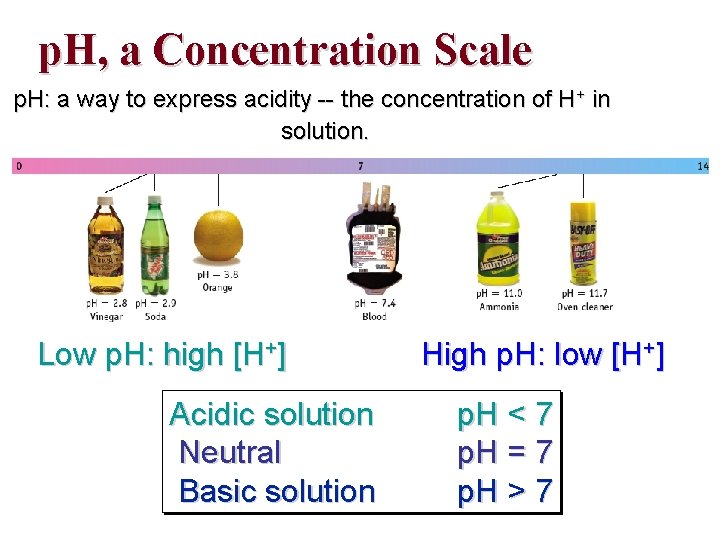
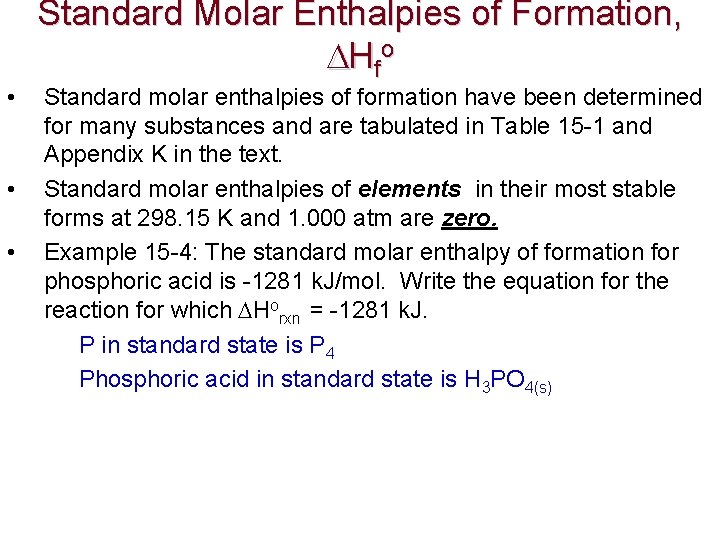
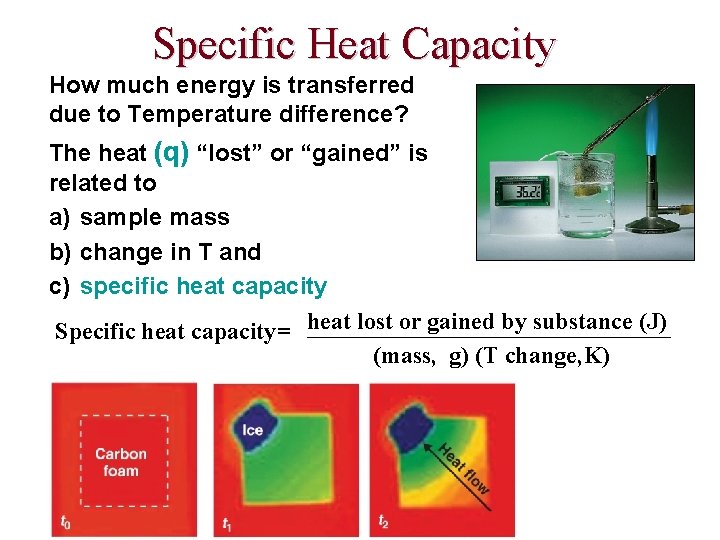
p. H, a Concentration Scale p. H: a way to express acidity -- the concentration of H+ in solution. Low p. H: high [H+] Acidic solution Neutral Basic solution High p. H: low [H+] p. H < 7 p. H = 7 p. H > 7
![The p H Scale p H log 1 H log H The p. H Scale p. H = log (1/ [H+]) = - log [H+]](https://slidetodoc.com/presentation_image_h/d517205590dc54fd56b8855defc8f534/image-31.jpg)
The p. H Scale p. H = log (1/ [H+]) = - log [H+] In a neutral solution, [H+] = [OH-] = 1. 00 x 10 -7 M at 25 o. C p. H = - log [H+] = If the [H+] of soda is 1. 6 x 10 -3 M, the p. H is ____. If the p. H of Coke is 3. 12, it is _____.

Acid-Base Strength Identification You should know the strong acids & bases

Oxidation-Reduction Reactions in Solution • Oxidation-reduction reactions — electrons are transferred from one substance or atom to another. • Oxidation-reduction reactions that occur in aqueous solution are complex, and their equations are very difficult to balance. • Two methods for balancing oxidation-reduction reactions in aqueous solution are: 1. Oxidation states — overall reaction is separated into an oxidation equation and a reduction equation 2. Half-reaction

Oxidation-Reduction Reactions The term oxidation was first used to describe reactions in which metals react with oxygen in air to produce metal oxides. – Metal acquires a positive charge by transferring electrons to the neutral oxygen atoms of an oxygen molecule. – Oxygen atoms acquire a negative charge and form oxide ions (O 2 -). – Metals lose electrons to oxygen and have been oxidized—oxidation is the loss of electrons. – Oxygen atoms have gained electrons and have been reduced—

Oxidation-Reduction Reactions • Oxidation and reduction reactions are now characterized by a change in the oxidation states of one or more elements in the reactants. • Oxidation states of each atom in a compound is the charge that atom would have if all of its bonding electrons were transferred to the atom with the greater attraction for electrons. Atoms in their elemental form are assigned an oxidation state of zero. • Oxidation-reduction reactions are called redox reactions, in which there is a net transfer of electrons from one reactant to another. The total number of electrons lost must equal the total number of electrons gained. • Oxidants and reductants – Oxidants – Compounds that are capable of accepting electrons are called oxidants, or oxidizing reagents, because they can oxidize other compounds. • An oxidant is reduced in the process of accepting electrons. – Reductants – Compounds that are capable of donating electrons are called reductants, or reducing agents, because they can cause the reduction of another compound.
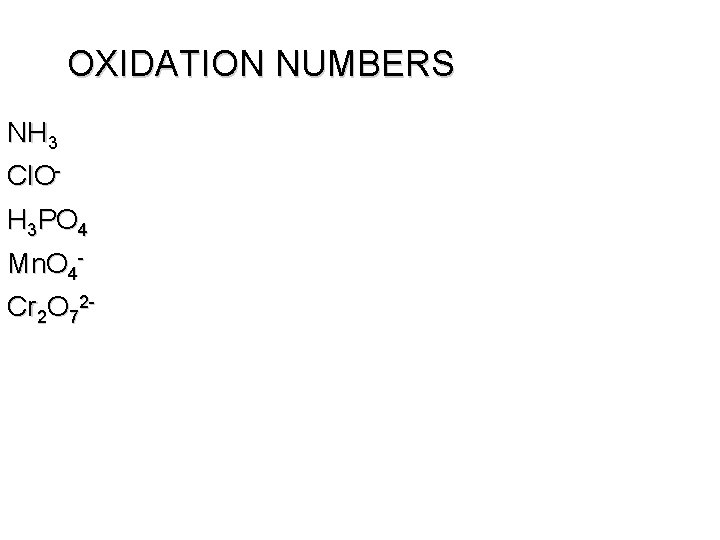
OXIDATION NUMBERS NH 3 Cl. OH 3 PO 4 Mn. O 4 Cr 2 O 72 -

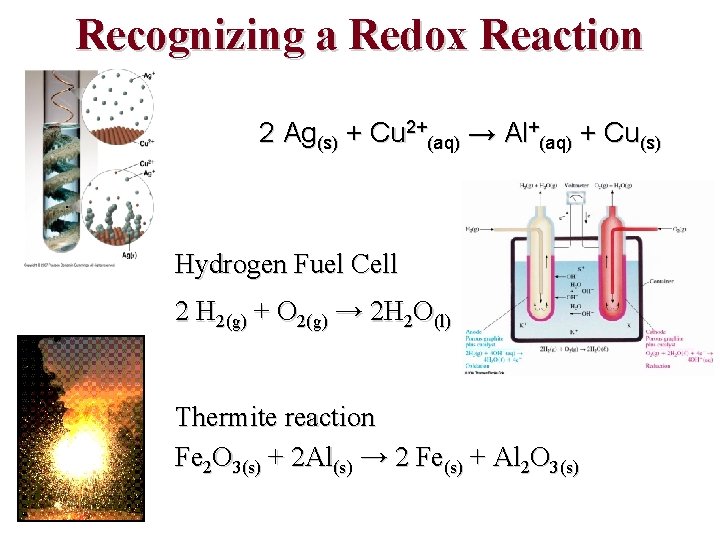
Recognizing a Redox Reaction 2 Ag(s) + Cu 2+(aq) → Al+(aq) + Cu(s) Hydrogen Fuel Cell 2 H 2(g) + O 2(g) → 2 H 2 O(l) Thermite reaction Fe 2 O 3(s) + 2 Al(s) → 2 Fe(s) + Al 2 O 3(s)

Balancing Redox Equations Using Oxidation States Will be covered in Chem 102

Quantitative Analysis: Titrations • Quantitative analysis — used to determine the amounts or concentrations of substances present in a sample by using a combination of chemical reactions and stoichiometric calculations • Titration – A method in which a measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a solution containing a compound whose concentration is to be determined (the unknown) – Reaction must be fast, complete, and specific (only the compound of interest should react with the titrant) – Equivalence point — point at which exactly enough reactant has been added for the reaction to go to completion (computed mathematically)

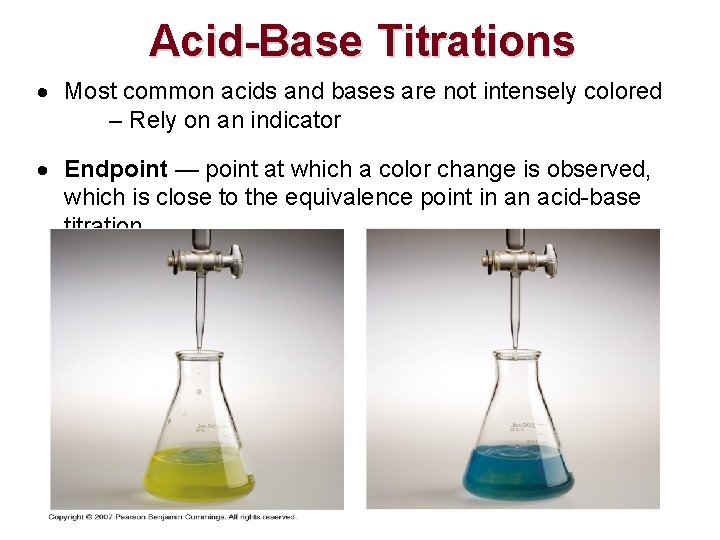
Acid-Base Titrations Most common acids and bases are not intensely colored – Rely on an indicator Endpoint — point at which a color change is observed, which is close to the equivalence point in an acid-base titration

Standard Solutions A solution of a primary standard whose concentration is known precisely A primary standard is non-hygroscopic, has a high mass, is fairly inexpensive compound, is of known reactive ability that can be accurately weighed for use as a titrant. A standard solution is used to determine the concentration of the titrant. Accuracy of any titration analysis depends on accurate knowledge of the concentration of the titrant. Most titrants are first standardized—their concentration is measured by titration with a standard solution.

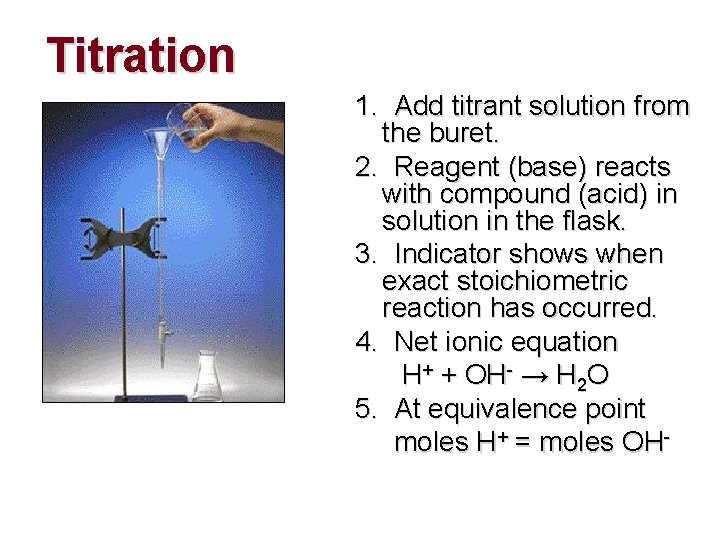
Titration 1. Add titrant solution from the buret. 2. Reagent (base) reacts with compound (acid) in solution in the flask. 3. Indicator shows when exact stoichiometric reaction has occurred. 4. Net ionic equation H+ + OH- → H 2 O 5. At equivalence point moles H+ = moles OH-

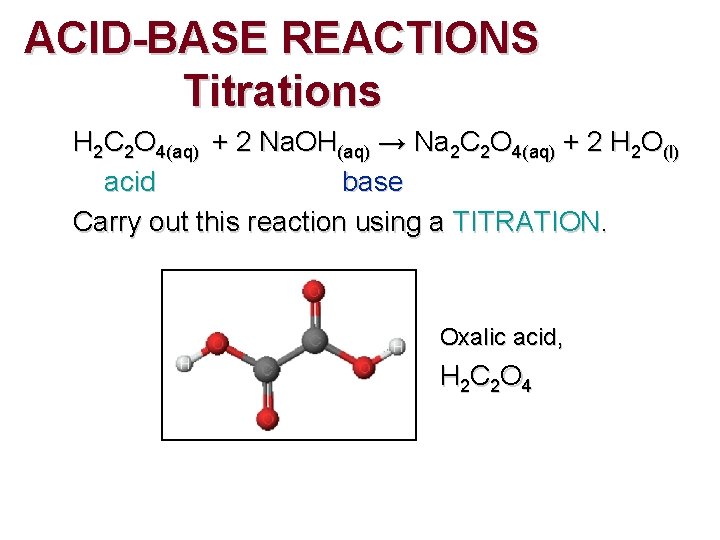
ACID-BASE REACTIONS Titrations H 2 C 2 O 4(aq) + 2 Na. OH(aq) → Na 2 C 2 O 4(aq) + 2 H 2 O(l) acid base Carry out this reaction using a TITRATION. Oxalic acid, H 2 C 2 O 4

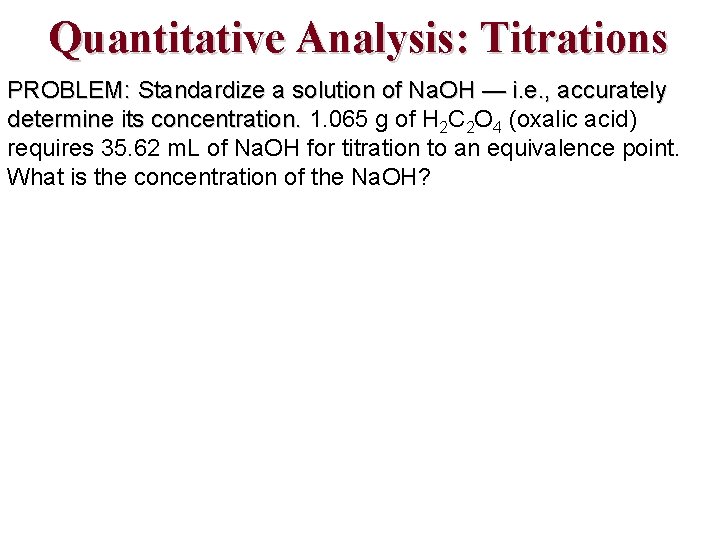
Quantitative Analysis: Titrations PROBLEM: Standardize a solution of Na. OH — i. e. , accurately determine its concentration. 1. 065 g of H 2 C 2 O 4 (oxalic acid) requires 35. 62 m. L of Na. OH for titration to an equivalence point. What is the concentration of the Na. OH?

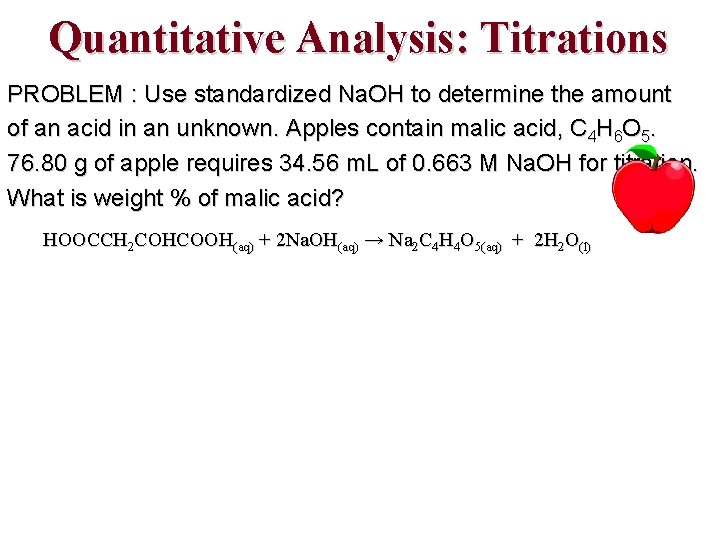
Quantitative Analysis: Titrations PROBLEM : Use standardized Na. OH to determine the amount of an acid in an unknown. Apples contain malic acid, C 4 H 6 O 5. 76. 80 g of apple requires 34. 56 m. L of 0. 663 M Na. OH for titration. What is weight % of malic acid? HOOCCH 2 COHCOOH(aq) + 2 Na. OH(aq) → Na 2 C 4 H 4 O 5(aq) + 2 H 2 O(l)

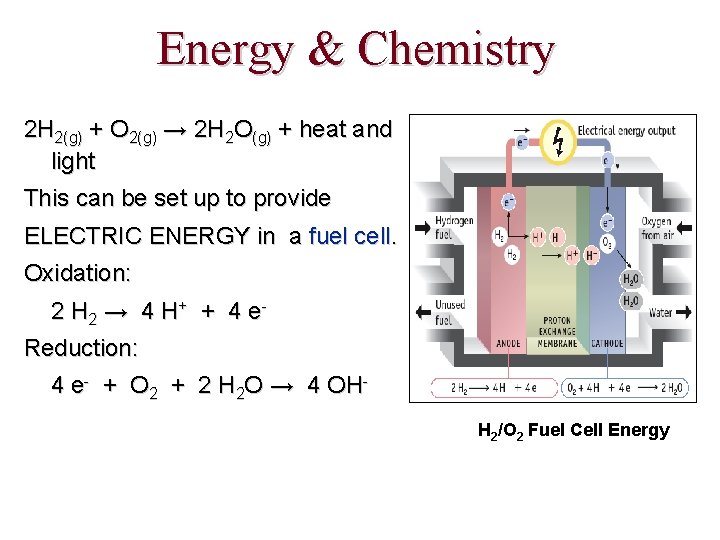
Energy & Chemistry 2 H 2(g) + O 2(g) → 2 H 2 O(g) + heat and light This can be set up to provide ELECTRIC ENERGY in a fuel cell. Oxidation: 2 H 2 → 4 H + + 4 e Reduction: 4 e- + O 2 + 2 H 2 O → 4 OHH 2/O 2 Fuel Cell Energy

Energy & Chemistry ENERGY is the capacity to do work (w) or transfer heat (q). HEAT is thermal energy that can be transferred from an object at one temperature to an object at another temperature – Net transfer of thermal energy stops when the two objects reach the same temperature. Other forms of energy — 1. Radiant (light) — energy in light, microwaves, and radio waves 2. Thermal (kinetic and potential) potential — results from atomic and molecular motion – Temperature of an object is a measure of thermal energy content 3. Chemical — results from the particular arrangement of atoms in a chemical compound; radiant and thermal energy produced in this reaction due to energy released during the breaking and reforming of chemical bonds 4. Nuclear — radiant and thermal energy released when

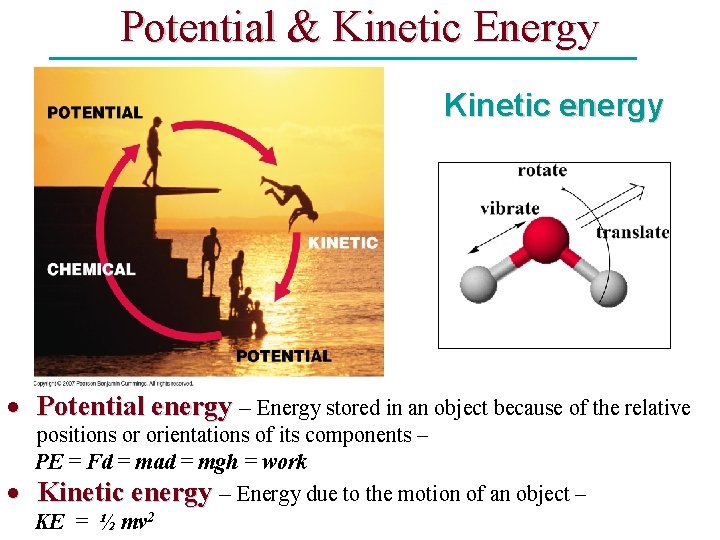
Potential & Kinetic Energy Kinetic energy Potential energy – Energy stored in an object because of the relative positions or orientations of its components – PE = Fd = mad = mgh = work Kinetic energy – Energy due to the motion of an object – KE = ½ mv 2

Potential & Kinetic Energy

Internal Energy (E) • PE + KE = Internal energy (E or U) • Internal Energy of a chemical system depends on • number of particles • type of particles • temperature • The higher the T the higher the internal energy • So, use changes in T (∆T) to monitor changes in E (∆E).

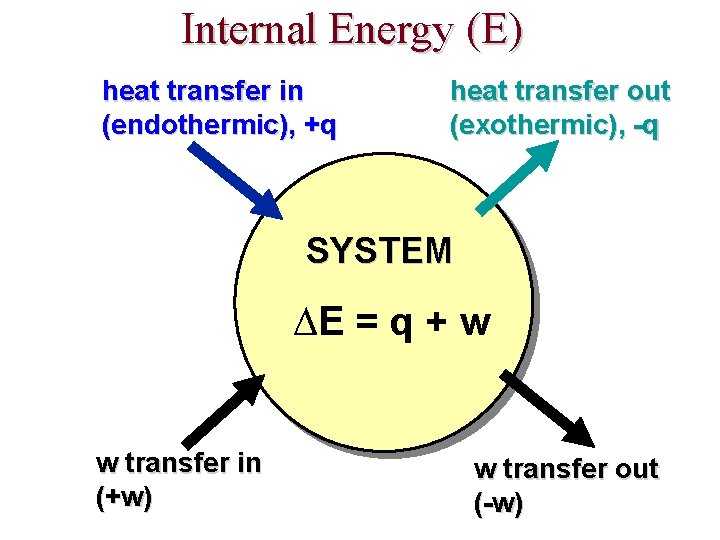
Internal Energy (E) heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM ∆E = q + w w transfer in (+w) w transfer out (-w)

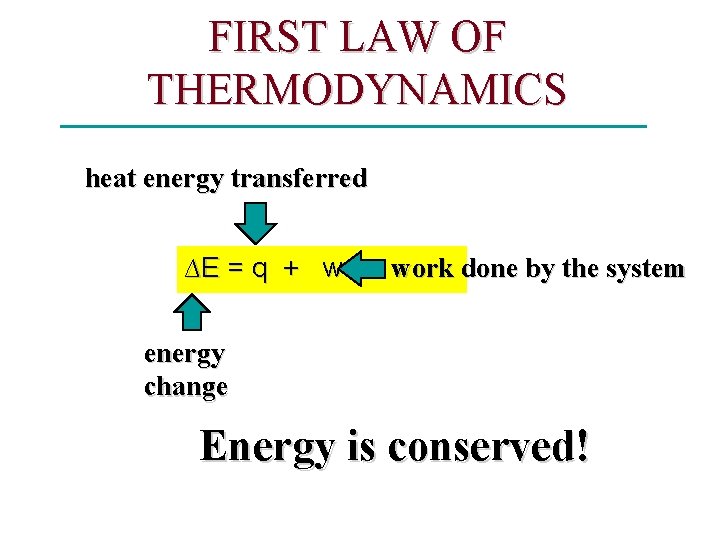
Energy & Chemistry All of thermodynamics depends on the law of CONSERVATION OF ENERGY. • The total energy is unchanged in a chemical reaction. • If PE of products is less than reactants, the difference must be released as KE. Energy Change in Chemical Processes Potential Energy of system dropped. Kinetic energy increased. Therefore, you often feel a Temperature increase.

Thermodynamics -Enthalpy • Thermodynamics – the science of heat (energy) transfer. Heat transfers until thermal equilibrium is established. ∆T measures energy transferred. • SYSTEM – The object under study 1. Open system — can exchange both matter and energy with its surroundings 2. Closed system — can exchange energy but not matter with its surroundings 3. Isolated system — exchanges neither energy nor matter with the surroundings; total energy of the system plus the surroundings is constant • SURROUNDINGS – Everything outside the system

FIRST LAW OF THERMODYNAMICS heat energy transferred ∆E = q + w work done by the system energy change Energy is conserved!

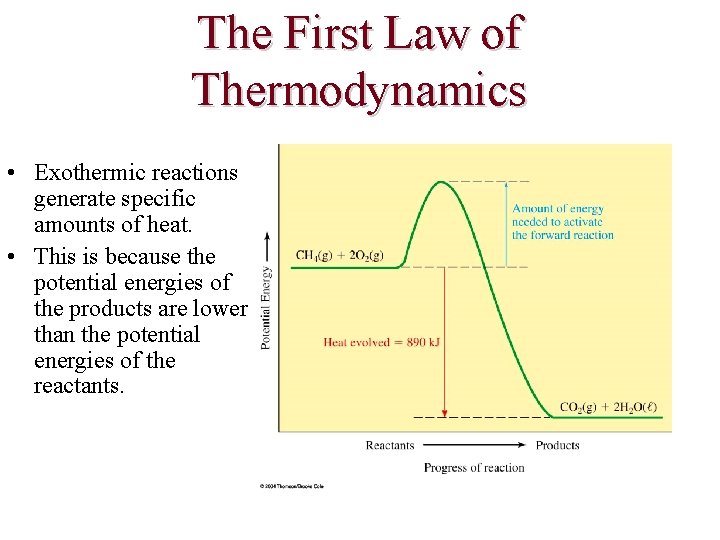
The First Law of Thermodynamics • Exothermic reactions generate specific amounts of heat. • This is because the potential energies of the products are lower than the potential energies of the reactants.

The First Law of Thermodynamics • There are two basic ideas of importance for thermodynamic systems. 1. Chemical systems tend toward a state of minimum potential energy. 2. Chemical systems tend toward a state of maximum disorder. • The first law is also known as the Law of Conservation of Energy. – Energy is neither created nor destroyed in chemical reactions and physical changes.

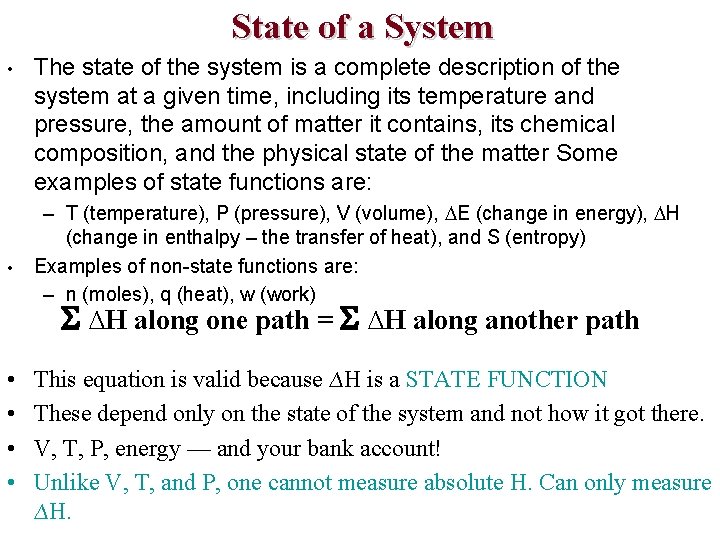
State of a System • • The state of the system is a complete description of the system at a given time, including its temperature and pressure, the amount of matter it contains, its chemical composition, and the physical state of the matter Some examples of state functions are: – T (temperature), P (pressure), V (volume), E (change in energy), H (change in enthalpy – the transfer of heat), and S (entropy) Examples of non-state functions are: – n (moles), q (heat), w (work) ∆H along one path = ∆H along another path • • This equation is valid because ∆H is a STATE FUNCTION These depend only on the state of the system and not how it got there. V, T, P, energy — and your bank account! Unlike V, T, and P, one cannot measure absolute H. Can only measure ∆H.

Standard States and Standard Enthalpy Changes • Thermochemical standard state conditions –The thermochemical standard T = 298. 15 K. –The thermochemical standard P = 1. 0000 atm. • Be careful not to confuse these values with STP. • Thermochemical standard states of matter –For pure substances in their liquid or solid phase the standard state is the pure liquid or solid. –For gases the standard state is the gas at 1. 00 atm of pressure. • For gaseous mixtures the partial pressure must be 1. 00 atm. –For aqueous solutions the standard state is 1. 00 M ∆H o =concentration. standard molar enthalpy of formation f • the enthalpy change when 1 mol of compound is formed from elements under standard conditions.

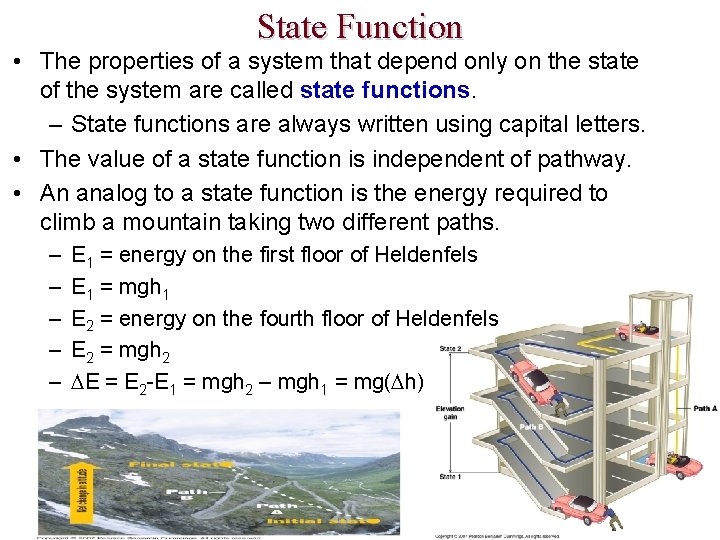
State Function • The properties of a system that depend only on the state of the system are called state functions. – State functions are always written using capital letters. • The value of a state function is independent of pathway. • An analog to a state function is the energy required to climb a mountain taking two different paths. – – – E 1 = energy on the first floor of Heldenfels E 1 = mgh 1 E 2 = energy on the fourth floor of Heldenfels E 2 = mgh 2 E = E 2 -E 1 = mgh 2 – mgh 1 = mg( h)

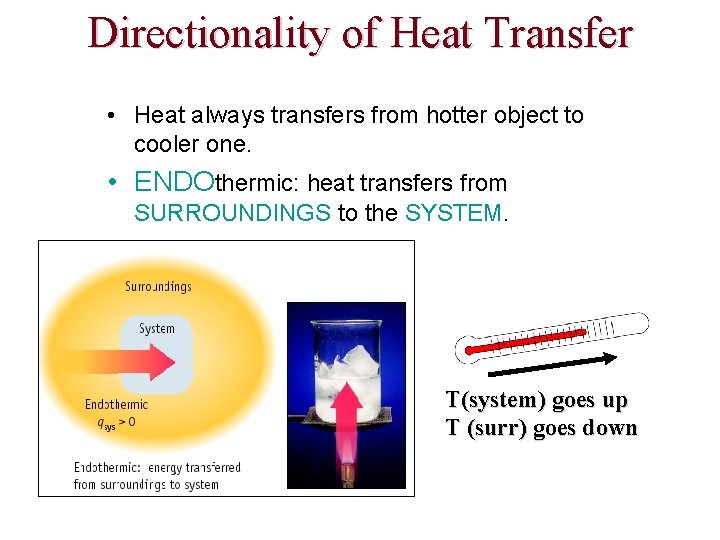
ENTHALPY Most chemical reactions occur at constant P, so Heat transferred at constant P = qp qp = ∆H where H = enthalpy and so ∆E = ∆H + w (and w is usually small) ∆H = heat transferred at constant P ≈ ∆E ∆H = change in heat content of the system ∆H = Hfinal - Hinitial

Directionality of Heat Transfer • Heat always transfer from hotter object to cooler one. • EXOthermic: heat transfers from SYSTEM to SURROUNDINGS. T(system) goes down T(surr) goes up

Directionality of Heat Transfer • Heat always transfers from hotter object to cooler one. • ENDOthermic: heat transfers from SURROUNDINGS to the SYSTEM. T(system) goes up T (surr) goes down

ENTHALPY ∆H = Hfinal - Hinitial If Hfinal > Hinitial then ∆H is positive Process is ENDOTHERMIC If Hfinal < Hinitial then ∆H is negative Process is EXOTHERMIC

USING ENTHALPY Consider the formation of water H 2(g) + 1/2 O 2(g) → H 2 O(g) + 241. 8 k. J Exothermic reaction — heat is a “product” and ∆H = – 241. 8 k. J

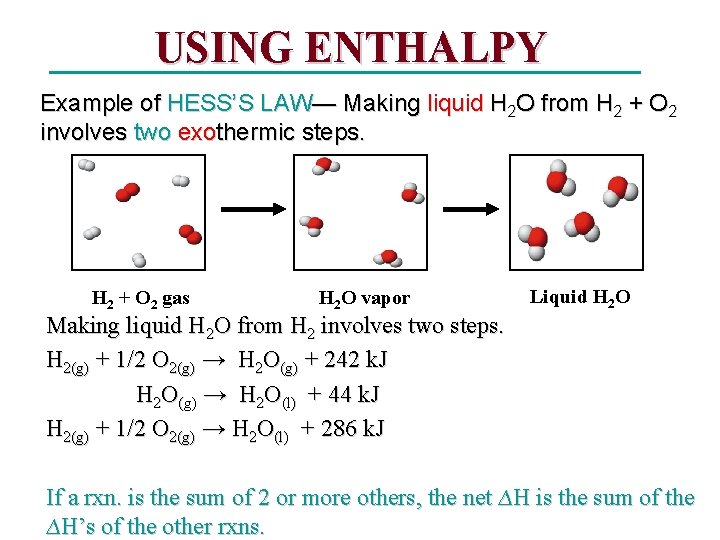
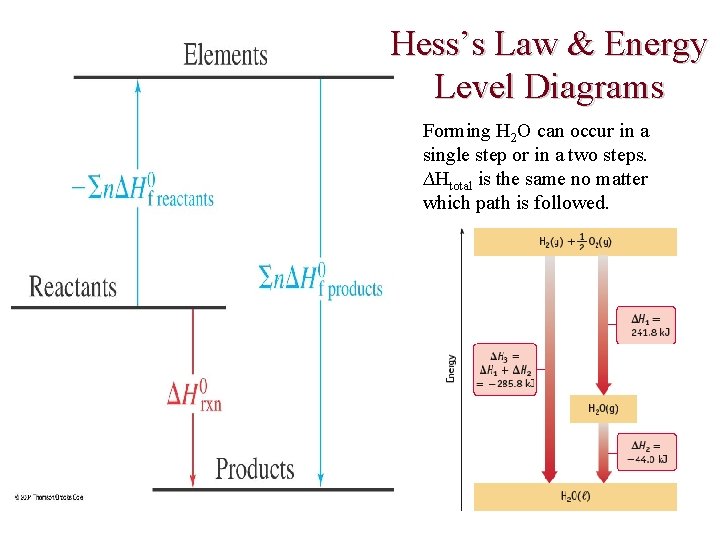
USING ENTHALPY Example of HESS’S LAW— Making liquid H 2 O from H 2 + O 2 involves two exothermic steps. H 2 + O 2 gas H 2 O vapor Liquid H 2 O Making liquid H 2 O from H 2 involves two steps. H 2(g) + 1/2 O 2(g) → H 2 O(g) + 242 k. J H 2 O(g) → H 2 O(l) + 44 k. J H 2(g) + 1/2 O 2(g) → H 2 O(l) + 286 k. J If a rxn. is the sum of 2 or more others, the net ∆H is the sum of the ∆H’s of the other rxns.

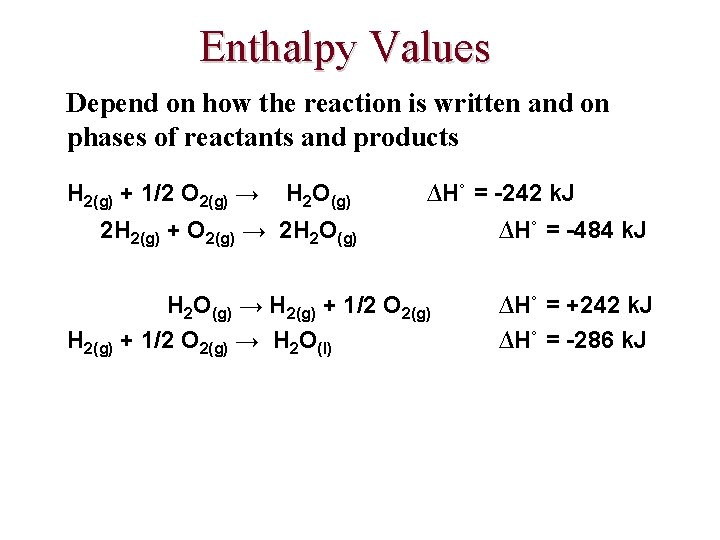
Enthalpy Values Depend on how the reaction is written and on phases of reactants and products H 2(g) + 1/2 O 2(g) → H 2 O(g) ∆H˚ = -242 k. J 2 H 2(g) + O 2(g) → 2 H 2 O(g) → H 2(g) + 1/2 O 2(g) → H 2 O(l) ∆H˚ = -484 k. J ∆H˚ = +242 k. J ∆H˚ = -286 k. J

Standard Molar Enthalpies of Formation, Hf o • • The standard molar enthalpy of formation is defined as the enthalpy for the reaction in which one mole of a substance is formed from its constituent elements. – The symbol for standard molar enthalpy of formation is Hfo. The standard molar enthalpy of formation for Mg. Cl 2 is:

• • • Standard Molar Enthalpies of Formation, Hf o Standard molar enthalpies of formation have been determined for many substances and are tabulated in Table 15 -1 and Appendix K in the text. Standard molar enthalpies of elements in their most stable forms at 298. 15 K and 1. 000 atm are zero. Example 15 -4: The standard molar enthalpy of formation for phosphoric acid is -1281 k. J/mol. Write the equation for the reaction for which Horxn = -1281 k. J. P in standard state is P 4 Phosphoric acid in standard state is H 3 PO 4(s)

Standard Molar Enthalpies of Formation, Hf o H 2(g) + ½ O 2(g) → H 2 O(g) ∆Hf˚ (H 2 O, g)= -241. 8 k. J/mol C(s) + ½ O 2(g) → CO(g) ∆Hf˚ of CO = - 111 Use ∆H˚’s to calculate enthalpy change for k. J/mol H O(g) + C(graphite) → H (g) + CO(g) By 2 definition, ∆Hfo = 0 for 2 elements in their standard states.

Standard Molar Enthalpies of Formation, Hfo • Calculate the enthalpy change for the reaction of one mole of H 2(g) with one mole of F 2(g) to form two moles of HF(g) at 25 o. C and one atmosphere.

Standard Molar Enthalpies of Formation, Hfo • Calculate the enthalpy change for the reaction in which 15. 0 g of aluminum reacts with oxygen to form Al 2 O 3 at 25 o. C and one atmosphere.

Hess’s Law – Enthalpies of Formation and Reaction Standard enthalpies of reaction (ΔH°xn) – The enthalpy that occurs when a reaction is carried out with all reactants and products in their standard states – General reaction: a. A + b. B c. C + d. D where A, B, C, and D are chemical substances and a, b, c, and d are their stoichiometric coefficients – Magnitude of ΔH°rxn is the sum of the standard enthalpies of formation of the products, each multiplied by its appropriate coefficient , minus the sum of the standard enthalpies of formation of the reactants, multiplied by their coefficients ΔH°rxn = [cΔH°f(C) + dΔH°f (D)] [aΔH°f(A) + bΔH°f(B)] or ΔH°rxn= mΔH°f(product) nΔH°f(reactants) where means “sum of” and m and n are the

Hess’s Law & Energy Level Diagrams Forming H 2 O can occur in a single step or in a two steps. ∆Htotal is the same no matter which path is followed.

Enthalpies of Formation Standard enthalpies of formation – The magnitude of ΔH for a reaction depends on the physical states of the reactants and products, the pressure of any gases present, and the temperature at which the reaction is carried out. – A specific set of conditions under which enthalpy changes are measured are used to ensure uniformity of reaction conditions and data. – Standard conditions: a pressure of 1 atmosphere (atm) for gases and a concentration of 1 M for species in solution (1 mol/L), each pure substance must be in its standard state, its most stable form at a pressure of 1 atm at a specified temperature. – Enthalpies of formation measured under standard conditions are called standard enthalpies of formation (ΔH°f). – Standard enthalpy of formation of any element in its

Enthalpies of Reaction Hess’s law allows the calculation of the enthalpy change for any conceivable chemical reaction by using a relatively small set of tabulated data 1. Enthalpy of combustion, ΔHcomb—enthalpy change that occurs when a substance is burned in excess oxygen 2. Enthalpy of fusion, ΔH fus—enthalpy change that accompanies the melting, or fusion, of 1 mol of a substance 3. Enthalpy of vaporization, ΔHvap—the enthalpy change that accompanies the vaporization of 1 mol of a substance 4. Enthalpy of solution, ΔHsoln—enthalpy change when a specified amount of solute dissolves in a given quantity of solvent 5. Enthalpy of formation, ΔHf—enthalpy change for

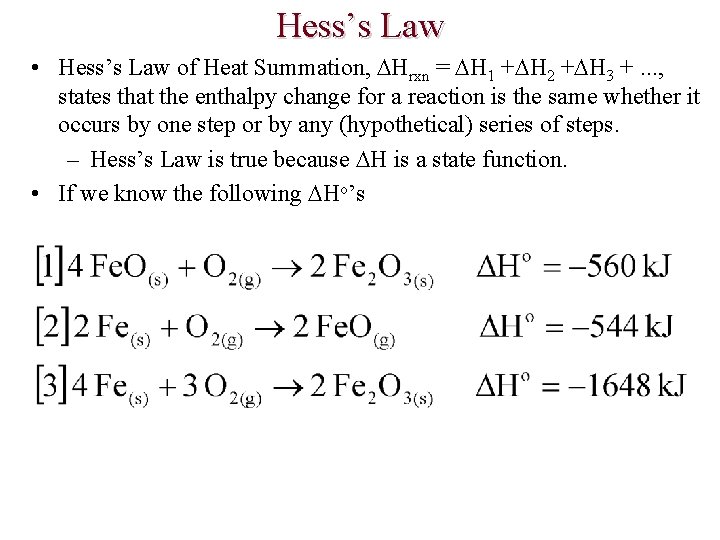
Hess’s Law • Hess’s Law of Heat Summation, Hrxn = H 1 + H 2 + H 3 +. . . , states that the enthalpy change for a reaction is the same whether it occurs by one step or by any (hypothetical) series of steps. – Hess’s Law is true because H is a state function. • If we know the following Ho’s
![Hesss Law For example we can calculate the Ho for reaction 1 Hess’s Law • • For example, we can calculate the Ho for reaction [1]](https://slidetodoc.com/presentation_image_h/d517205590dc54fd56b8855defc8f534/image-77.jpg)
Hess’s Law • • For example, we can calculate the Ho for reaction [1] by properly adding (or subtracting) the Ho’s for reactions [2] and [3]. Notice that reaction [1] has Fe. O and O 2 as reactants and Fe 2 O 3 as a product. – Arrange reactions [2] and [3] so that they also have Fe. O and O 2 as reactants and Fe 2 O 3 as a product. • Each reaction can be doubled, tripled, or multiplied by half, etc. • The Ho values are also doubled, tripled, etc. • If a reaction is reversed the sign of the Ho is changed.

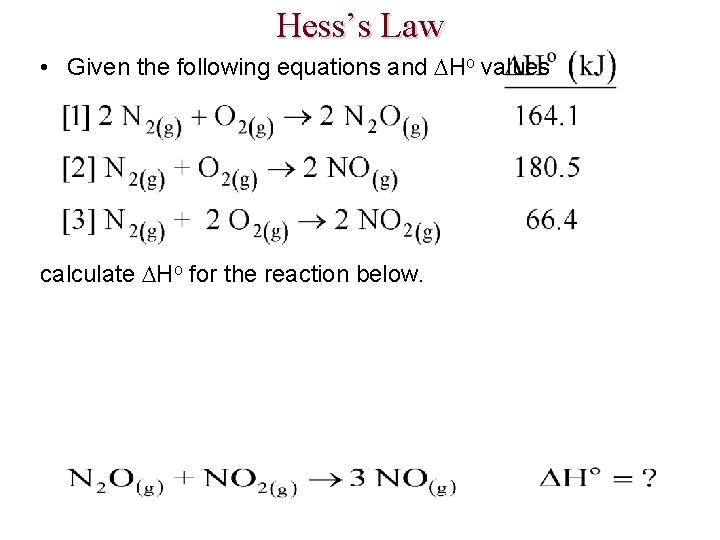
Hess’s Law • Given the following equations and Ho values calculate Ho for the reaction below.

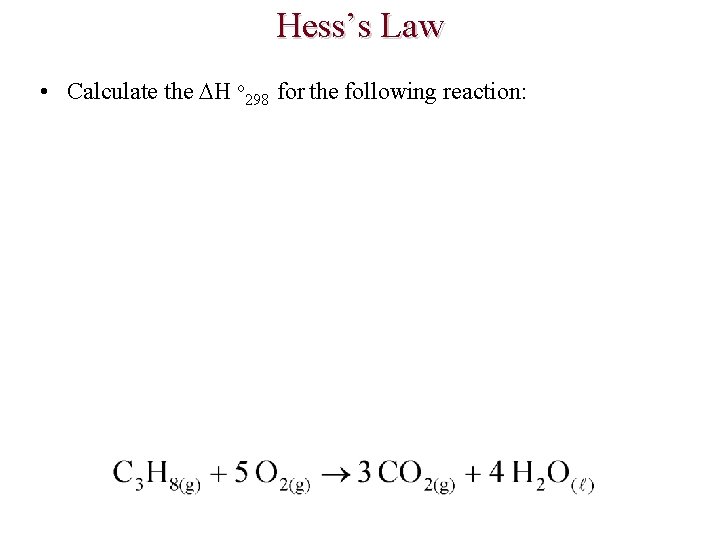
Hess’s Law • Calculate the H o 298 for the following reaction:

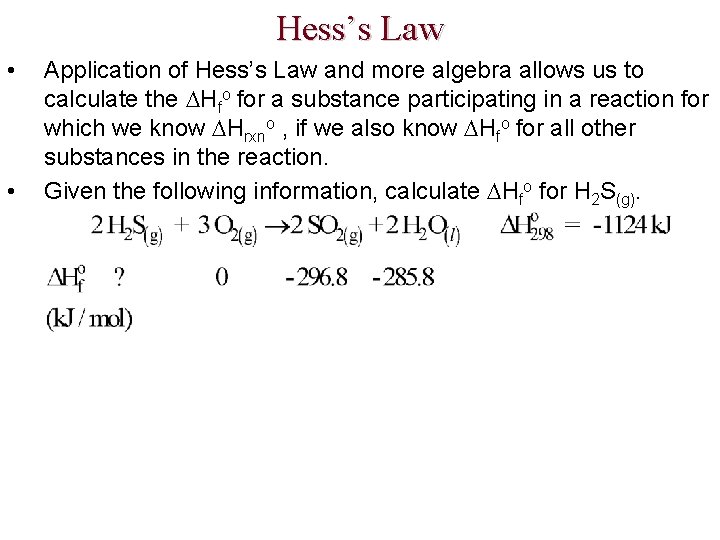
Hess’s Law • • Application of Hess’s Law and more algebra allows us to calculate the Hfo for a substance participating in a reaction for which we know Hrxno , if we also know Hfo for all other substances in the reaction. Given the following information, calculate Hfo for H 2 S(g).

HEAT CAPACITY The heat required to raise an object’s T by 1 ˚C. Which has the larger heat capacity? Thermal energy cannot be measured easily. Temperature change caused by the flow of thermal energy between objects or substances can be measured. Calorimetry is the set of techniques employed to measure enthalpy changes in chemical processes using calorimeters.

Specific Heat Capacity How much energy is transferred due to Temperature difference? The heat (q) “lost” or “gained” is related to a) sample mass b) change in T and c) specific heat capacity Specific heat capacity= heat lost or gained by substance (J) (mass, g) (T change, K)

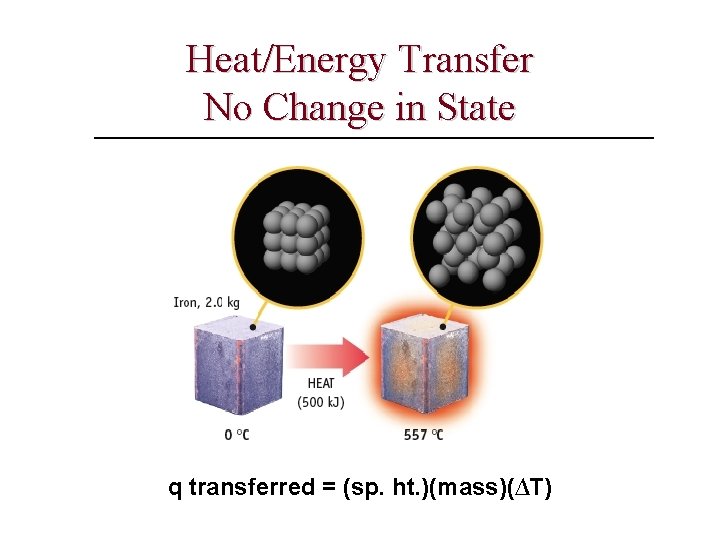
Table of specific heat capacities Heat capacity of an object depends on both its mass and its composition. Molar heat capacity (Cp) — amount of energy needed to increase the temperature of 1 mol of a substance by 1°C; units of Cp are J/(mol • °C) Specific heat (Cs) — amount of energy needed to increase the temperature of 1 g of a substance by 1°C, units are J/(g • °C) The quantity of a substance, the amount of heat transferred, its heat capacity, and the temperature change are related in two ways: q = n. CpΔT where n = number of moles of substance q = m. CsΔT where m = mass of substance in grams Substance Phase Air (typ. room conditions) gas 1. 012 29. 19 Aluminium solid 0. 897 24. 2 Argon gas 0. 5203 20. 7862 Copper solid 0. 385 24. 47 Diamond solid 0. 5091 6. 115 Ethanol liquid 2. 44 112 Gold solid 0. 1291 25. 42 Graphite solid 0. 710 8. 53 Helium gas 5. 1932 20. 7862 Hydrogen gas 14. 30 28. 82 Iron solid 0. 450 25. 1 Lithium solid 3. 58 24. 8 Mercury liquid 0. 1395 27. 98 Nitrogen gas 1. 040 29. 12 Neon gas 1. 0301 20. 7862 Oxygen gas 0. 918 29. 38 Uranium solid 0. 116 27. 7 gas (100 °C) 2. 080 37. 47 liquid (25 °C) 4. 1813 75. 327 2. 114 38. 09 Water solid (0 °C) Aluminum cp J g-1 K-1 Cp J mol-1 K-1 All measurements are at 25 °C unless noted. Notable minimums and maximums are shown in maroon text.

Specific Heat Capacity If 25. 0 g of Al cool from 310 o. C to 37 o. C, how many joules of heat energy are lost by the Al?

Heat/Energy Transfer No Change in State q transferred = (sp. ht. )(mass)(∆T)

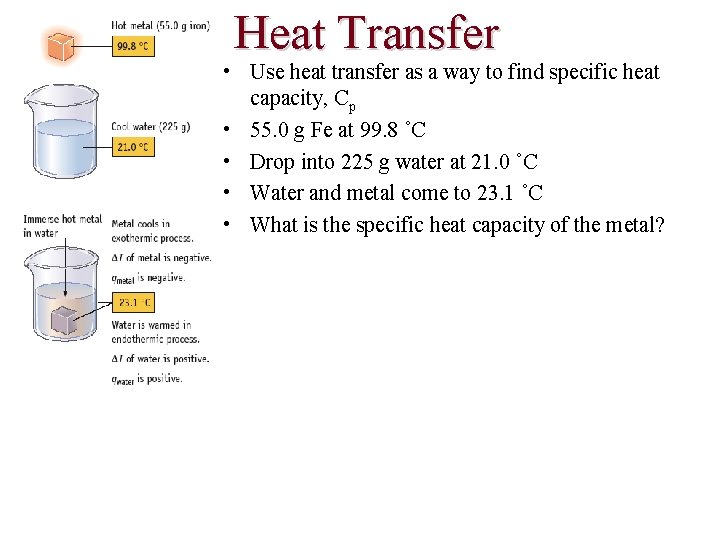
Heat Transfer • Use heat transfer as a way to find specific heat capacity, Cp • 55. 0 g Fe at 99. 8 ˚C • Drop into 225 g water at 21. 0 ˚C • Water and metal come to 23. 1 ˚C • What is the specific heat capacity of the metal?

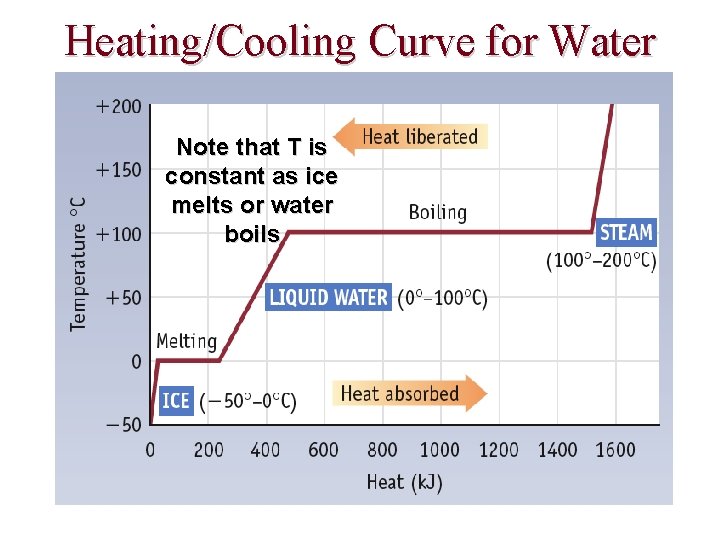
Heating/Cooling Curve for Water Note that T is constant as ice melts or water boils

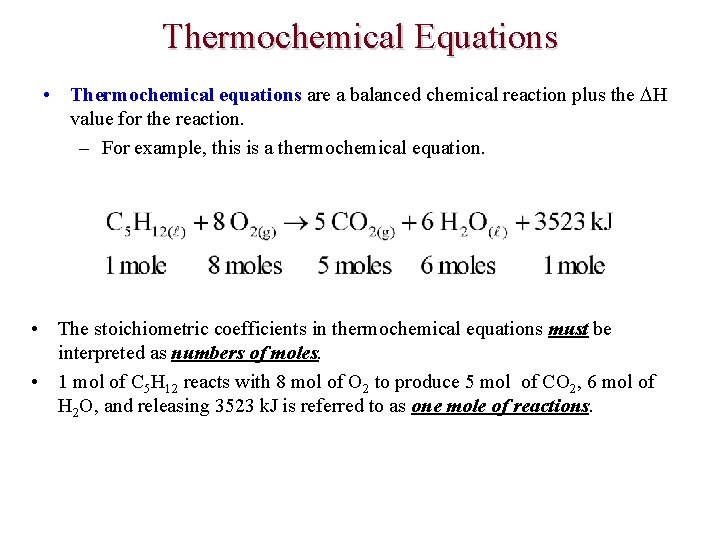
Thermochemical Equations • Thermochemical equations are a balanced chemical reaction plus the H value for the reaction. – For example, this is a thermochemical equation. • The stoichiometric coefficients in thermochemical equations must be interpreted as numbers of moles. • 1 mol of C 5 H 12 reacts with 8 mol of O 2 to produce 5 mol of CO 2, 6 mol of H 2 O, and releasing 3523 k. J is referred to as one mole of reactions.

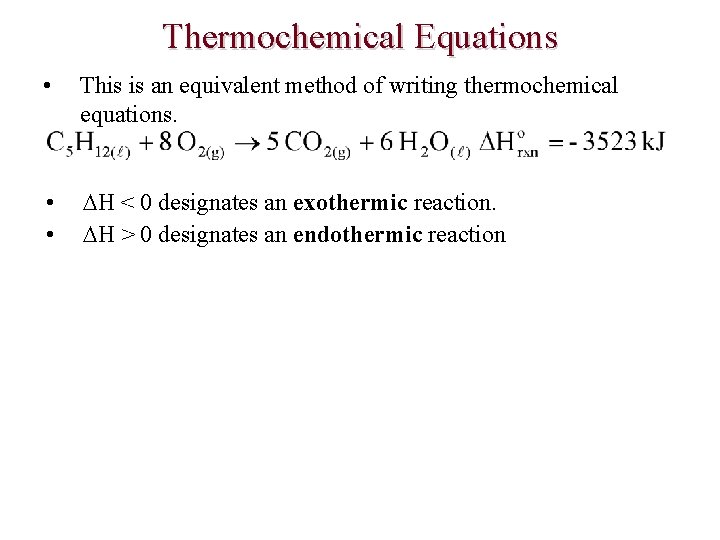
Thermochemical Equations • This is an equivalent method of writing thermochemical equations. • • H < 0 designates an exothermic reaction. H > 0 designates an endothermic reaction

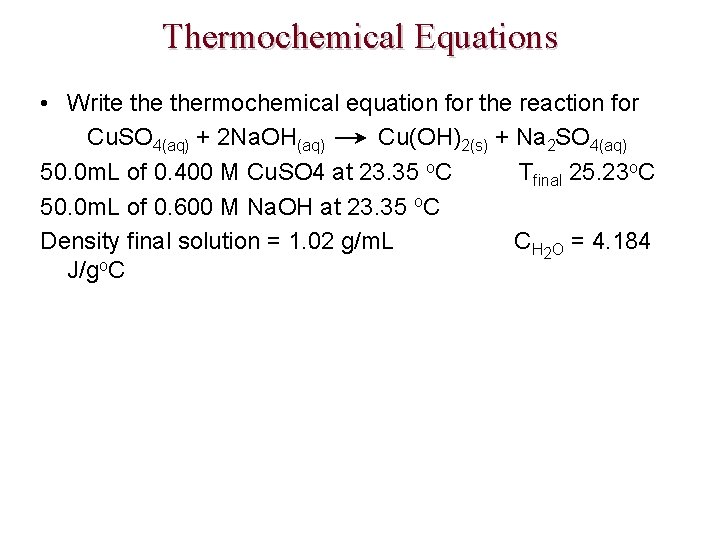
Thermochemical Equations • Write thermochemical equation for the reaction for Cu. SO 4(aq) + 2 Na. OH(aq) Cu(OH)2(s) + Na 2 SO 4(aq) 50. 0 m. L of 0. 400 M Cu. SO 4 at 23. 35 o. C Tfinal 25. 23 o. C 50. 0 m. L of 0. 600 M Na. OH at 23. 35 o. C Density final solution = 1. 02 g/m. L CH 2 O = 4. 184 J/go. C

Gas Laws At the macroscopic level, a complete physical description of a sample of a gas requires four quantities: 1. Temperature (expressed in K) 2. Volume (expressed in liters) 3. Amount (expressed in moles) 4. Pressure (given in atmospheres) • These variables are not independent — if the values of any three of these quantities are known, the fourth can be calculated. Standard Temperature and Pressure • Standard temperature and pressure is given the symbol STP. – It is a reference point for some gas calculations. • Standard P 1. 00000 atm or 101. 3 k. Pa • Standard T 273. 15 K or 0. 00 o. C – Gas laws must use the Kelvin scale to be correct. • Relationship between Kelvin and centigrade.

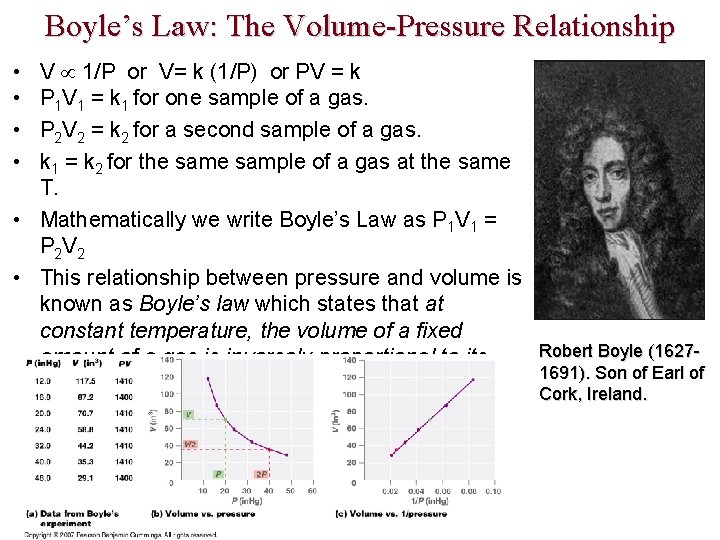
Boyle’s Law: The Volume-Pressure Relationship V 1/P or V= k (1/P) or PV = k P 1 V 1 = k 1 for one sample of a gas. P 2 V 2 = k 2 for a second sample of a gas. k 1 = k 2 for the sample of a gas at the same T. • Mathematically we write Boyle’s Law as P 1 V 1 = P 2 V 2 • This relationship between pressure and volume is known as Boyle’s law which states that at constant temperature, the volume of a fixed Robert Boyle (1627 amount of a gas is inversely proportional to its 1691). Son of Earl of pressure. Cork, Ireland. • •

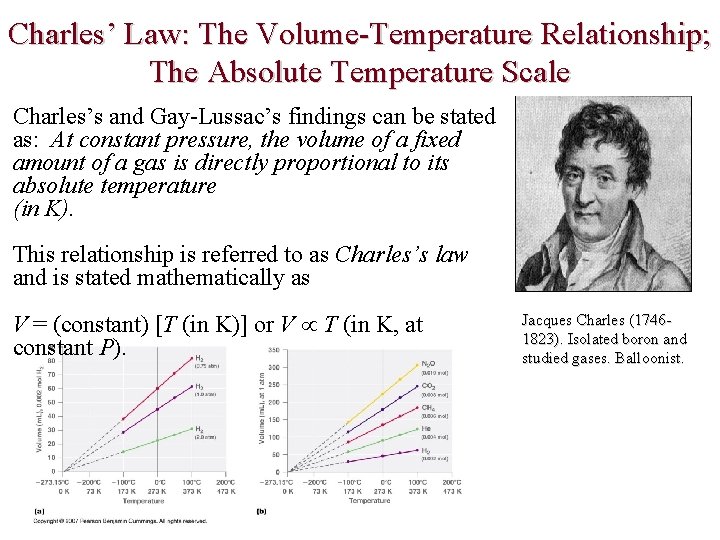
Charles’ Law: The Volume-Temperature Relationship; The Absolute Temperature Scale Charles’s and Gay-Lussac’s findings can be stated as: At constant pressure, the volume of a fixed amount of a gas is directly proportional to its absolute temperature (in K). This relationship is referred to as Charles’s law and is stated mathematically as V = (constant) [T (in K)] or V T (in K, at constant P). Jacques Charles (17461823). Isolated boron and studied gases. Balloonist.

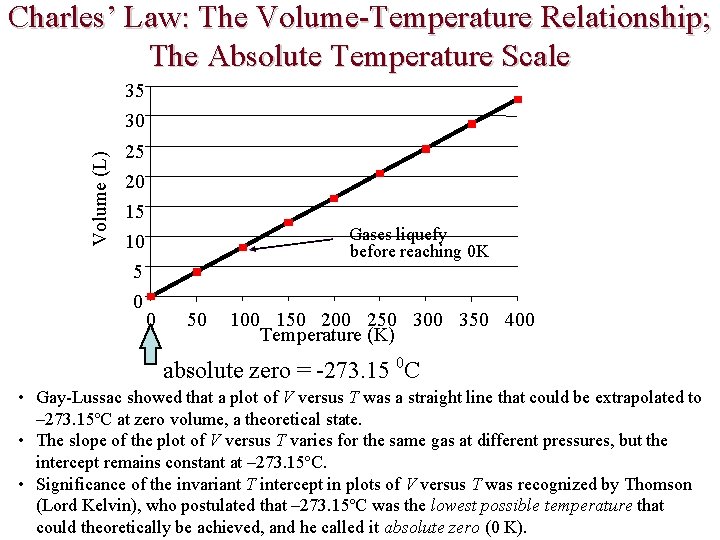
Charles’ Law: The Volume-Temperature Relationship; The Absolute Temperature Scale Volume (L) 35 30 25 20 15 Gases liquefy before reaching 0 K 10 5 0 0 50 100 150 200 250 300 350 400 Temperature (K) absolute zero = -273. 15 0 C • Gay-Lussac showed that a plot of V versus T was a straight line that could be extrapolated to – 273. 15ºC at zero volume, a theoretical state. • The slope of the plot of V versus T varies for the same gas at different pressures, but the intercept remains constant at – 273. 15ºC. • Significance of the invariant T intercept in plots of V versus T was recognized by Thomson (Lord Kelvin), who postulated that – 273. 15ºC was the lowest possible temperature that could theoretically be achieved, and he called it absolute zero (0 K).

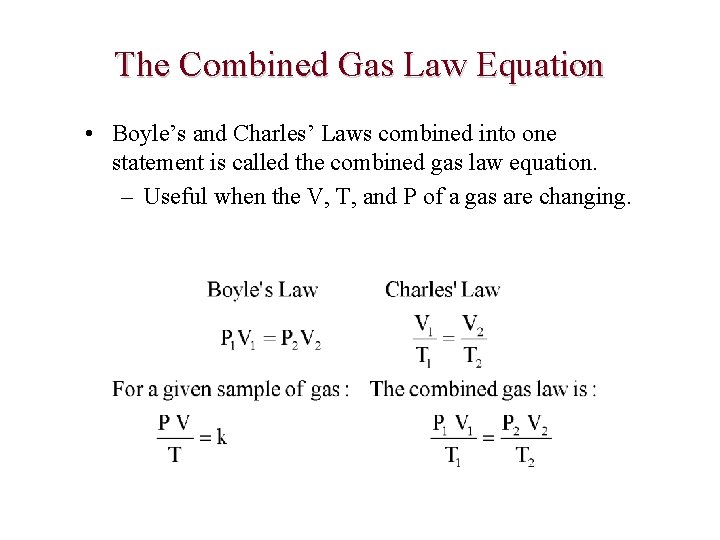
The Combined Gas Law Equation • Boyle’s and Charles’ Laws combined into one statement is called the combined gas law equation. – Useful when the V, T, and P of a gas are changing.

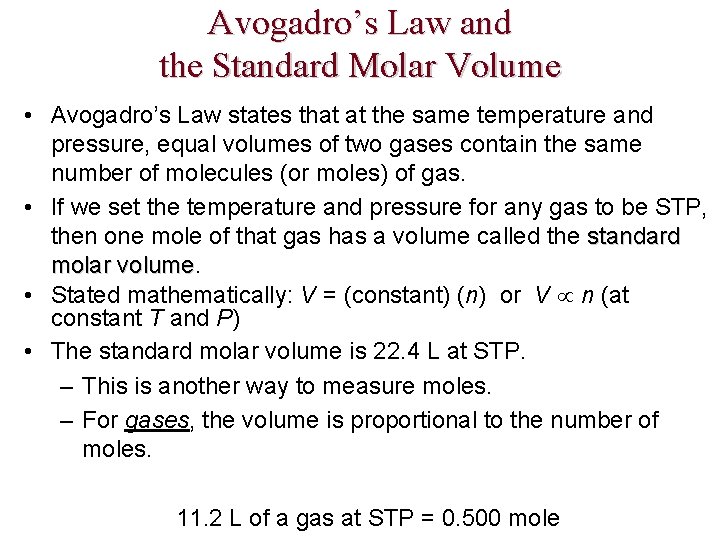
Avogadro’s Law and the Standard Molar Volume • Avogadro’s Law states that at the same temperature and pressure, equal volumes of two gases contain the same number of molecules (or moles) of gas. • If we set the temperature and pressure for any gas to be STP, then one mole of that gas has a volume called the standard molar volume • Stated mathematically: V = (constant) (n) or V n (at constant T and P) • The standard molar volume is 22. 4 L at STP. – This is another way to measure moles. – For gases, the volume is proportional to the number of moles. 11. 2 L of a gas at STP = 0. 500 mole

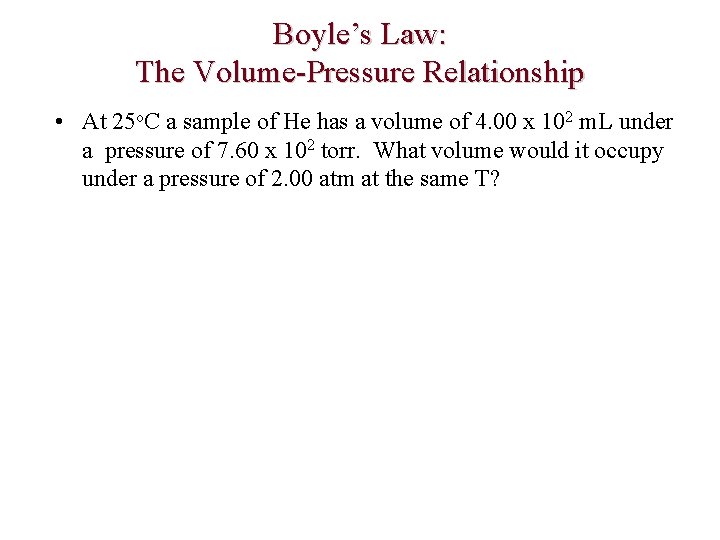
Boyle’s Law: The Volume-Pressure Relationship • At 25 o. C a sample of He has a volume of 4. 00 x 102 m. L under a pressure of 7. 60 x 102 torr. What volume would it occupy under a pressure of 2. 00 atm at the same T?

Charles’ Law: The Volume-Temperature Relationship; The Absolute Temperature Scale • A sample of hydrogen, H 2, occupies 1. 00 x 102 m. L at 25. 0 o. C and 1. 00 atm. What volume would it occupy at 50. 0 o. C under the same pressure?

The Combined Gas Law Equation • A sample of nitrogen gas, N 2, occupies 7. 50 x 102 m. L at 75. 00 C under a pressure of 8. 10 x 102 torr. What volume would it occupy at STP?

Avogadro’s Law and the Standard Molar Volume • One mole of a gas occupies 36. 5 L and its density is 1. 36 g/L at a given temperature and pressure. (a) What is its molar mass? (b) What is its density at STP?

Summary of Gas Laws: The Ideal Gas Law • What volume would 50. 0 g of ethane, C 2 H 6, occupy at 1. 40 x 102 o. C under a pressure of 1. 82 x 103 torr?

Summary of Gas Laws: The Ideal Gas Law • Calculate the number of moles in, and the mass of, an 8. 96 L sample of methane, CH 4, measured at standard conditions?

Dalton’s Law of Partial Pressures 1. The ideal gas law assumes that all gases behave identically. If V, T, and n for each gas in a mixture are known, the pressure of each gas, its partial pressure, can be calculated. Holding V and T constant, P is directly proportional to n: P = n(RT/V) = n(constant) 2. Generally, for a mixture of i components, the total pressure is given by Pt = (n 1 + n 2 + n 3 + - - - +ni) (RT/V). 3. The above equation makes it clear that, at constant T and V, P depends on only the total number of moles of gas present, whether the gas is a single chemical species or a mixture of gaseous species. Nothing in the equation depends on the nature of the gas, only on the John Dalton 1766 -1844 quantity. 4. The total pressure exerted by a mixture of gases is the sum of the partial pressures of component gases. This law is known as Dalton’s law of partial pressures and can be written mathematically as Pt = P 1 + P 2 + P 3 - - - + Pi • Vapor the pressure and exerted by a terms are the partial where. Pressure Pt is theistotal the other substance’s over the substance’s liquid at pressures ofvapor the individual gases. equilibrium. • Partial pressure is the pressure the gas would exert if it were the only one present (at the same temperature and volume).

Dalton’s Law of Partial Pressures • If 1. 00 x 102 m. L of hydrogen, measured at 25. 0 o. C and 3. 00 atm pressure, and 1. 00 x 102 m. L of oxygen, measured at 25. 0 o. C and 2. 00 atm pressure, were forced into one of the containers at 25. 0 o. C, what would be the pressure of the mixture of gases?

Dalton’s Law of Partial Pressures • A sample of hydrogen was collected by displacement of water at 25. 0 o. C. The atmospheric pressure was 748 torr. What pressure would the dry hydrogen exert in the same container?

Mass-Volume Relationships in Reactions Involving Gases • In this section we are looking at reaction stoichiometry, like in Chapter 3, just including gases in the calculations. 2 mol KCl. O 3 mol O 2 2(122. 6 g) (32. 0 g) yields 2 mol KCl 2 (74. 6 g) Those 3 moles of O 2 can also be thought of as: 3(22. 4 L) or and 3

Mass-Volume Relationships in Reactions Involving Gases • What volume of oxygen measured at STP, can be produced by thermal decomposition of 120. 0 g of KCl. O 3?

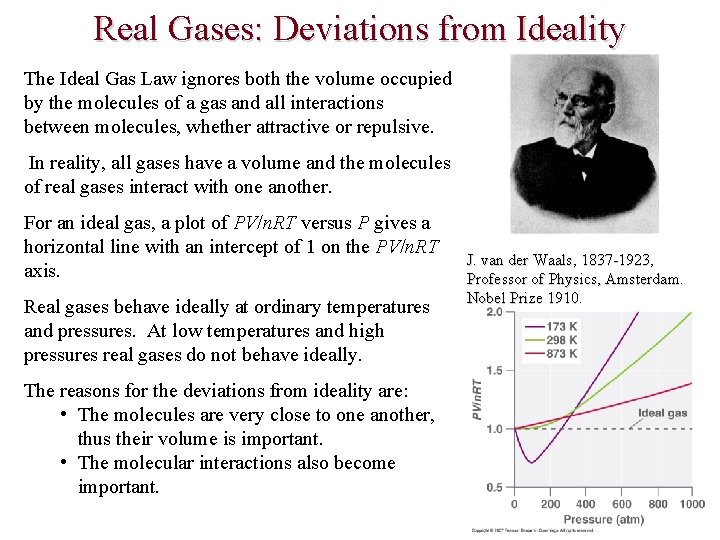
Real Gases: Deviations from Ideality The Ideal Gas Law ignores both the volume occupied by the molecules of a gas and all interactions between molecules, whether attractive or repulsive. In reality, all gases have a volume and the molecules of real gases interact with one another. For an ideal gas, a plot of PV/n. RT versus P gives a horizontal line with an intercept of 1 on the PV/n. RT axis. Real gases behave ideally at ordinary temperatures and pressures. At low temperatures and high pressures real gases do not behave ideally. The reasons for the deviations from ideality are: • The molecules are very close to one another, thus their volume is important. • The molecular interactions also become important. J. van der Waals, 1837 -1923, Professor of Physics, Amsterdam. Nobel Prize 1910.

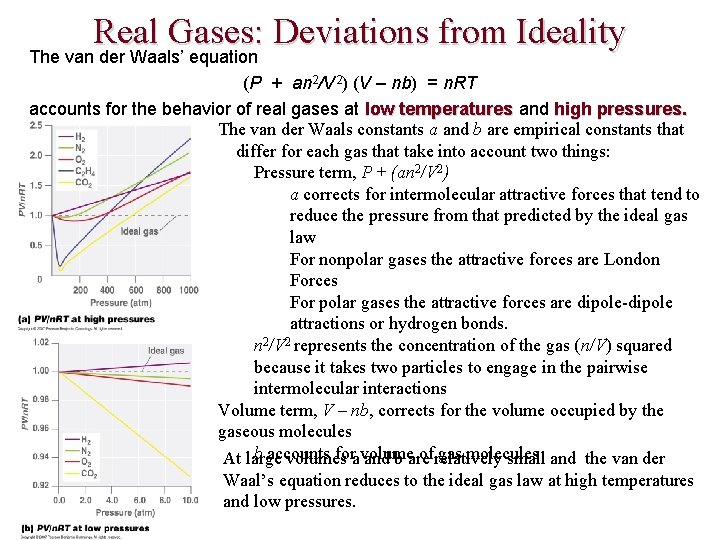
Real Gases: Deviations from Ideality The van der Waals’ equation (P + an 2/V 2) (V – nb) = n. RT accounts for the behavior of real gases at low temperatures and high pressures. The van der Waals constants a and b are empirical constants that differ for each gas that take into account two things: Pressure term, P + (an 2/V 2) a corrects for intermolecular attractive forces that tend to reduce the pressure from that predicted by the ideal gas law For nonpolar gases the attractive forces are London Forces For polar gases the attractive forces are dipole-dipole attractions or hydrogen bonds. n 2/V 2 represents the concentration of the gas (n/V) squared because it takes two particles to engage in the pairwise intermolecular interactions Volume term, V – nb, corrects for the volume occupied by the gaseous molecules b accounts fora volume of relatively gas molecules At large volumes and b are small and the van der Waal’s equation reduces to the ideal gas law at high temperatures and low pressures.

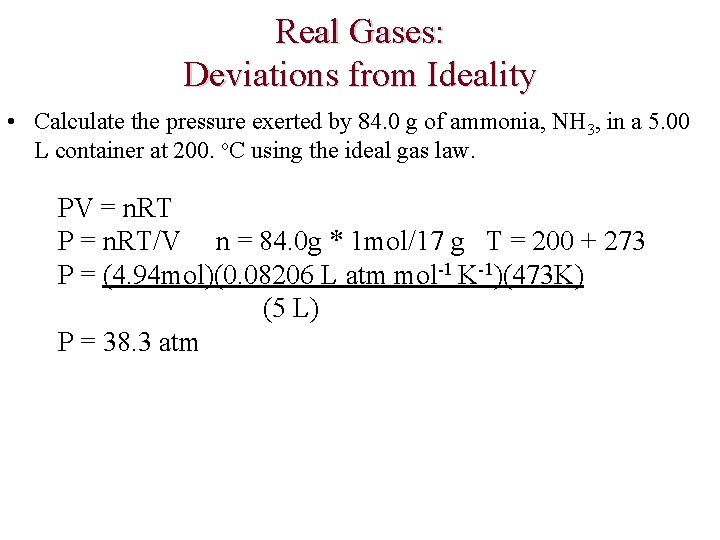
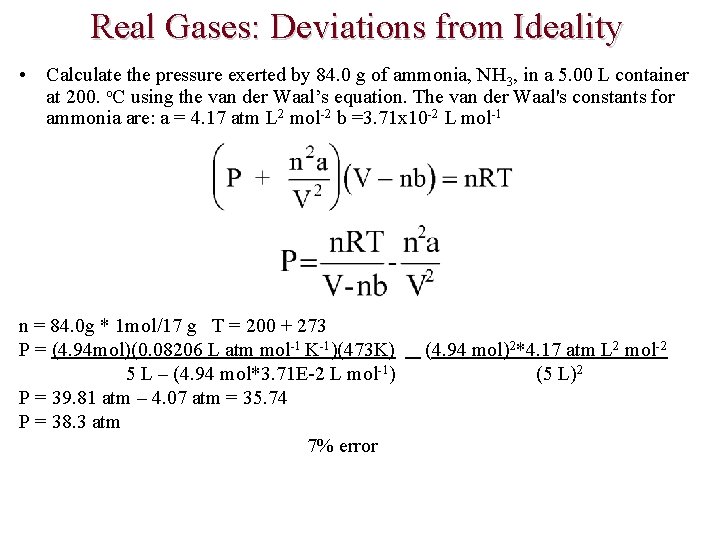
Real Gases: Deviations from Ideality • Calculate the pressure exerted by 84. 0 g of ammonia, NH 3, in a 5. 00 L container at 200. o. C using the ideal gas law. PV = n. RT P = n. RT/V n = 84. 0 g * 1 mol/17 g T = 200 + 273 P = (4. 94 mol)(0. 08206 L atm mol-1 K-1)(473 K) (5 L) P = 38. 3 atm

Real Gases: Deviations from Ideality • Calculate the pressure exerted by 84. 0 g of ammonia, NH 3, in a 5. 00 L container at 200. o. C using the van der Waal’s equation. The van der Waal's constants for ammonia are: a = 4. 17 atm L 2 mol-2 b =3. 71 x 10 -2 L mol-1 n = 84. 0 g * 1 mol/17 g T = 200 + 273 P = (4. 94 mol)(0. 08206 L atm mol-1 K-1)(473 K) 5 L – (4. 94 mol*3. 71 E-2 L mol-1) P = 39. 81 atm – 4. 07 atm = 35. 74 P = 38. 3 atm 7% error (4. 94 mol)2*4. 17 atm L 2 mol-2 (5 L)2