Chapter 7 Solutions Sodium Chloride Liquid Solution Section































- Slides: 35

Chapter 7: Solutions Sodium Chloride Liquid Solution Section 1: Solutions VS Mixtures Air is a Gaseous Solution

What is a mixture? • Mixtures of different substances exist all around us. – Many of these mixtures are invisible, and we never notice them. – However, some mixtures can easily be identified.

What is a mixture? • All matter is either a pure substance or a mixture of other substances. – Definition: pure substance – matter that has a fixed chemical composition. • For example: Water is a pure substance. It is ALWAYS H 2 O. Kool-Aid is a mixture of water and other substances.

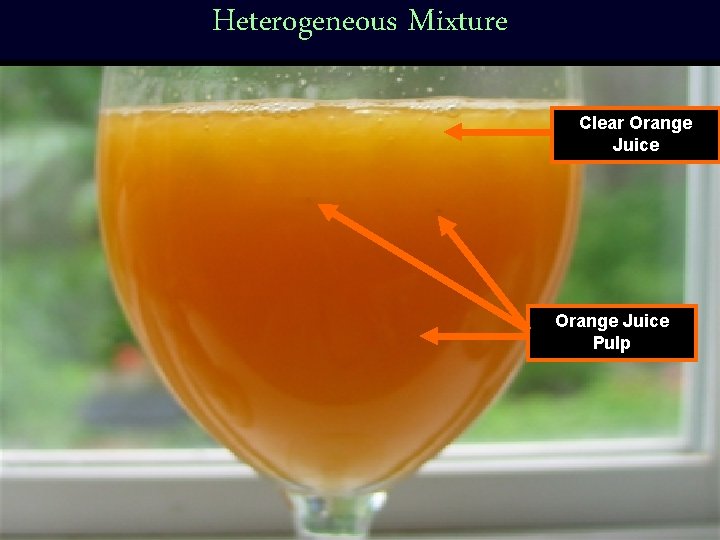
Types of Mixtures • Mixtures can be categorized into two groups: – Heterogeneous and Homogeneous – Definition: heterogeneous mixture – a mixture made of visibly different substances. • The particles in a het. Mixture are not spread evenly. • Orange juice with pulp is an example of a heterogeneous mixture.

Heterogeneous Mixture Clear Orange Juice Pulp

Heterogeneous Mixtures Different fragments make up this rock. This rock is a heterogeneous mixture.

Homogeneous Mixtures • Homogeneous mixtures look the same all over. – Definition: homogeneous mixture – a mixture in which the particles are spread evenly. – Sweet tea is an example of a homogenous mixture. – There are several different substances (tea, water, and sugar) but you cannot see them. The tea looks uniform (the same) throughout.


The tea looks the same throughout the pitcher.

Solutions • We can call homogeneous mixtures by another name: solutions. – Definition: solution - a homogenous mixture of 2 or more substances that are evenly dispersed.

Solutions • Many solutions are formed by dissolving one substance into another substance. – These tablets are dissolving in the water to form a solution.

What is in a Solution? • It is important to know what makes up a solution. • All solutions are made of solutes and solvents. – Definition: solute - a substance that dissolves into another substance. – Definition: solvent - a substance that a solute is dissolved into.

Solvents • For example: – When you stir sugar into water, the sugar dissolves. – The water is the solvent. – The sugar is the solute. = + Solute Solvent Solution

The Universal Solvent • Water is very good at dissolving things. – Water is known as “The Universal Solvent”

Other types of Solutions • Not all solutions contain water! • Other states of matter can be solutions. – Gases & Solids can form solutions also. – Air is an example of a gaseous solution. – Air is composed of lots of different gases that we cannot see.

Metal Alloys • 2 or more solids can form solutions also. – Metal alloys are homogenous mixtures that contain a metal mixed with another substance. – Some examples are: • Steel – iron and carbon • Brass – copper and zinc • Bronze – copper and tin

Metal Alloys • In order to make an alloy, the metals must be melted. – While melted, the metals are mixed to form a solution. Bronze + Tin Copper

Chapter 7: Solutions Section 2: How Substances Dissolve

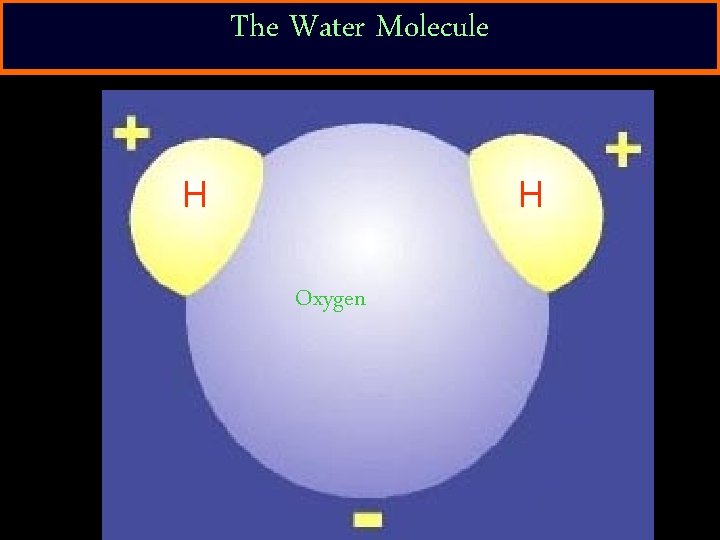
How do things dissolve? • Water can dissolve ionic compounds because of its structure. – The electrons in the hydrogen atoms are pulled toward the oxygen atom. – This gives the oxygen atom a slight negative charge. – The hydrogen atoms gain a slight positive charge.

The Water Molecule H H Oxygen

Polar Compound • Because water’s + and – charges are not spread out evenly, it becomes “polar”. – Definition: polar compound – a molecule that has a positive side and a negative side. • Because water is a polar compound, it is a good solvent.

Like dissolves Like – In chemistry, a rule of thumb is that “like dissolves like. ” – Water is a polar compound, so it can dissolve other polar compounds. – If water cannot dissolve a substance, then that substance is “nonpolar”.

Nonpolar Compound – Definition: nonpolar compound – a compound that has no charge on its molecules. . • Nonpolar compounds can only dissolve other nonpolar substances. • Example – oil-based paint will not dissolve in water. A nonpolar solvent must be used.

Polar vs. Nonpolar Oil is Nonpolar Water is Polar They cannot mix.

The Dissolving Process • We have all seen solutes dissolve into solvents before. • And we all *probably* know some ways to speed up the process.

The Dissolving Process • Making a solute smaller makes it dissolve faster. – By crushing up a solute, you increase the surface area. Crushed Salt Rock Salt

The Dissolving Process • Stirring or shaking will make a solute dissolve faster.

The Dissolving Process • Increasing the solvent’s temperature will make the solute dissolve faster. Which one will Dissolve sugar Fastest?

The Dissolving Process: Gases • Did you know that liquids can dissolve gases? • Fish and other aquatic life breath oxygen that has dissolved into water. • Liquids dissolve gases best when they are cold.

The Dissolving Process: Gases • Don’t believe it? Well, consider this… – Which makes a louder “whoosh” sound when opened… a hot soda or a cold one?

Concentration • The amount of solute dissolved in a solvent affects its concentration. – Think of concentration as being how “strong” a solution is. – Definition: concentration – the amount of a substance in a certain amount of solution.

Concentration • “Concentrated” substances have lots of solute. • “Diluted” substances only have a little solute.

Saturated / Unsaturated • When a solvent can no longer hold any more solute, we called it “saturated”. – Definition: saturated solution – a solution that cannot dissolve any more of a given solute. – Definition: unsaturated – a solution that CAN hold more solute.

Super Saturated • Sometimes, a solvent can be made to hold more solute than normal. – The solution is called “supersaturated”. – Definition: supersaturated – a solution that has more solute than normal.

Super Saturated • Supersaturated solutions are unstable. – The extra solute can “fall out” at any time.