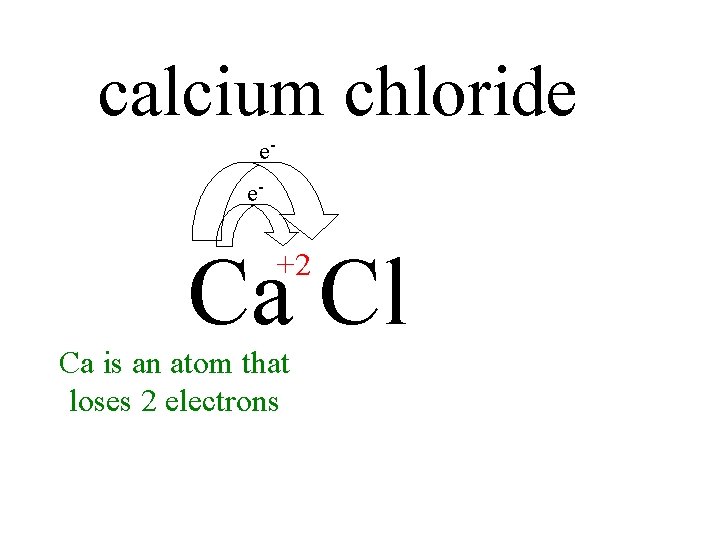
calcium chloride Ca Cl calcium chloride ee Ca


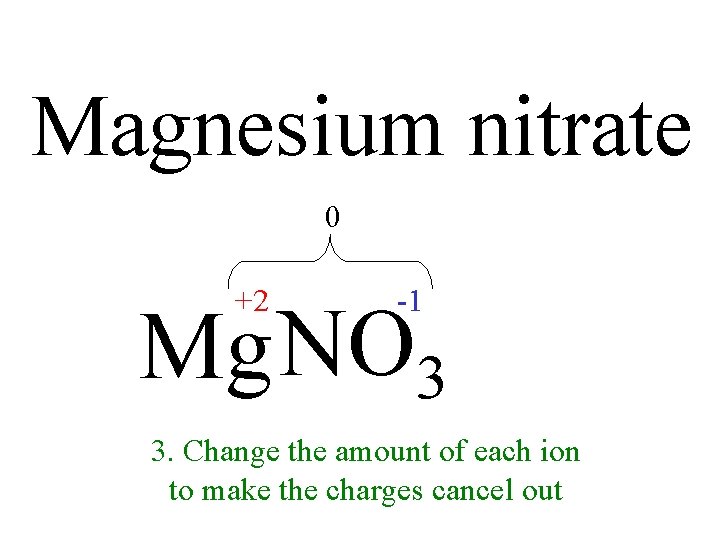
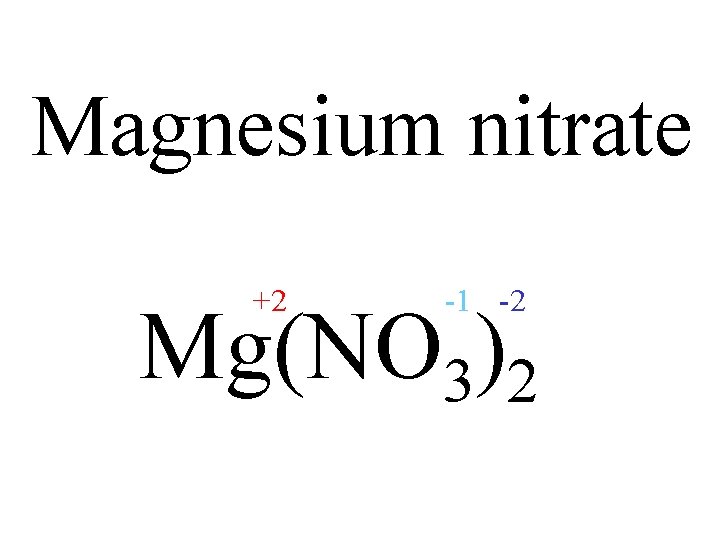
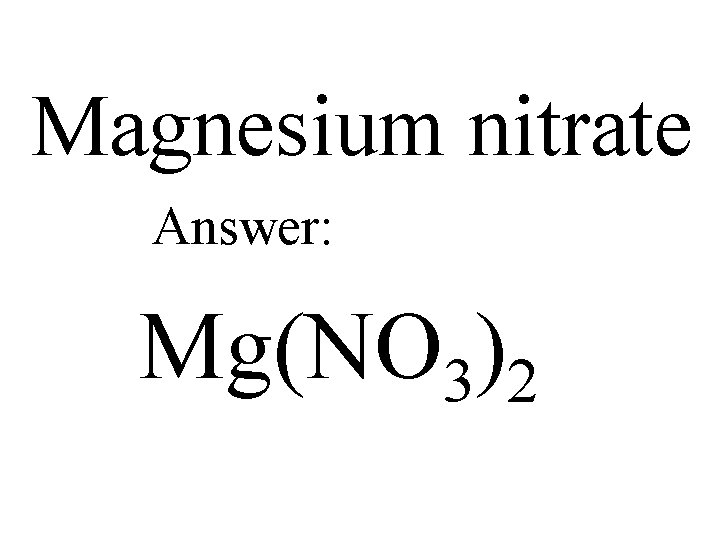
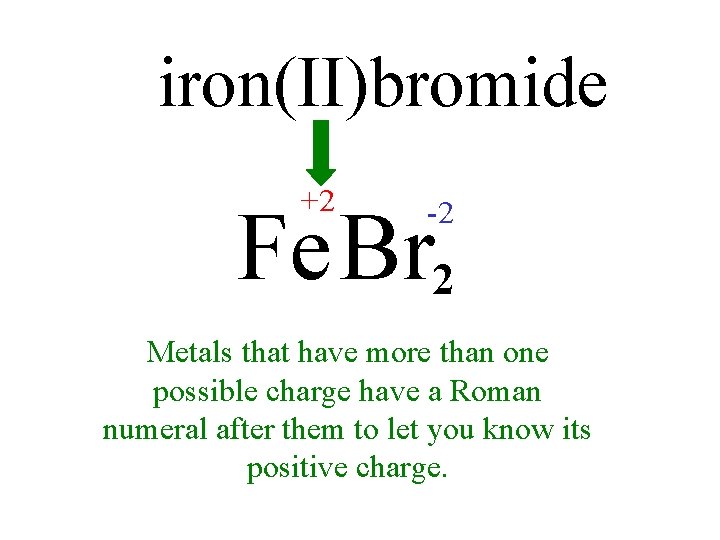
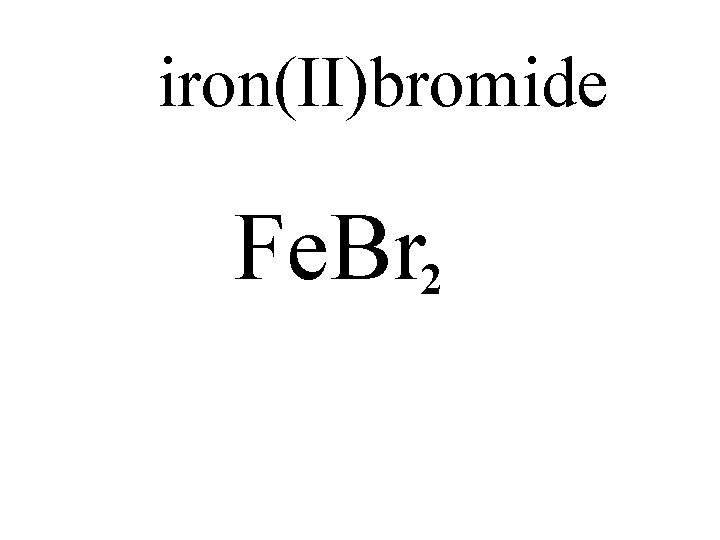

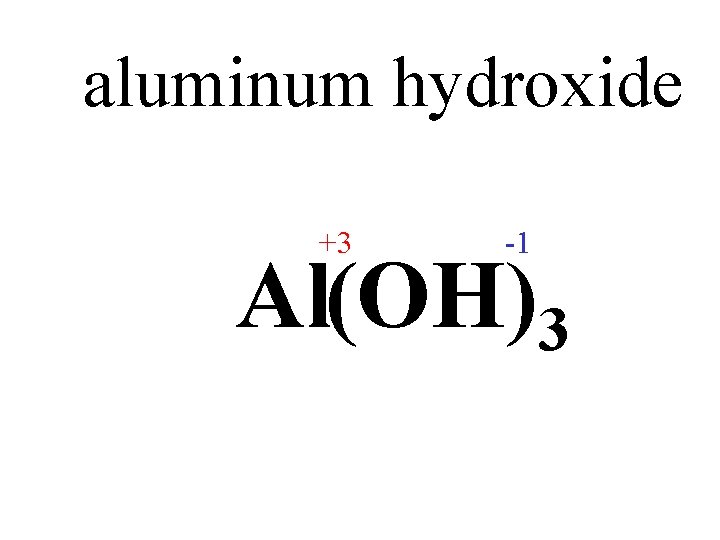





- Slides: 28


calcium chloride Ca Cl

calcium chloride ee- Ca Cl +2 Ca is an atom that loses 2 electrons

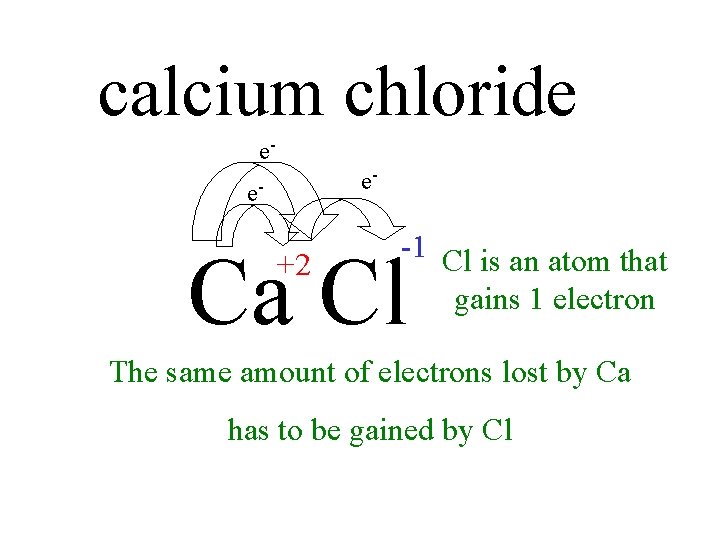
calcium chloride e- e- e- -1 Cl is an atom that gains 1 electron Ca Cl +2 The same amount of electrons lost by Ca has to be gained by Cl

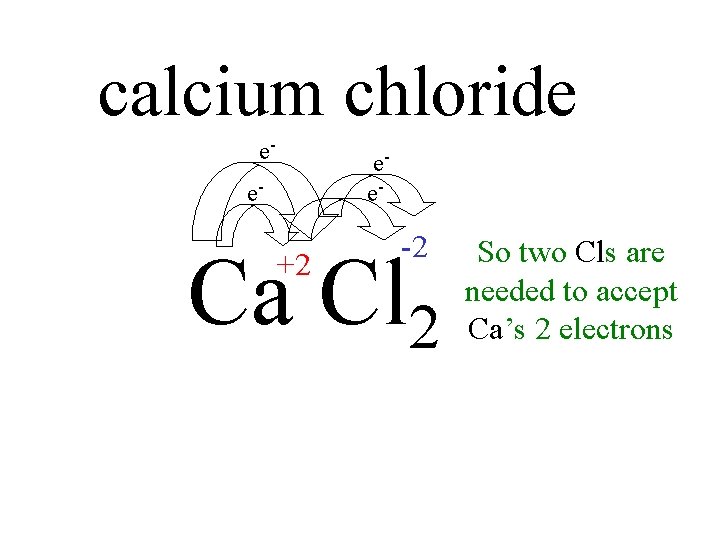
calcium chloride e- e- -2 Ca Cl 2 +2 So two Cls are needed to accept Ca’s 2 electrons

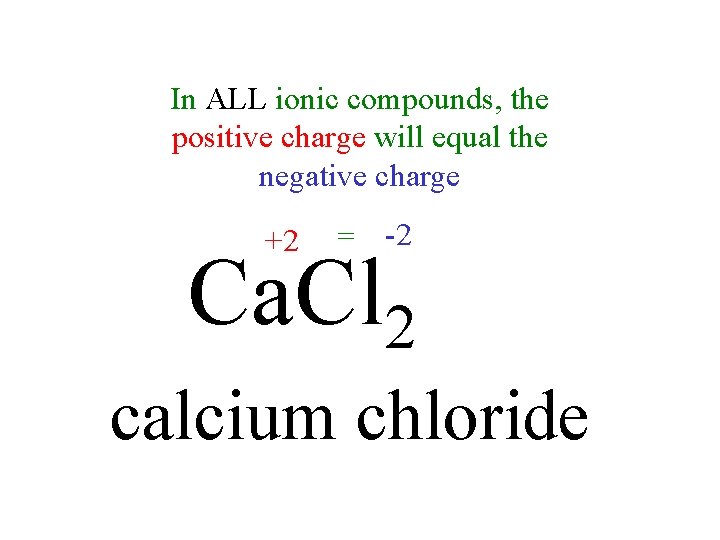
In ALL ionic compounds, the positive charge will equal the negative charge +2 = -2 Ca. Cl 2 calcium chloride

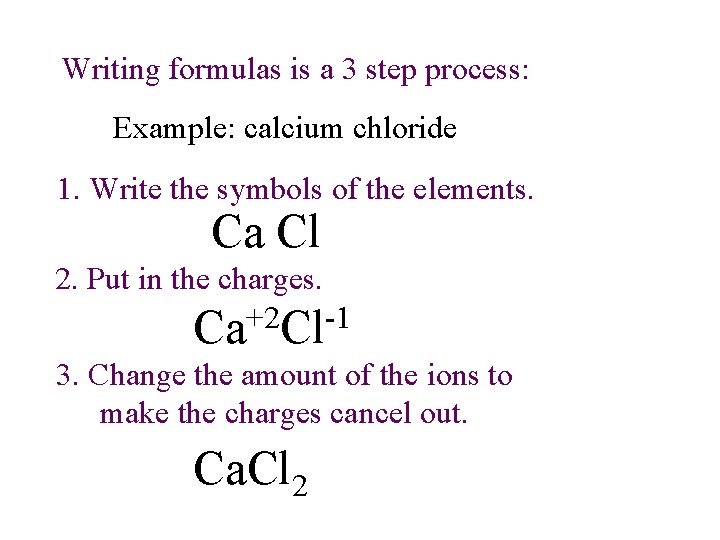
Writing formulas is a 3 step process: Example: calcium chloride 1. Write the symbols of the elements. Ca Cl 2. Put in the charges. Ca+2 Cl-1 3. Change the amount of the ions to make the charges cancel out. Ca. Cl 2
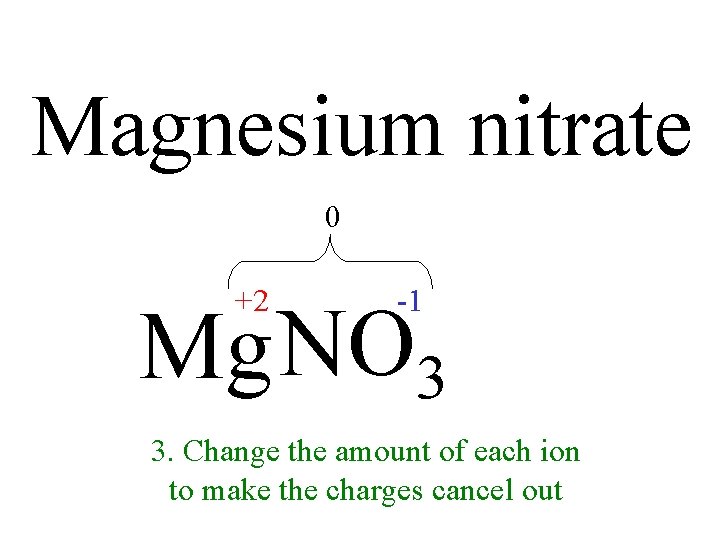
Magnesium nitrate 0 +2 -1 Mg NO 3 3. 1. Change thein amount of each ion Write the formula of 2. Put the charges to make the charges cancel out
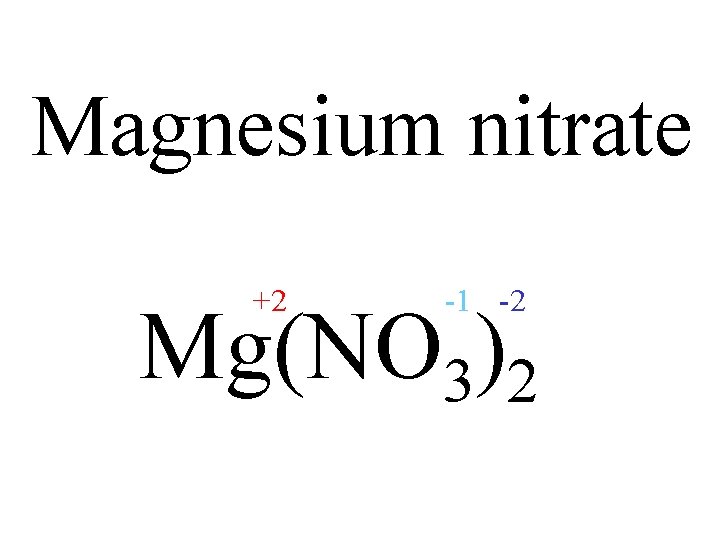
Magnesium nitrate +2 -1 -2 Mg(NO 3)2
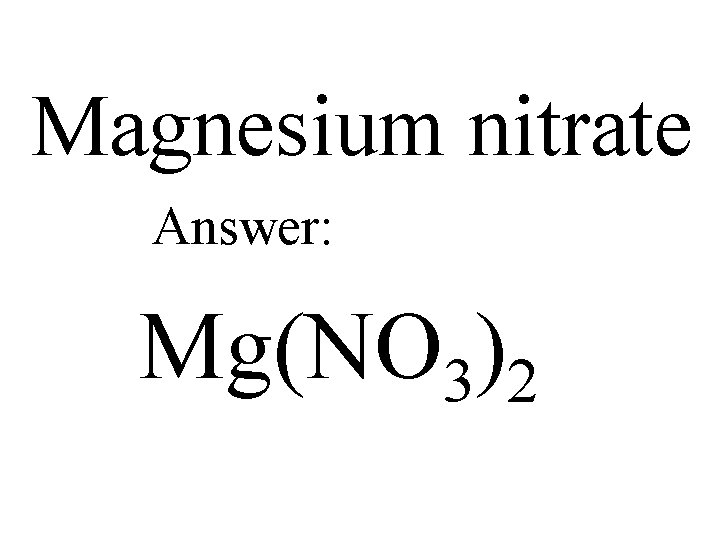
Magnesium nitrate Answer: Mg(NO 3)2
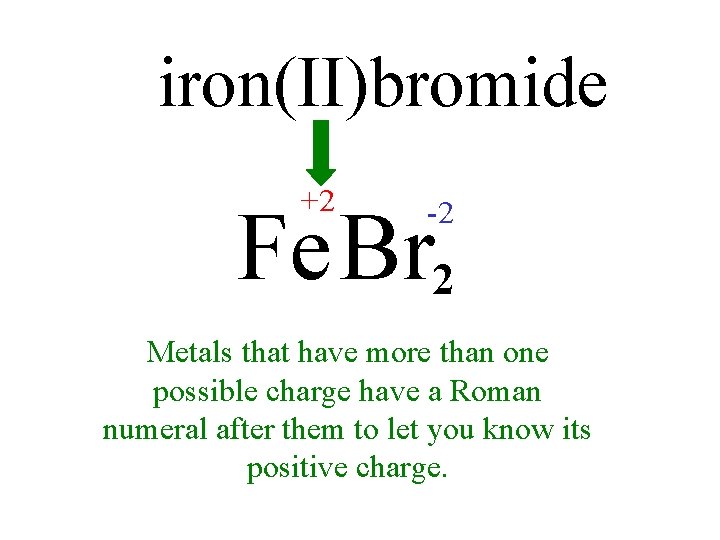
iron(II)bromide +2 Fe Br 2 -2 -1 Metals that have more than one possible charge have a Roman numeral after them to let you know its positive charge.
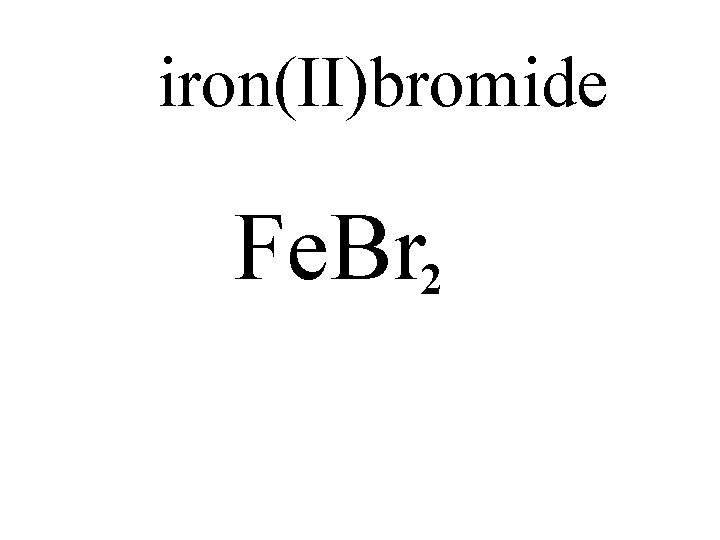
iron(II)bromide Fe. Br 2

aluminum hydroxide +3 -1 Al OH
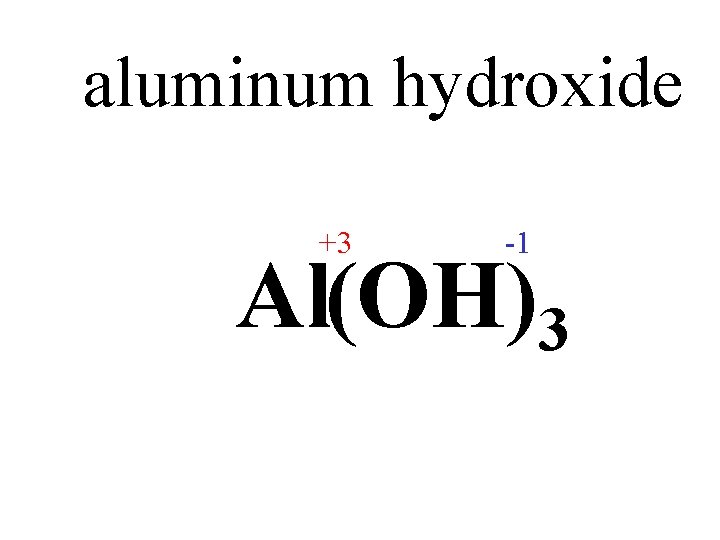
aluminum hydroxide +3 -1 Al(OH)3

aluminum hydroxide Al(OH)3

Example: calcium sulfide +2 -2 Ca. S

Example: calcium sulfide Ca. S

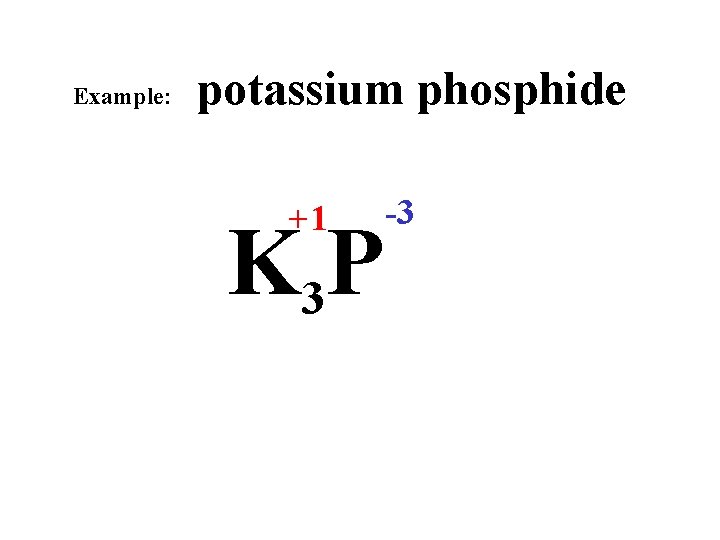
Example: potassium phosphide +1 K 3 P -3

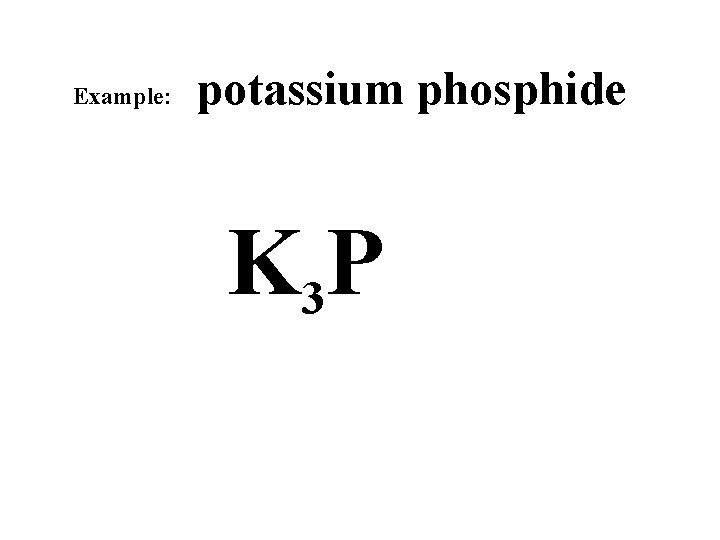
Example: potassium phosphide K 3 P

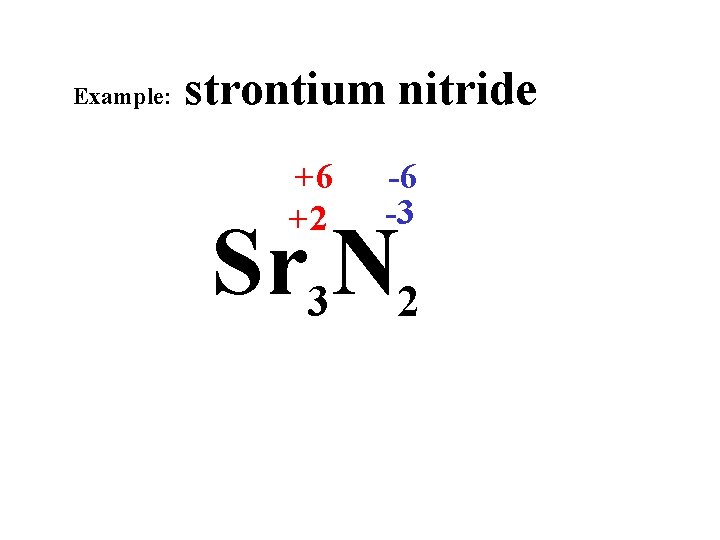
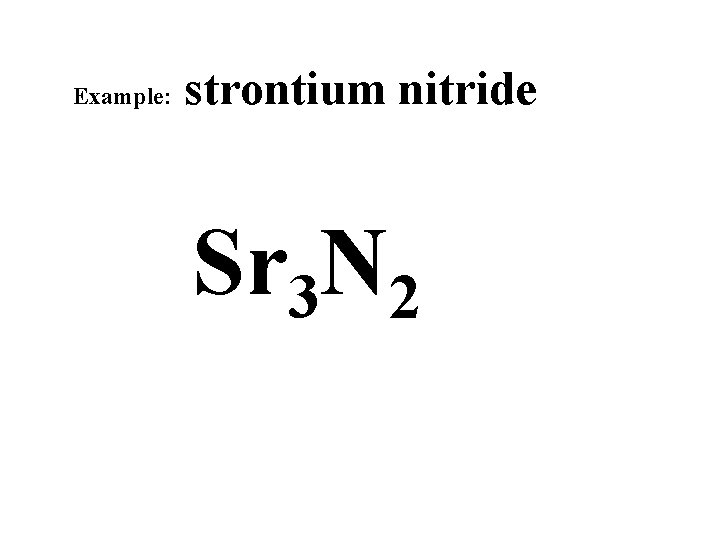
Example: strontium nitride +6 +2 -6 -3 Sr 3 N 2

Example: strontium nitride Sr 3 N 2

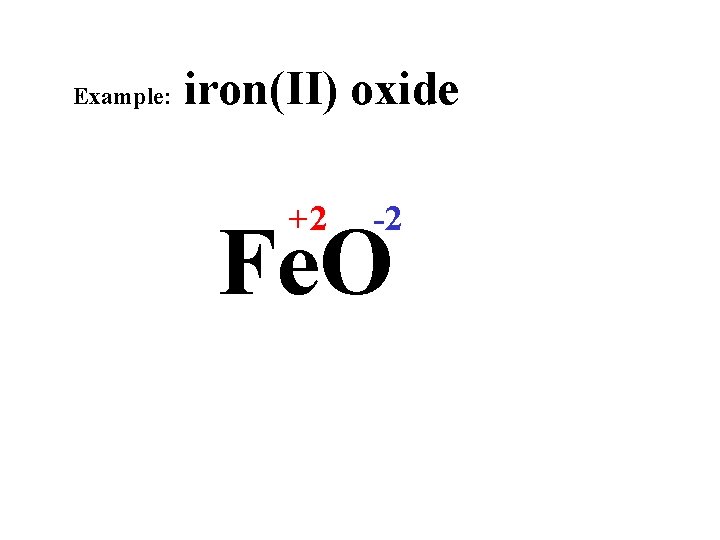
Example: iron(II) oxide +2 -2 Fe. O

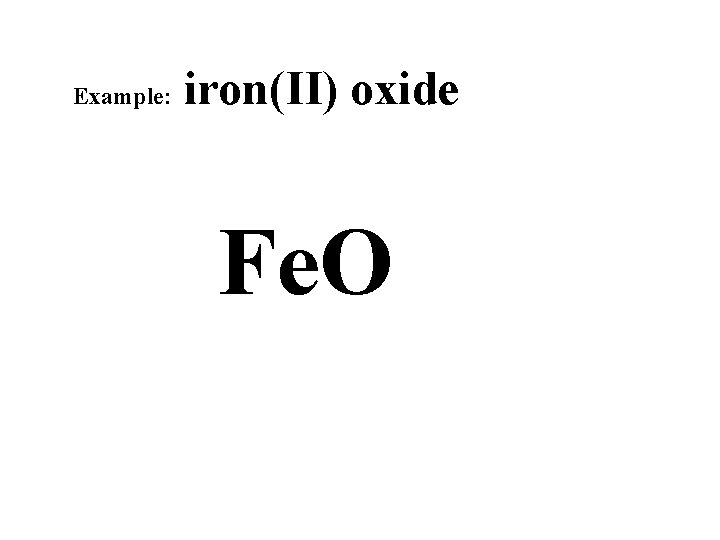
Example: iron(II) oxide Fe. O

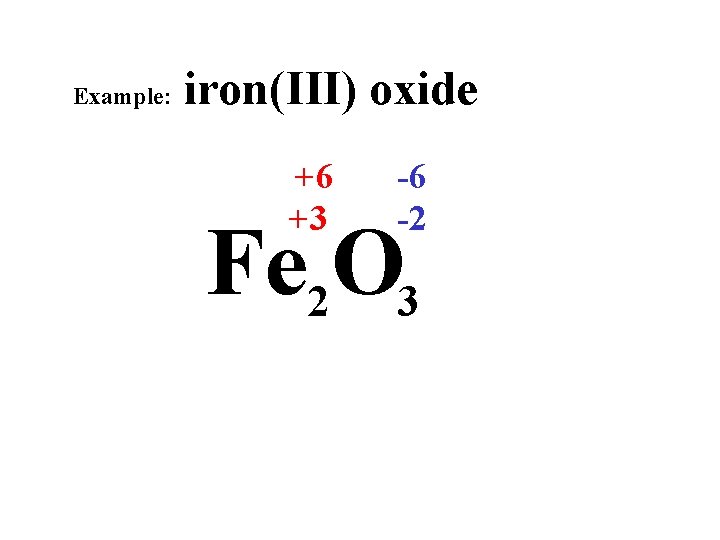
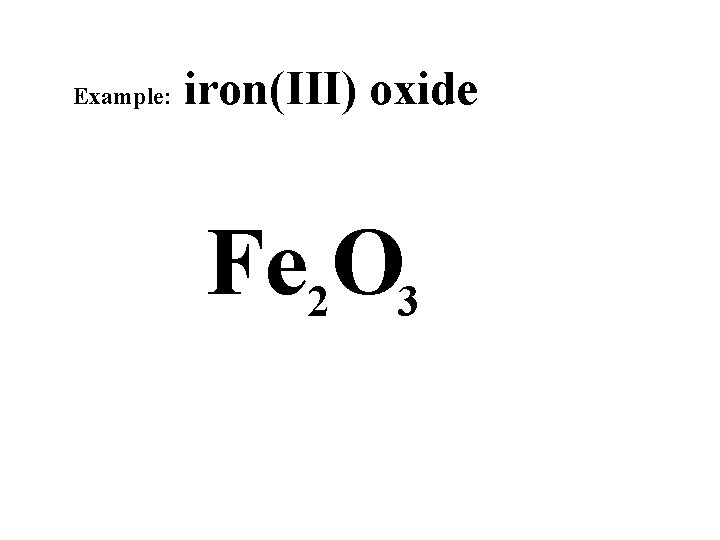
Example: iron(III) oxide +6 +3 -6 -2 Fe 2 O 3

Example: iron(III) oxide Fe 2 O 3

Example: dinitrogen trioxide N 2 O 3

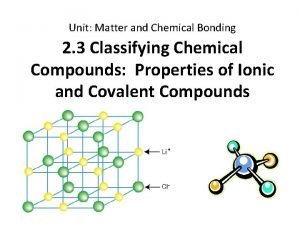
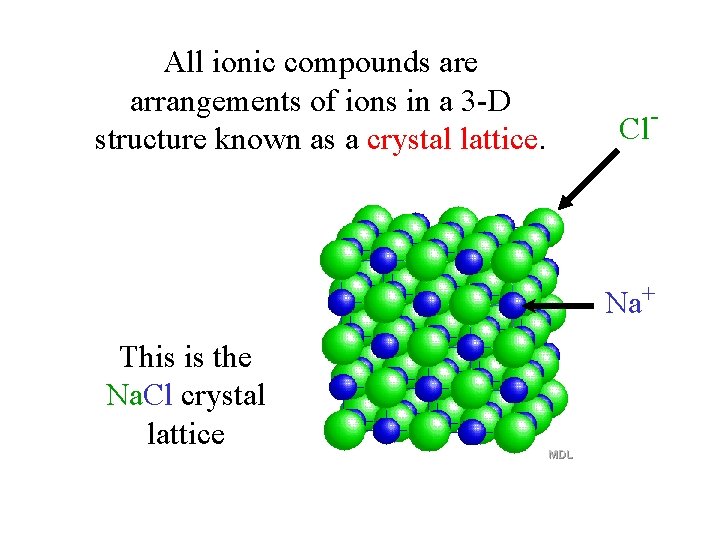
All ionic compounds are arrangements of ions in a 3 -D structure known as a crystal lattice. Cl- Na+ This is the Na. Cl crystal lattice

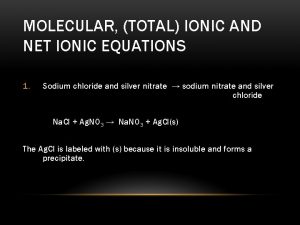
 Molecular total and net ionic equations
Molecular total and net ionic equations Sodium chloride chemical equation
Sodium chloride chemical equation Sodium chloride lewis structure
Sodium chloride lewis structure Can fused calcium chloride dry ammonia
Can fused calcium chloride dry ammonia Synthesis of sodium chloride
Synthesis of sodium chloride Empirical formula of copper chloride lab
Empirical formula of copper chloride lab Copper chloride formula
Copper chloride formula Silicon(iv) chloride dot and cross diagram
Silicon(iv) chloride dot and cross diagram Buffer solution uses
Buffer solution uses Discuss the phase diagram of ferric chloride water system
Discuss the phase diagram of ferric chloride water system Red river chloride control project
Red river chloride control project Mercurimetric titration
Mercurimetric titration Barium nitrate criss cross method
Barium nitrate criss cross method Oxalyl chloride reaction with amine
Oxalyl chloride reaction with amine Silver nitrate and potassium chloride
Silver nitrate and potassium chloride Atoms tend to gain lose or share electrons
Atoms tend to gain lose or share electrons Gettler chloride test
Gettler chloride test Sodium chloride chemical equation
Sodium chloride chemical equation Magnesium ribbon formula
Magnesium ribbon formula Triammine triaquachromium (iii) chloride
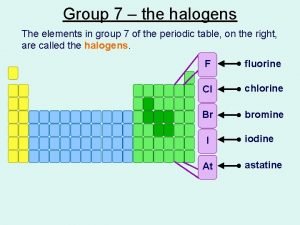
Triammine triaquachromium (iii) chloride Elements in group 7
Elements in group 7 Alcohol to alkyl halide
Alcohol to alkyl halide Dibal h reduction
Dibal h reduction Benzene with ethanoyl chloride
Benzene with ethanoyl chloride Chloride half equation
Chloride half equation Empirical formula of copper chloride
Empirical formula of copper chloride Aminolysis of acid chlorides
Aminolysis of acid chlorides Boiling point of sodium chloride
Boiling point of sodium chloride Ammonium chloride criss cross method
Ammonium chloride criss cross method