The Octet Rule Atoms tend to gain lose














- Slides: 34

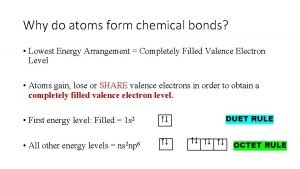
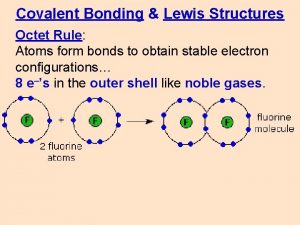
The Octet Rule Atoms tend to gain, lose, or share electrons until they have eight valence electrons. OCTET RULE Rule in which all elements want to satisfy. All elements want to have a full or empty outermost shell/energy level. They want to have 8 valence electrons to become stable. Valence Electrons - Outermost electrons on the outermost shell/energy level Valence electrons are the available electrons for bonding. The Group number tells you the number of valence electrons. 8

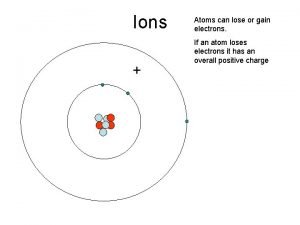
Atomic Structure • ATOMS – Differ by number of protons • ISOTOPES – Differ by number of neutrons • IONS – Differ by number of electrons

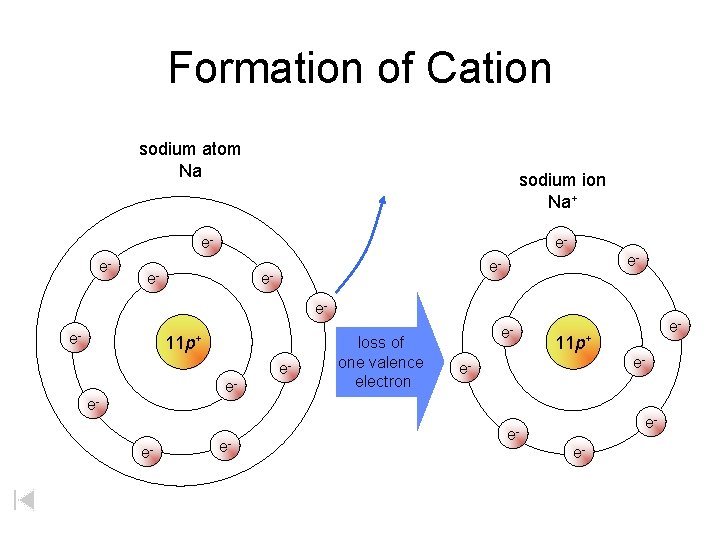
Formation of Cation sodium atom Na sodium ion Na+ ee- e- 11 p+ ee- loss of one valence electron e- e- 11 p+ e- e- ee-

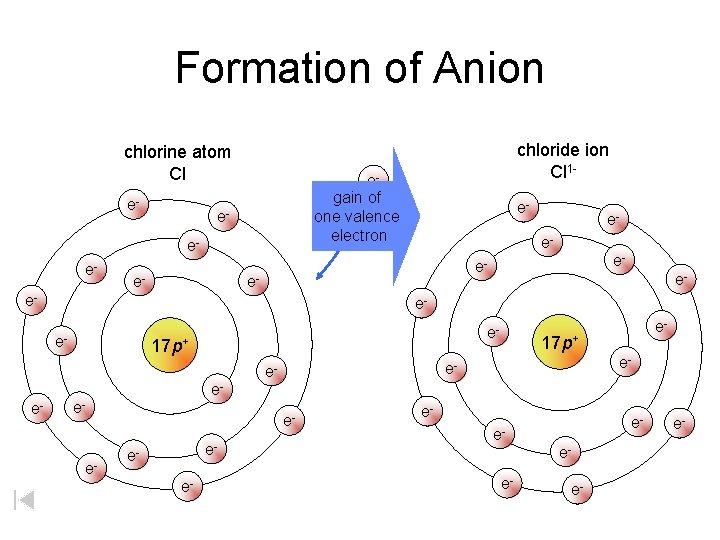
Formation of Anion chlorine atom Cl e- egain of one valence electron ee- e- e- chloride ion Cl 1 e- eee- e- 17 p+ e- e- ee- e- e- eee- e- e-

Formation of Ionic Bond chloride ion Cl 1 - sodium ion Na+ e- e- ee- e- 11 p+ e- e- e- 17 p+ e- ee- e- e-

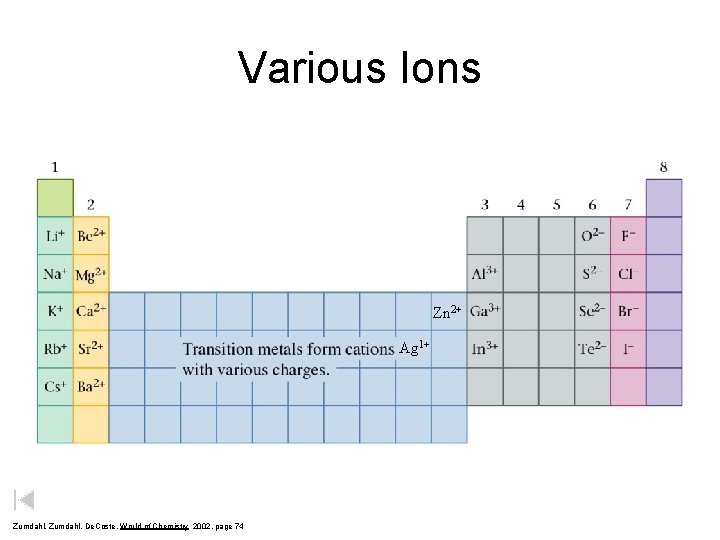
Various Ions Zn 2+ Ag 1+ Zumdahl, De. Coste, World of Chemistry 2002, page 74

Periodic Table with Group Names

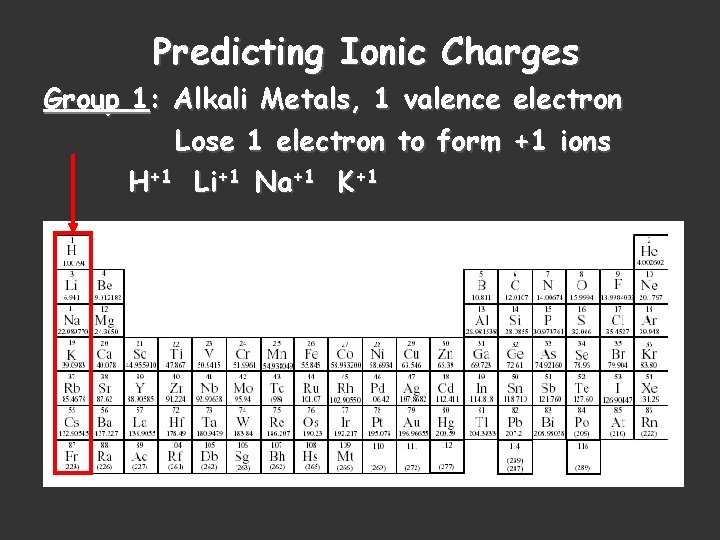
Predicting Ionic Charges Group 1: Alkali Metals, 1 valence electron Lose 1 electron to form +1 ions H+1 Li+1 Na+1 K+1

Predicting Ionic Charges Group 2: Alkaline Earth Metals, 2 valence e. Loses 2 electrons to form +2 ions Be+2 Mg+2 Ca+2 Sr+2 Ba+2

Predicting Ionic Charges Groups 3 - 12: Transition Metals Many transition elements have more than one possible oxidation state. Iron(II) = Fe+2 Iron(III) = Fe+3

Predicting Ionic Charges B+3 Group 13: Boron Group, 3 valence e. Loses 3 electrons +3 +3 Al Ga to form +3 ions

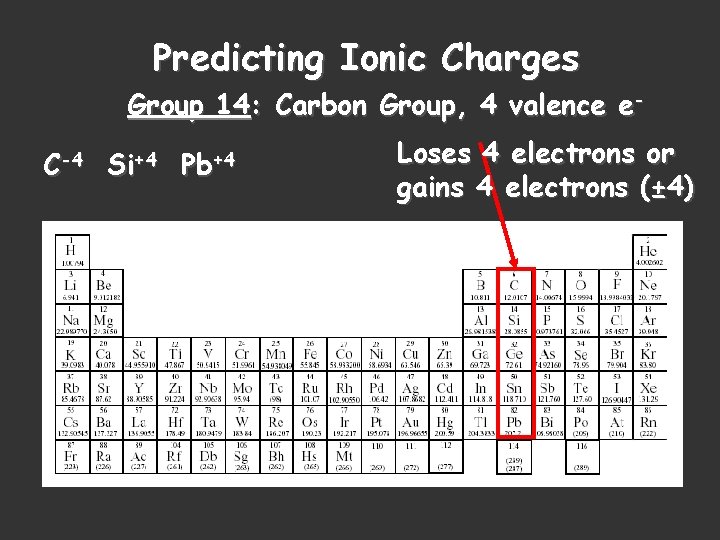
Predicting Ionic Charges Group 14: Carbon Group, 4 valence e. C-4 Si+4 Pb+4 Loses 4 electrons or gains 4 electrons (± 4)

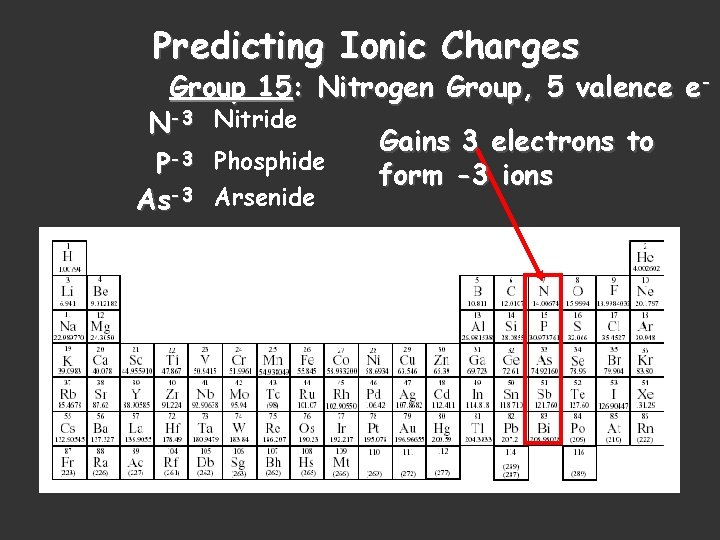
Predicting Ionic Charges Group 15: Nitrogen Group, 5 valence e. N-3 Nitride Gains 3 electrons to P-3 Phosphide form -3 ions As-3 Arsenide

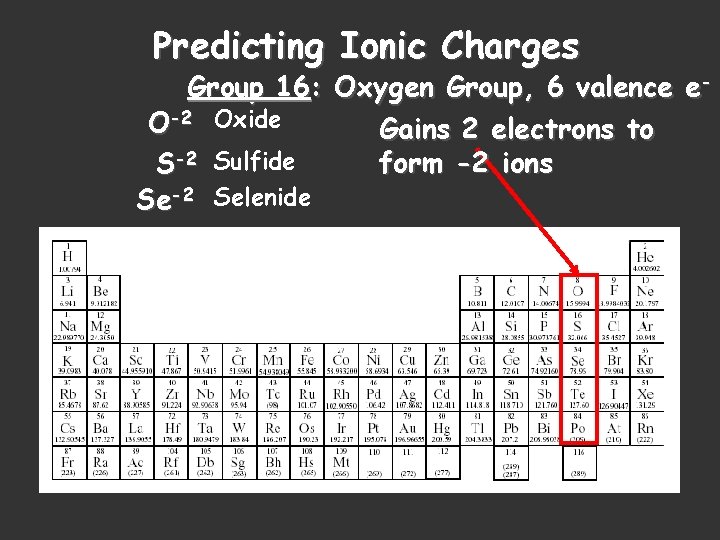
Predicting Ionic Charges Group 16: Oxygen Group, 6 valence e. O-2 Oxide Gains 2 electrons to form -2 ions S-2 Sulfide Se-2 Selenide

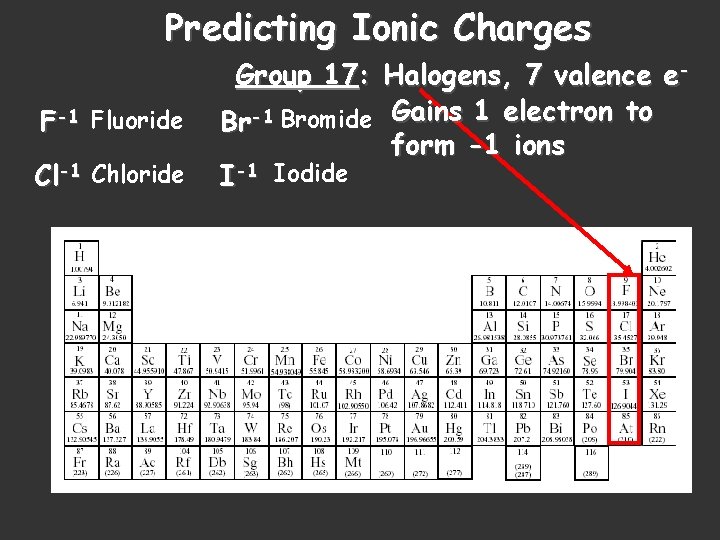
Predicting Ionic Charges F-1 Fluoride Cl-1 Chloride Group 17: Halogens, 7 valence e. Br-1 Bromide Gains 1 electron to form -1 ions I-1 Iodide

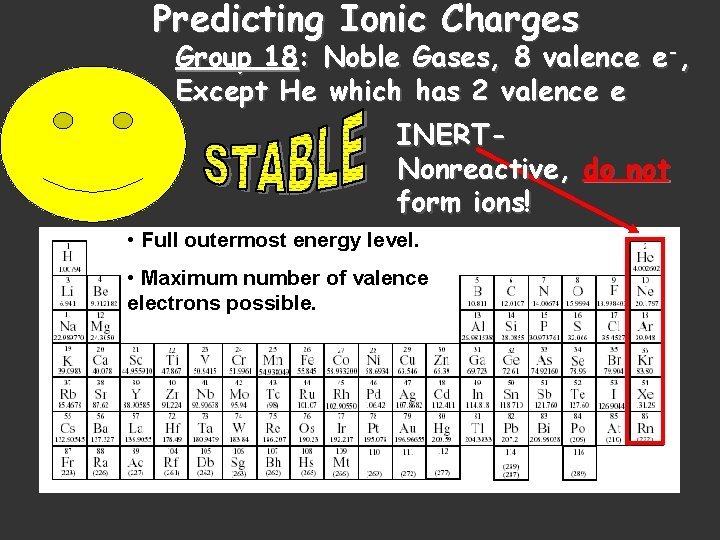
Predicting Ionic Charges Group 18: Noble Gases, 8 valence e-, Except He which has 2 valence e INERTNonreactive, do not form ions! • Full outermost energy level. • Maximum number of valence electrons possible.

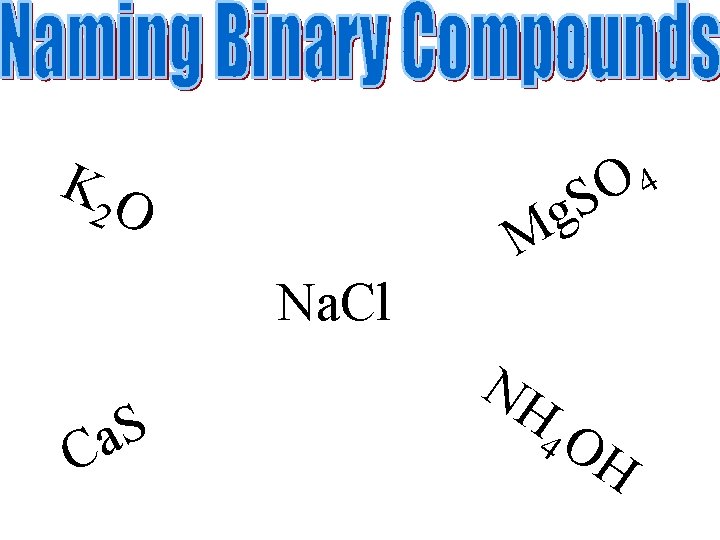
KO 4 O S g M 2 Na. Cl S a C NH 4 O H

First - write the name of the metal. (Cation) Name does not change Then - write the name of the nonmetal (Anion) with an -ide ending. Examples: Na. Cl - sodium chloride Ca. I 2 - calcium iodide Li 2 O - lithium oxide Ag 3 N – silver nitride Try These: Mg. S - Magnesium sulfide Zn. Br 2 - Zinc bromide Al 2 O 3 - Aluminum oxide Fe 2 O 3 - Iron(III) oxide (rust)

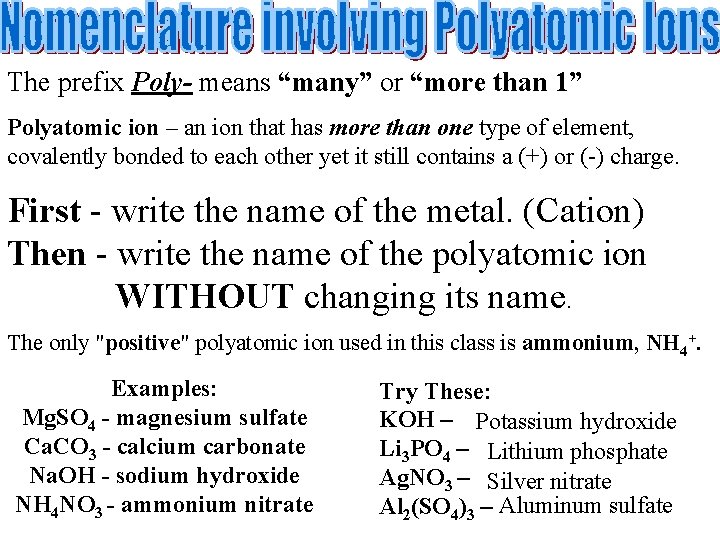
The prefix Poly- means “many” or “more than 1” Polyatomic ion – an ion that has more than one type of element, covalently bonded to each other yet it still contains a (+) or (-) charge. First - write the name of the metal. (Cation) Then - write the name of the polyatomic ion WITHOUT changing its name. The only "positive" polyatomic ion used in this class is ammonium, NH 4+. Examples: Mg. SO 4 - magnesium sulfate Ca. CO 3 - calcium carbonate Na. OH - sodium hydroxide NH 4 NO 3 - ammonium nitrate Try These: KOH – Potassium hydroxide Li 3 PO 4 – Lithium phosphate Ag. NO 3 – Silver nitrate Al 2(SO 4)3 – Aluminum sulfate

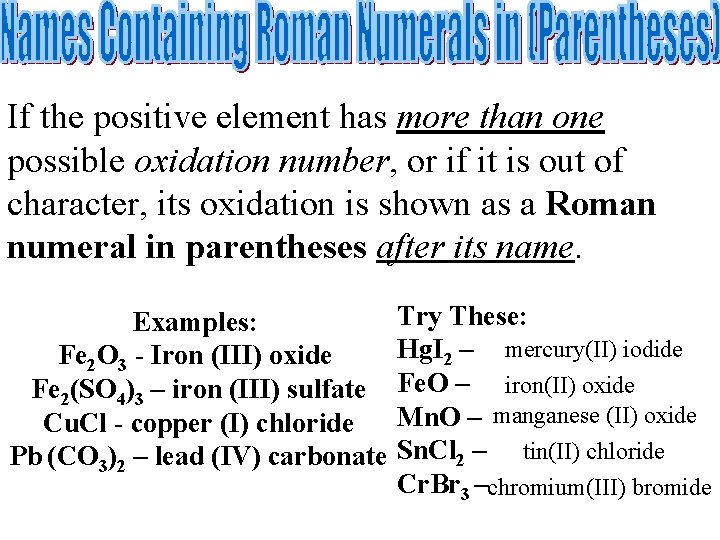
If the positive element has more than one possible oxidation number, or if it is out of character, its oxidation is shown as a Roman numeral in parentheses after its name. Examples: Fe 2 O 3 - Iron (III) oxide Fe 2(SO 4)3 – iron (III) sulfate Cu. Cl - copper (I) chloride Pb (CO 3)2 – lead (IV) carbonate Try These: Hg. I 2 – mercury(II) iodide Fe. O – iron(II) oxide Mn. O – manganese (II) oxide Sn. Cl 2 – tin(II) chloride Cr. Br 3 – chromium(III) bromide

Concept Understanding: Write the correct name for each of these compounds: 1. Fe(OH)2 Iron(II) hydroxide 2. (NH 4)3 PO 4 Ammonium phosphate Aluminum phosphate 3. Al. PO 4 4. Cu(C 2 H 3 O 2)2 Copper(II) acetate 5. Ca. CO 3 Calcium carbonate 6. NH 4 OH Ammonium Hydroxide 7. Cr 2(SO 4)3 Chromium(III) sulfate 8. Mg(NO 3)2 Magnesium nitrate

“Perhaps one of you gentlemen would mind telling me just what is outside the window that you find so attractive. . ? ” Image courtesy Nearing. Zero. net

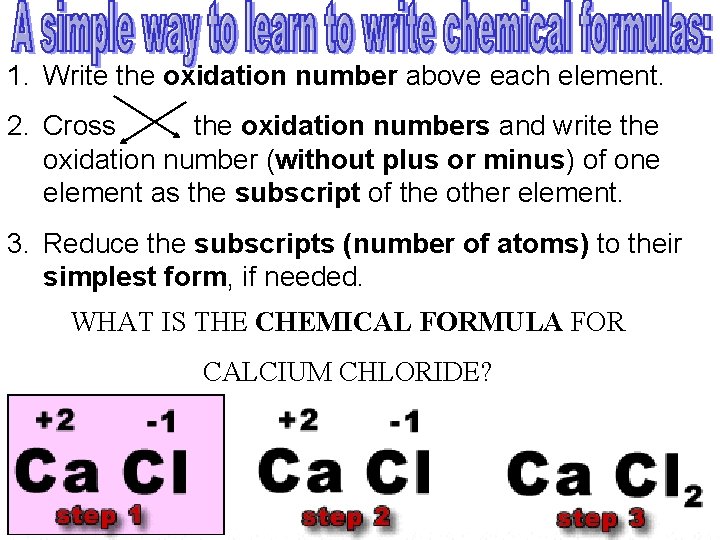
1. Write the oxidation number above each element. 2. Cross the oxidation numbers and write the oxidation number (without plus or minus) of one element as the subscript of the other element. 3. Reduce the subscripts (number of atoms) to their simplest form, if needed. WHAT IS THE CHEMICAL FORMULA FOR CALCIUM CHLORIDE?

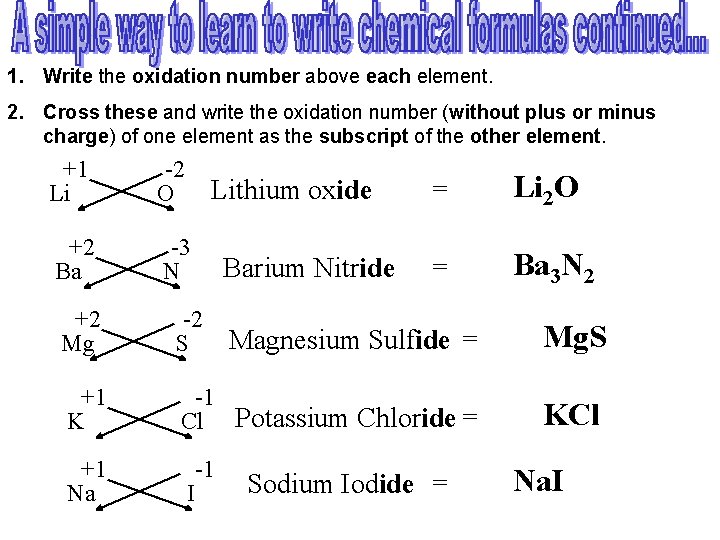
1. Write the oxidation number above each element. 2. Cross these and write the oxidation number (without plus or minus charge) of one element as the subscript of the other element. +1 Li -2 O +2 Ba -3 N Barium Nitride +2 Mg -2 S Magnesium Sulfide = Mg. S Lithium oxide = Li 2 O = Ba 3 N 2 +1 K -1 Cl Potassium Chloride = KCl +1 Na -1 I Sodium Iodide = Na. I

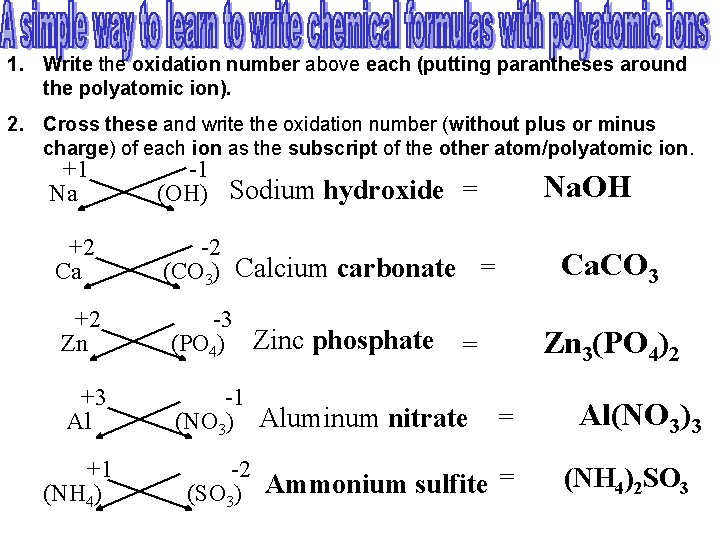
1. Write the oxidation number above each (putting parantheses around the polyatomic ion). 2. Cross these and write the oxidation number (without plus or minus charge) of each ion as the subscript of the other atom/polyatomic ion. +1 Na -1 (OH) Sodium hydroxide = Na. OH +2 Ca -2 (CO 3) Calcium carbonate +2 Zn -3 (PO 4) Zinc phosphate +3 Al +1 (NH 4) = Ca. CO 3 = Zn 3(PO 4)2 -1 (NO 3) Aluminum nitrate = Al(NO 3)3 -2 = (NH 4)2 SO 3 Ammonium sulfite (SO 3)

1. Na. Cl = Sodium Chloride 2. K 2 O = Potassium Oxide 3. Ca. F 2 = Calcium Fluoride 4. Fe 2 O 3= Iron (III) Oxide 5. Cu. Cl = Copper (I) Chloride 6. Na. OH = Sodium Hydroxide 7. Al. PO 4 = Aluminum Phosphate 8. Mg(NO 3)2 = Magnesium Nitrate

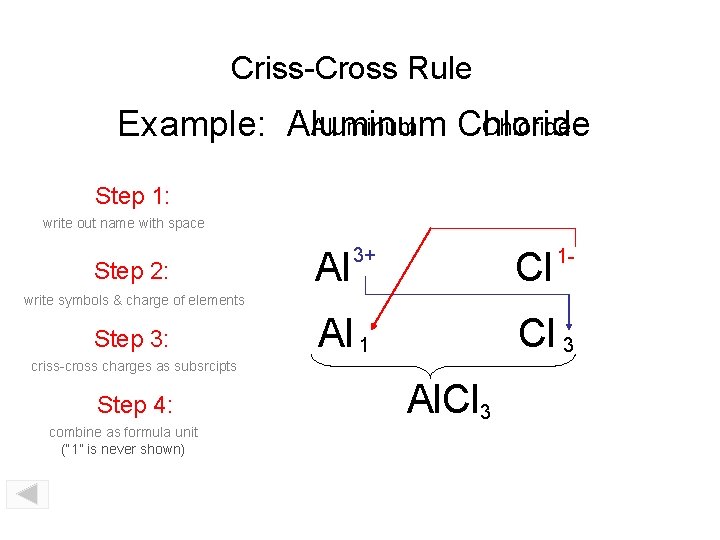
Criss-Cross Rule Aluminum Chloride Example: Aluminum Chloride Step 1: write out name with space Step 2: Al 3+ Cl 1 - write symbols & charge of elements Step 3: Al 1 Cl 3 criss-cross charges as subsrcipts Step 4: combine as formula unit (“ 1” is never shown) Al. Cl 3

Criss-Cross Rule Example: Aluminum Chloride Step 1: Aluminum Chloride Step 2: Al 3+ Cl 1 - Step 3: Al 1 Cl 3 Step 4: Al. Cl 3

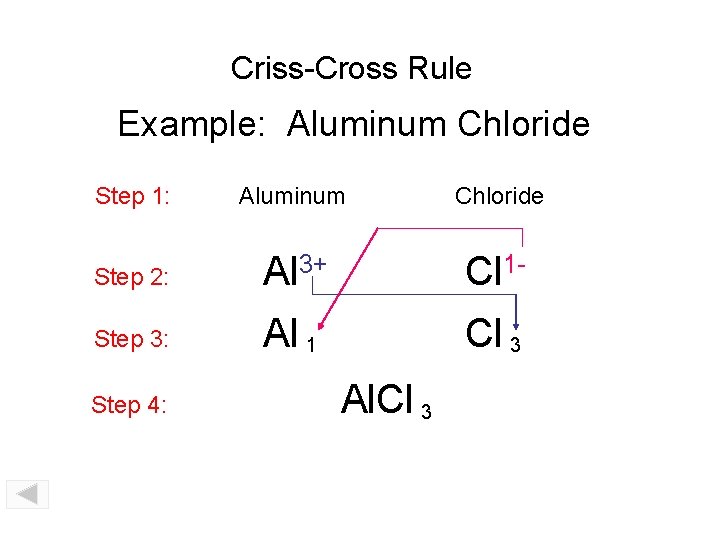
Criss-Cross Rule Example: Aluminum Oxide Step 1: Aluminum Oxide Step 2: Al 3+ O 2 - Step 3: Al 2 O 3 Step 4: Al 2 O 3

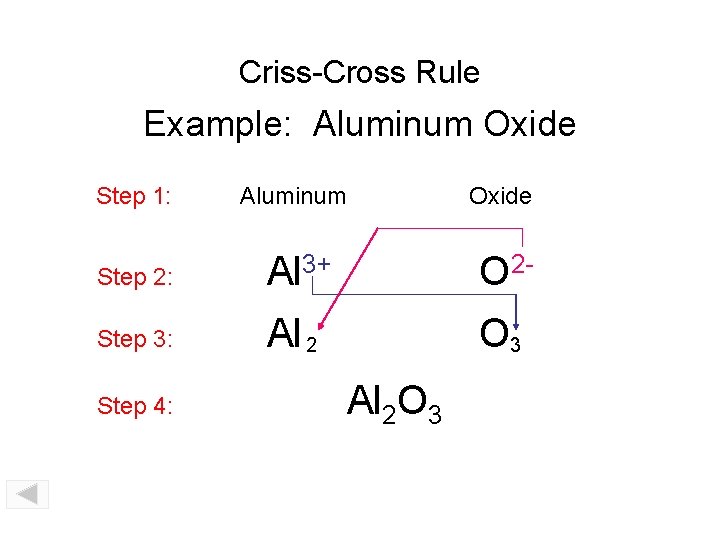
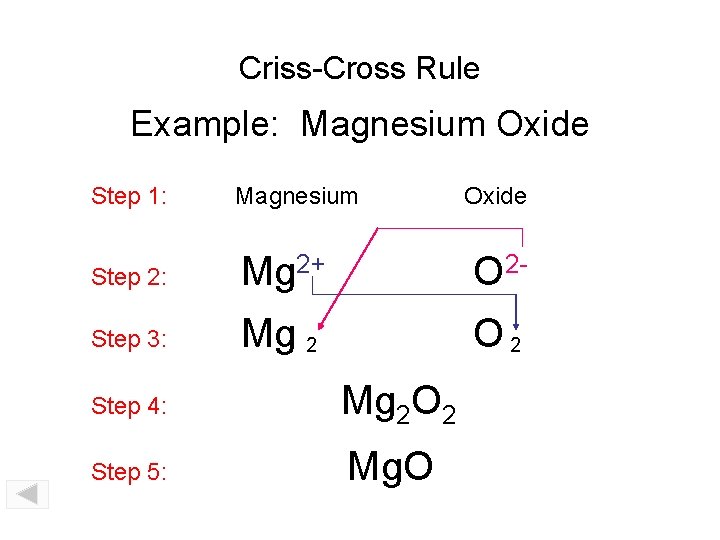
Criss-Cross Rule Example: Magnesium Oxide Step 1: Magnesium Step 2: Mg 2+ O 2 - Step 3: Mg 2 O 2 Step 4: Mg 2 O 2 Step 5: Mg. O Oxide

Ionic Compounds: Formed from oppositely charged ions Cations: Positively charged ions (METALS) give e- away Anions: Negatively charged ions (NONMETALS) take e- in Positive ions (Cations) take the names of the metal from which they are derived: Na+ = Sodium ion, Ca+2 = Calcium ion, Al+3 = Aluminum ion (The name DOES NOT CHANGE) Negative ions (Anions) are named by adding the suffix -ide to the stem of the name of the nonmetal from which they are derived: Cl- = Chloride ion, O -2 = Oxide ion, N-3 = Nitride ion (-ide is added to the end)

1. Cation (Metal) first, then Anion (Nonmetal) 2. Monatomic Cation = name of the element • Ca+2 = calcium ion • Na+ = sodium ion • Al+3 = aluminum ion 3. Monatomic Anion = root name + -ide • Cl- = chloride • O-2 = oxide • Ca. Cl 2 = calcium chloride • Na 2 O = sodium oxide

When a metal forms more than one ion (as the transition metals do), it is necessary to distinguish between these ions. The accepted practice today is to indicate the charge of the ion by a ROMAN NUMERAL in (parentheses) immediately following the name of the metal. The following are the only metals with multiple ions you need to know: Fe+2 = Iron (II) Fe+3 = Iron (III) Hg+ = Mercury (I) Hg+2 = Mercury (II) Cu+ = Copper (I) Cu+2 = Copper (II) Mn+2 = Manganese (II) Mn+3 = Manganese (III) Pb+2 = Lead (II) Pb+4 = Lead (IV) Cr+2 = Chromium (II) Cr+3 = Chromium (III)

Polyatomic Ions – An ion consisting of a group of bonded atoms. Polyatomic ions have a suffix ending in –ate or –ite (except for OH- = hydroxide ion, NH 4+ = Ammonium) The difference in the polyatomic ending, is in the number of Oxygen atoms. Polyatomic ions with more Oxygen atoms will take on the –ate suffix. Sulfate = (SO 4 -2) Nitrate (NO 3 -) Polyatomic ions with less Oxygen atoms will take on the –ite suffix. Sulfite = (SO 3 -2) Nitrite = (NO 2 -)
 Atoms tend to gain lose or share electrons
Atoms tend to gain lose or share electrons Win win win lose lose lose
Win win win lose lose lose Win win situacija
Win win situacija Win lose the totem pole
Win lose the totem pole What atoms can have an expanded octet
What atoms can have an expanded octet Expanded octet
Expanded octet Non bonding electrons examples
Non bonding electrons examples Win win examples
Win win examples Win-win conflict resolution
Win-win conflict resolution N n avogadro
N n avogadro Does carbon gain or lose electrons
Does carbon gain or lose electrons Periodic table 6
Periodic table 6 Unstable nuclei and radioactive decay
Unstable nuclei and radioactive decay Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally The octet rule states that
The octet rule states that Oono- lewis structure
Oono- lewis structure Whats ionic bond
Whats ionic bond Mg octet rule
Mg octet rule Octet rule
Octet rule The octet rule states that
The octet rule states that Ionic bond
Ionic bond Exceptions to the octet rule
Exceptions to the octet rule Lesson 5 eight is enough octet rule answer key
Lesson 5 eight is enough octet rule answer key Lewis structure and vsepr
Lewis structure and vsepr Mayan periodic table
Mayan periodic table The octet rule states that
The octet rule states that Hybridization exceptions
Hybridization exceptions Magnesium electron dot diagram
Magnesium electron dot diagram Mason's gain rule
Mason's gain rule Charge formelle
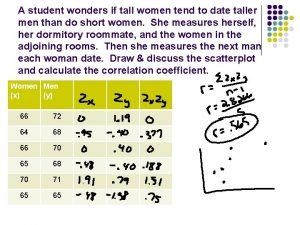
Charge formelle A student wonders if tall women tend to date taller men
A student wonders if tall women tend to date taller men Li atomic mass
Li atomic mass First octet of ip address
First octet of ip address Children tend to learn english
Children tend to learn english Ominous octet
Ominous octet