UNIT 4 Chemical Names Formulas Rxns Chapter 9




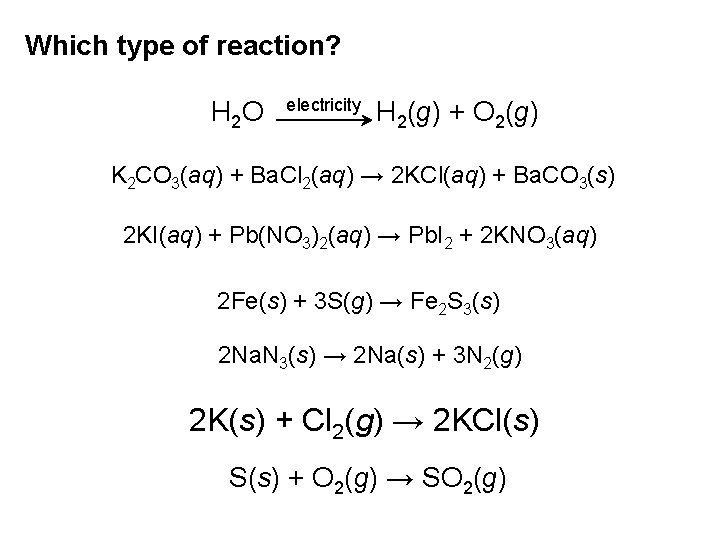
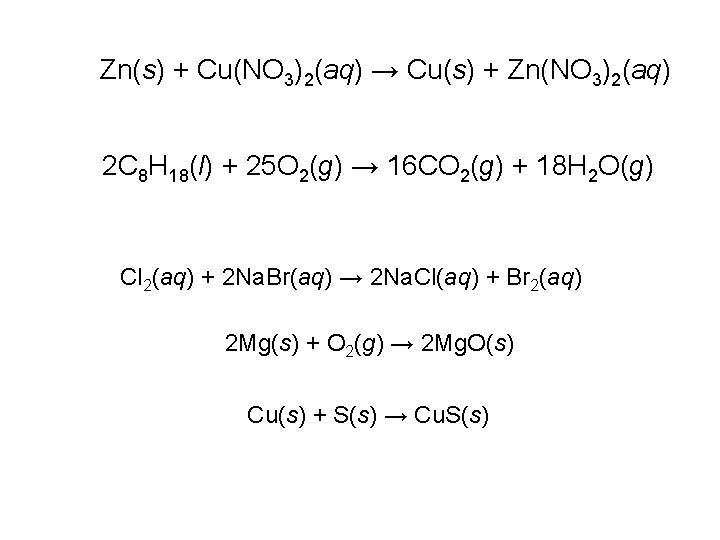

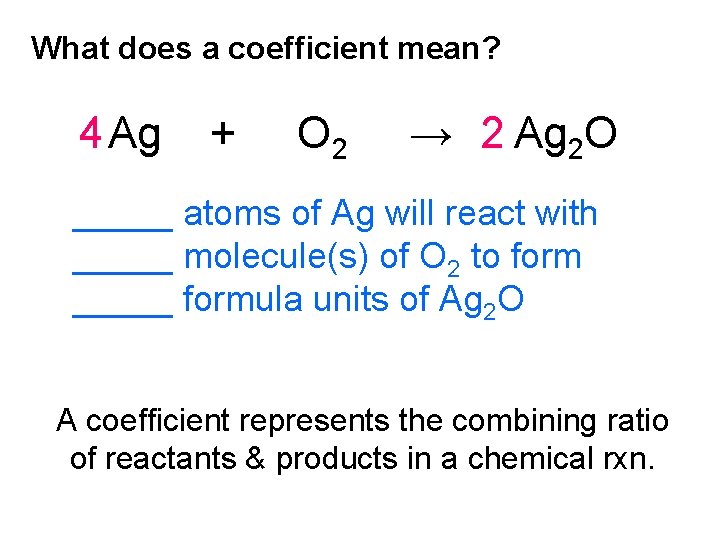
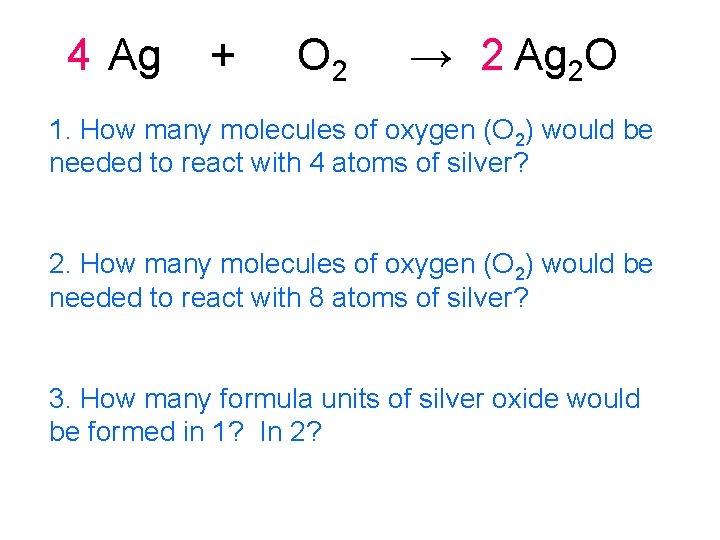
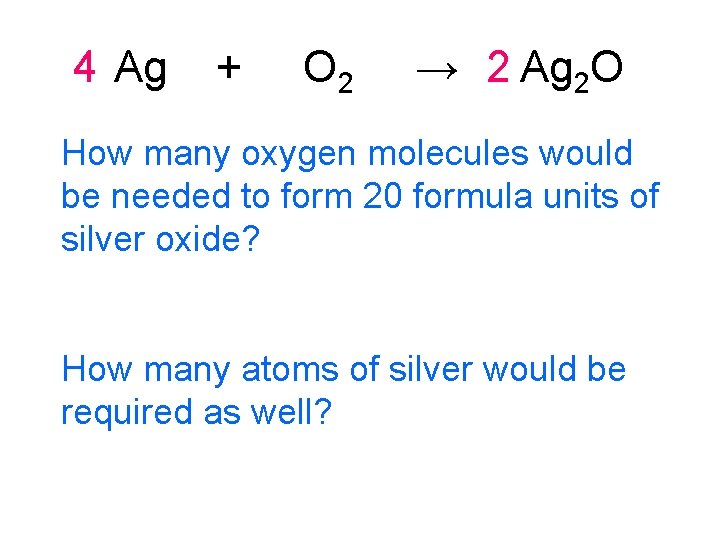
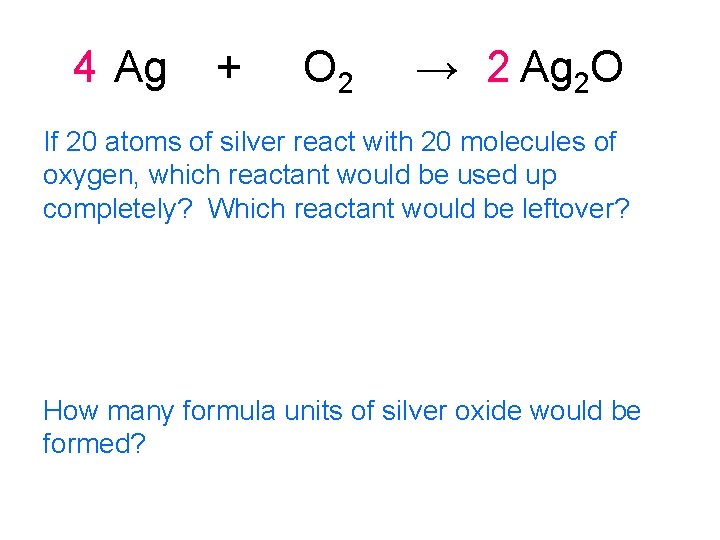

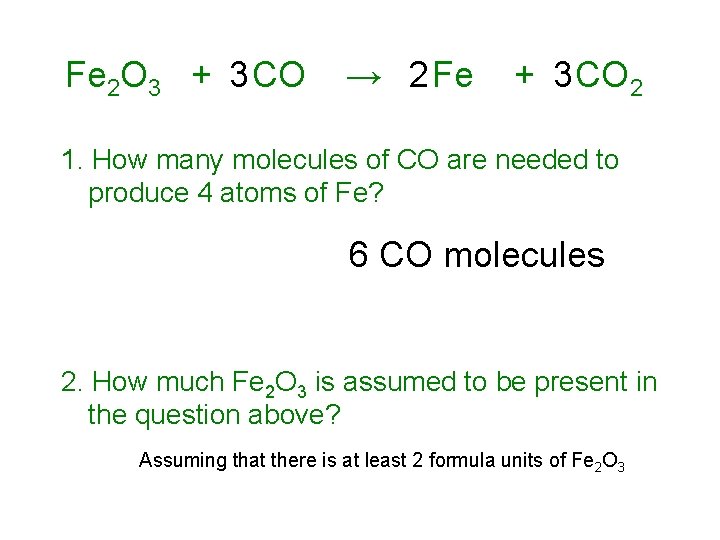

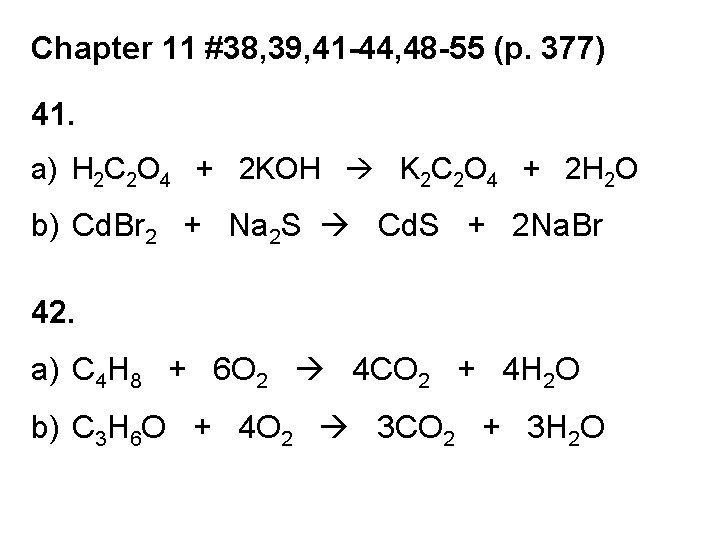
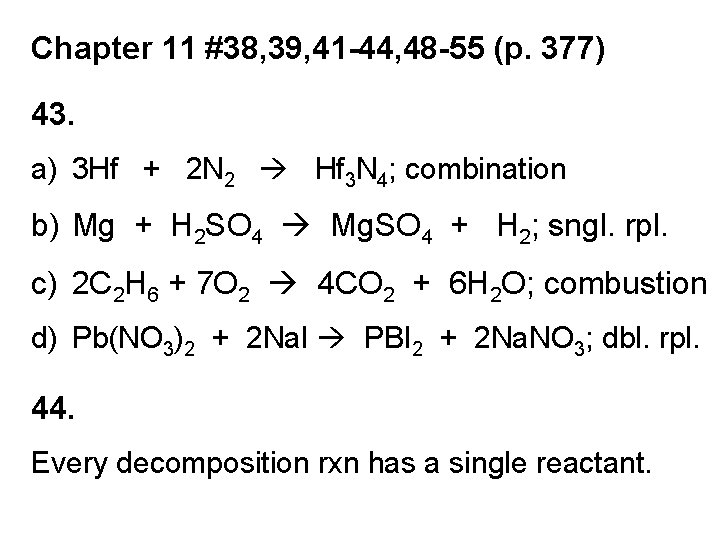


- Slides: 40

UNIT 4 – Chemical Names, Formulas & Rxns Chapter 9 – Chemical Names and Formulas Chapter 11 – Chemical Reactions Chapter 11 Chemical Reactions Anything in black letters = write it in your notes (‘knowts’)

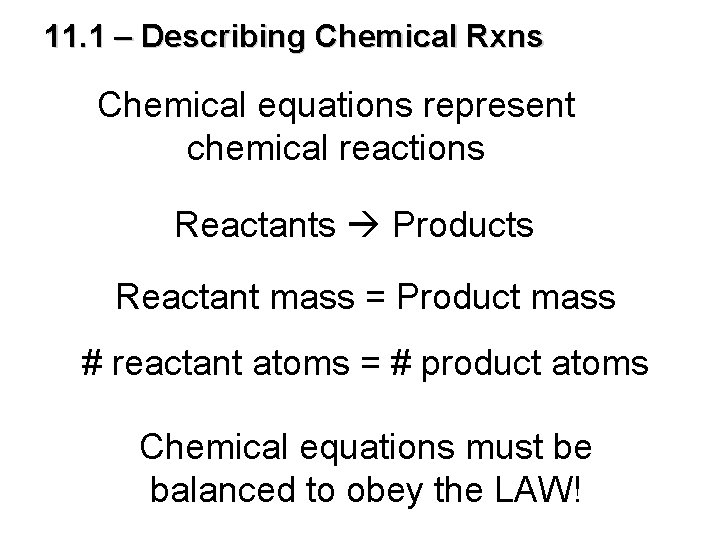
11. 1 – Describing Chemical Rxns Chemical equations represent chemical reactions Reactants Products Reactant mass = Product mass # reactant atoms = # product atoms Chemical equations must be balanced to obey the LAW!

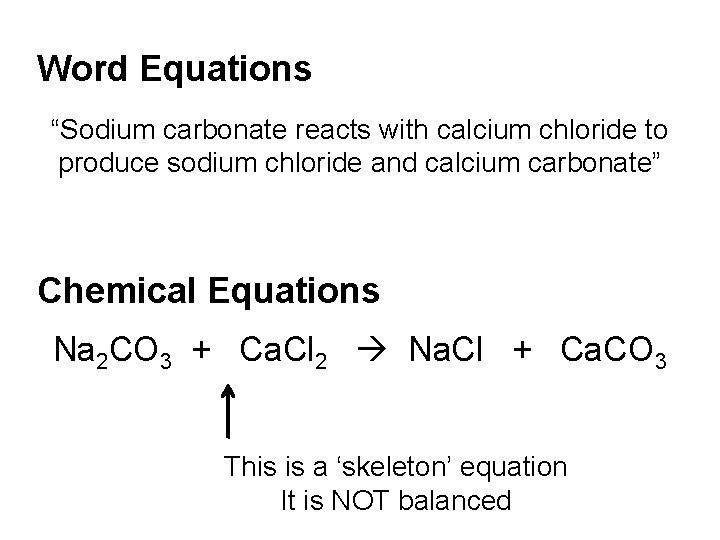
Word Equations “Sodium carbonate reacts with calcium chloride to produce sodium chloride and calcium carbonate” Chemical Equations Na 2 CO 3 + Ca. Cl 2 Na. Cl + Ca. CO 3 This is a ‘skeleton’ equation It is NOT balanced

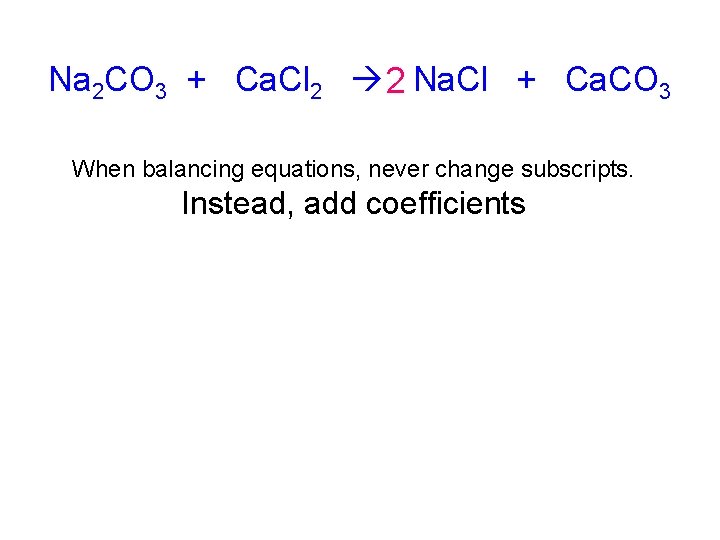
Na 2 CO 3 + Ca. Cl 2 2 Na. Cl + Ca. CO 3 When balancing equations, never change subscripts. Instead, add coefficients

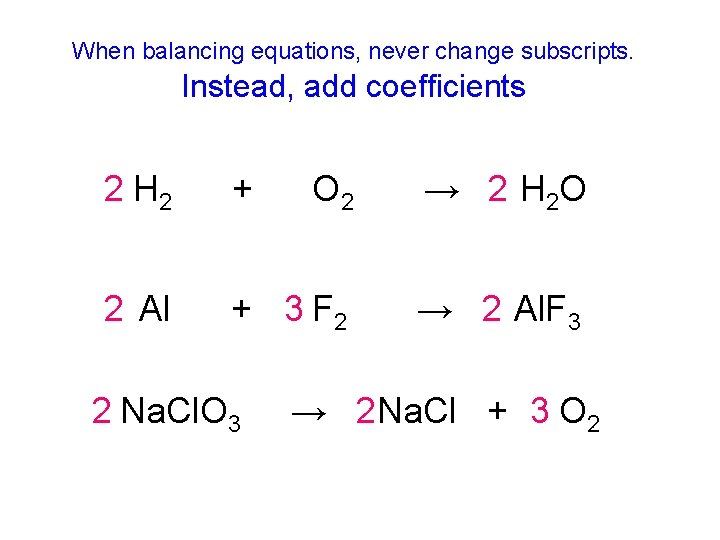
When balancing equations, never change subscripts. Instead, add coefficients 2 H 2 + O 2 → 2 H 2 O 2 Al + 3 F 2 → 2 Al. F 3 2 Na. Cl. O 3 → 2 Na. Cl + 3 O 2

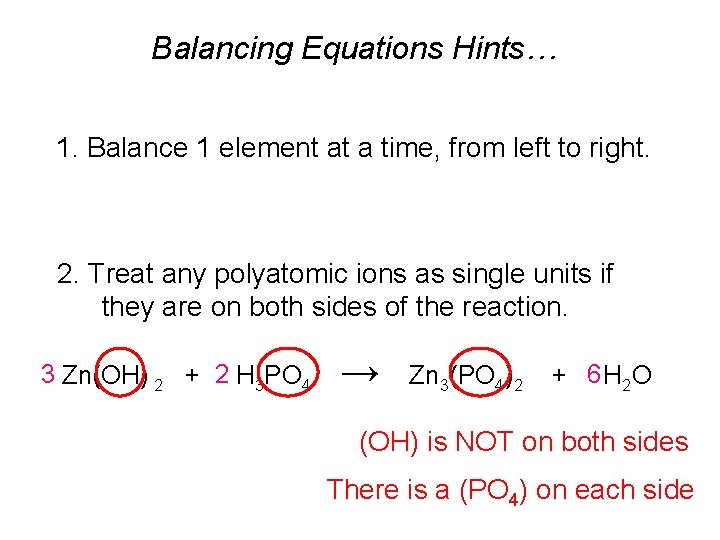
Balancing Equations Hints… 1. Balance 1 element at a time, from left to right. 2. Treat any polyatomic ions as single units if they are on both sides of the reaction. 3 Zn(OH) 2 + 2 H 3 PO 4 → Zn 3(PO 4)2 + 6 H 2 O (OH) is NOT on both sides There is a (PO 4) on each side

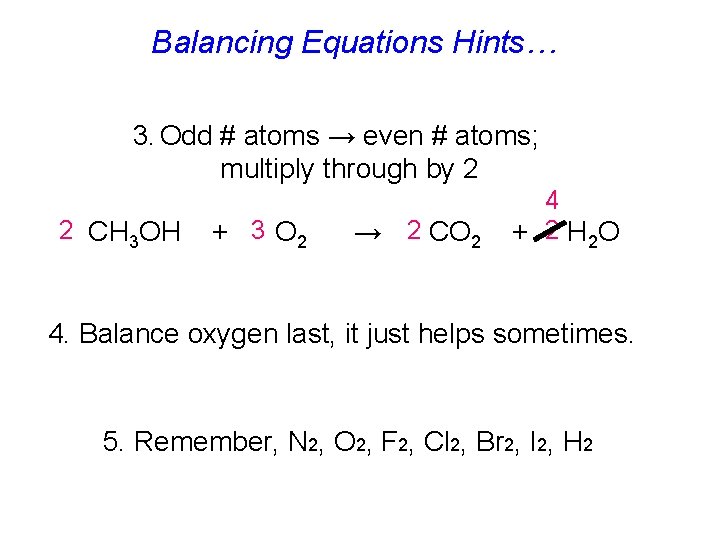
Balancing Equations Hints… 3. Odd # atoms → even # atoms; multiply through by 2 2 CH 3 OH + 3 O 2 → 2 CO 2 4 + 2 H 2 O 4. Balance oxygen last, it just helps sometimes. 5. Remember, N 2, O 2, F 2, Cl 2, Br 2, I 2, H 2

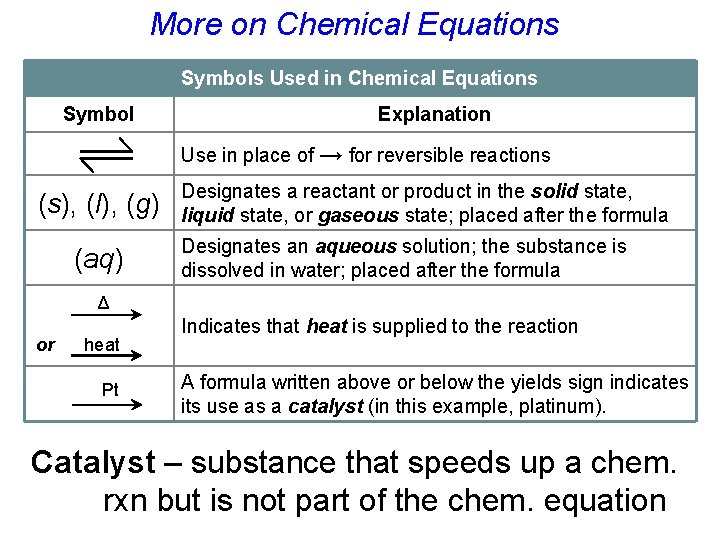
More on Chemical Equations Symbols Used in Chemical Equations Symbol Explanation Use in place of → for reversible reactions (s), (l), (g) (aq) Designates a reactant or product in the solid state, liquid state, or gaseous state; placed after the formula Designates an aqueous solution; the substance is dissolved in water; placed after the formula Δ or heat Pt Indicates that heat is supplied to the reaction A formula written above or below the yields sign indicates its use as a catalyst (in this example, platinum). Catalyst – substance that speeds up a chem. rxn but is not part of the chem. equation

This is on page 352 Rules for Writing and Balancing Equations 1. Determine the correct formulas for all the reactants and products. 4. Balance the elements one at a time by using coefficients. When no coefficient is written, it is assumed to 2. Write the skeleton equation by be 1. Begin by balancing elements placing the formulas for the that appear only once on each side reactants on the left and the of the equation. Never balance an formulas for the products on the equation by changing the subscripts right with a yields sign (→) in in a chemical formula. Each between. If two or more reactants or substance only has one correct products are involved, separate their formulas with plus signs. 5. Check each atom or polyatomic ion 3. Determine the number of atoms of to be sure that the number is equal each element in the reactants and on both sides of the equation. products. Count a polyatomic ion as a single unit if it appears unchanged 6. Make sure all the coefficients are in the lowest possible ratio. on both sides of the equation.

ASSIGNMENT: Chapter 11 #1 -11 (p. 349 – 354)

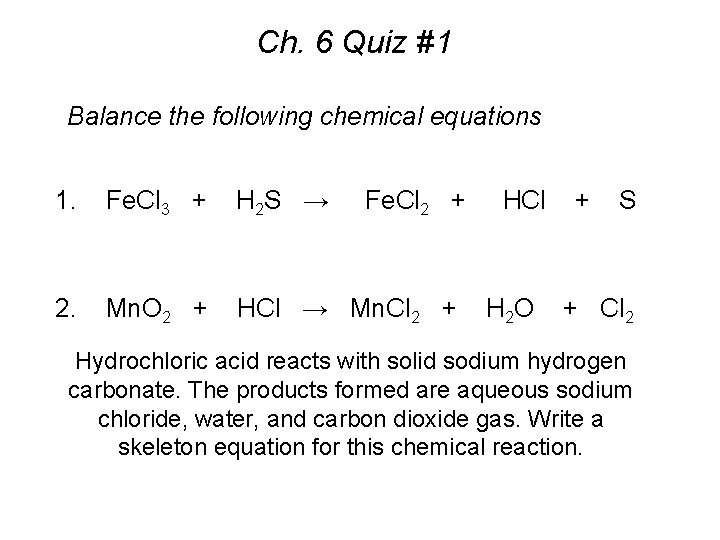
Ch. 6 Quiz #1 Balance the following chemical equations 1. Fe. Cl 3 + H 2 S → Fe. Cl 2 + 2. Mn. O 2 + HCl → Mn. Cl 2 + HCl H 2 O + S + Cl 2 Hydrochloric acid reacts with solid sodium hydrogen carbonate. The products formed are aqueous sodium chloride, water, and carbon dioxide gas. Write a skeleton equation for this chemical reaction.

11. 2 – Types of Chemical Rxns Most chemical rxns will fit into 1 of 5 types. 1. Combination 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion

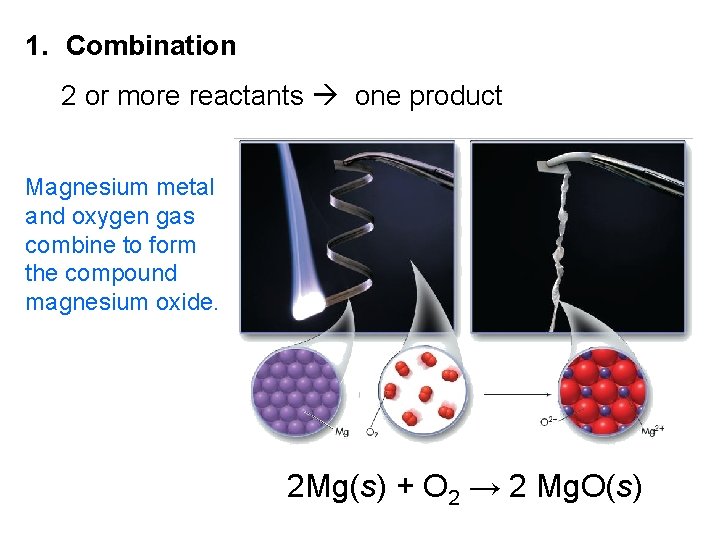
1. Combination 2 or more reactants one product Magnesium metal and oxygen gas combine to form the compound magnesium oxide. 2 Mg(s) + O 2 → 2 Mg. O(s)

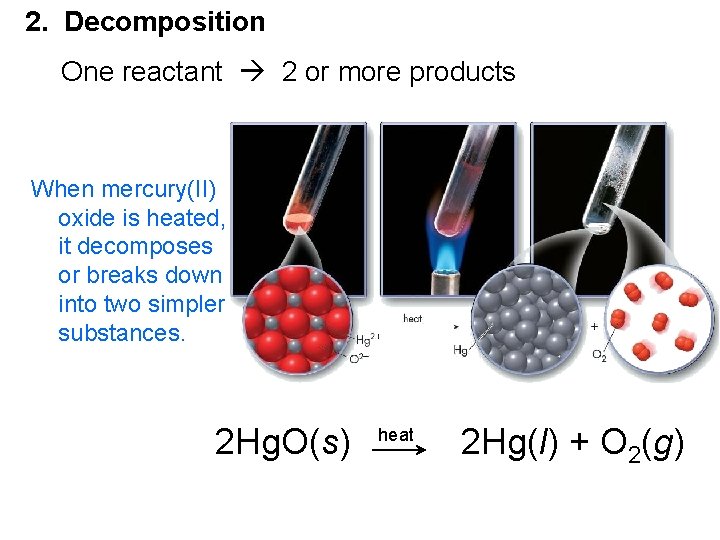
2. Decomposition One reactant 2 or more products When mercury(II) oxide is heated, it decomposes or breaks down into two simpler substances. 2 Hg. O(s) heat 2 Hg(l) + O 2(g)

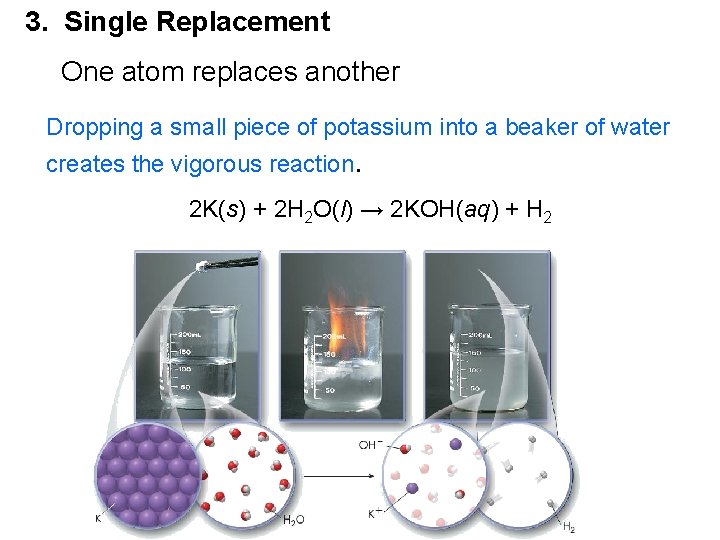
3. Single Replacement One atom replaces another Dropping a small piece of potassium into a beaker of water creates the vigorous reaction. 2 K(s) + 2 H 2 O(l) → 2 KOH(aq) + H 2

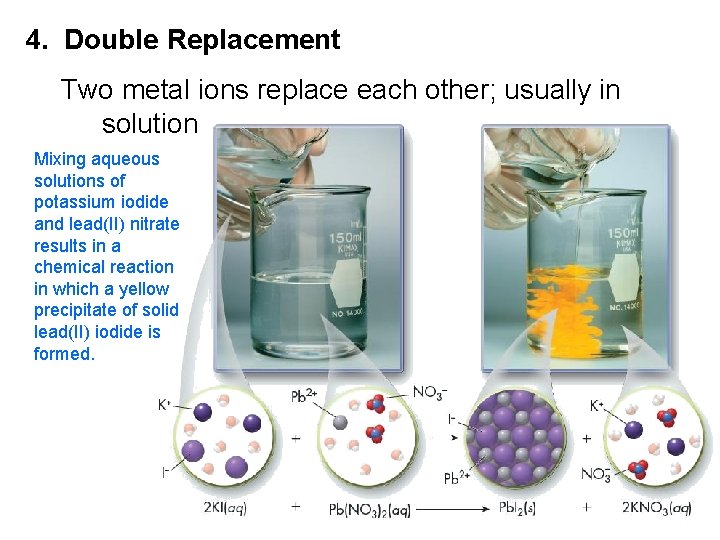
4. Double Replacement Two metal ions replace each other; usually in solution Mixing aqueous solutions of potassium iodide and lead(II) nitrate results in a chemical reaction in which a yellow precipitate of solid lead(II) iodide is formed.

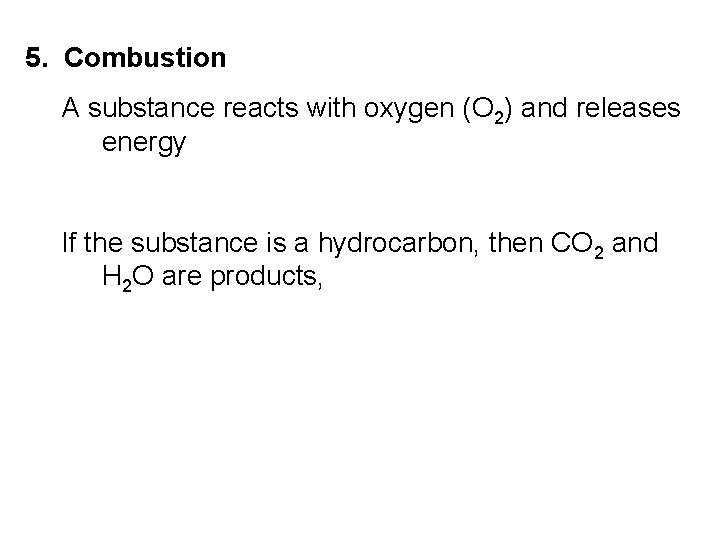
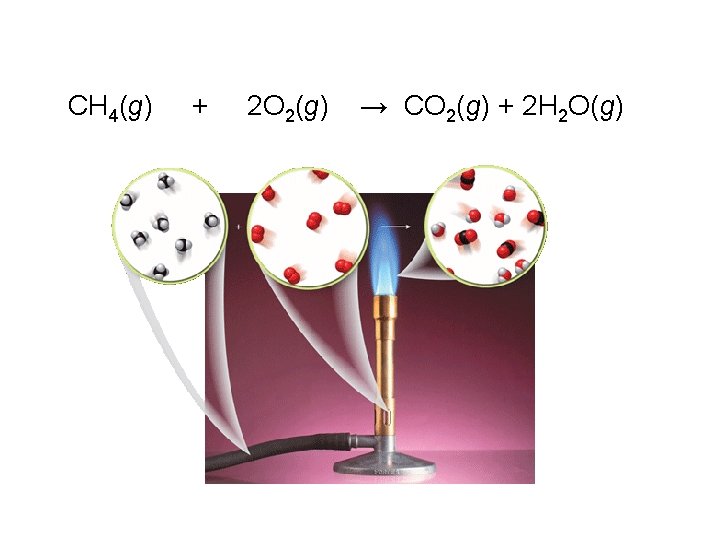
5. Combustion A substance reacts with oxygen (O 2) and releases energy If the substance is a hydrocarbon, then CO 2 and H 2 O are products,

CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g)
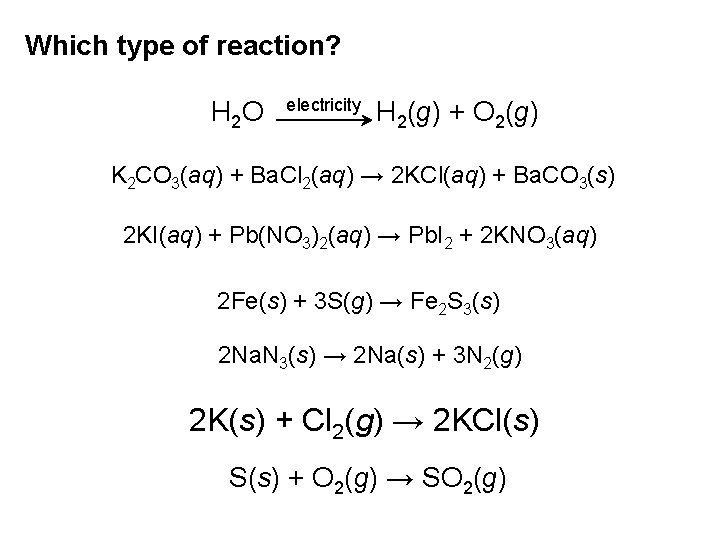
Which type of reaction? H 2 O electricity H 2(g) + O 2(g) K 2 CO 3(aq) + Ba. Cl 2(aq) → 2 KCl(aq) + Ba. CO 3(s) 2 KI(aq) + Pb(NO 3)2(aq) → Pb. I 2 + 2 KNO 3(aq) 2 Fe(s) + 3 S(g) → Fe 2 S 3(s) 2 Na. N 3(s) → 2 Na(s) + 3 N 2(g) 2 K(s) + Cl 2(g) → 2 KCl(s) S(s) + O 2(g) → SO 2(g)
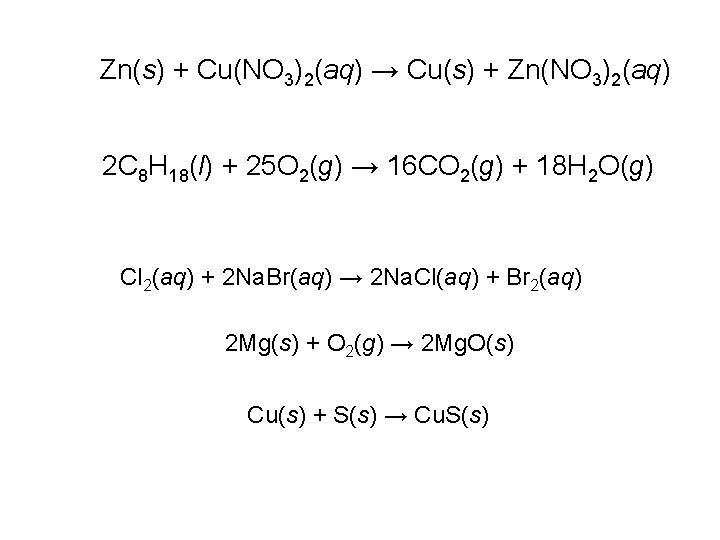
Zn(s) + Cu(NO 3)2(aq) → Cu(s) + Zn(NO 3)2(aq) 2 C 8 H 18(l) + 25 O 2(g) → 16 CO 2(g) + 18 H 2 O(g) Cl 2(aq) + 2 Na. Br(aq) → 2 Na. Cl(aq) + Br 2(aq) 2 Mg(s) + O 2(g) → 2 Mg. O(s) Cu(s) + S(s) → Cu. S(s)

ASSIGNMENT: Chapter 11 Worksheet #1

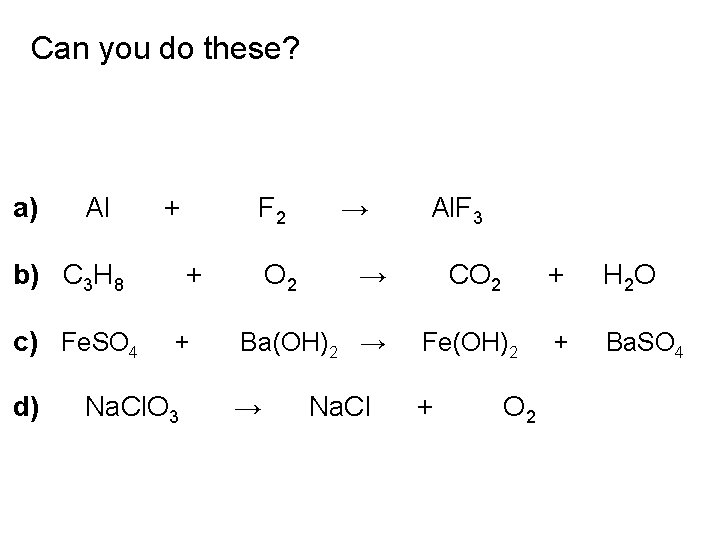
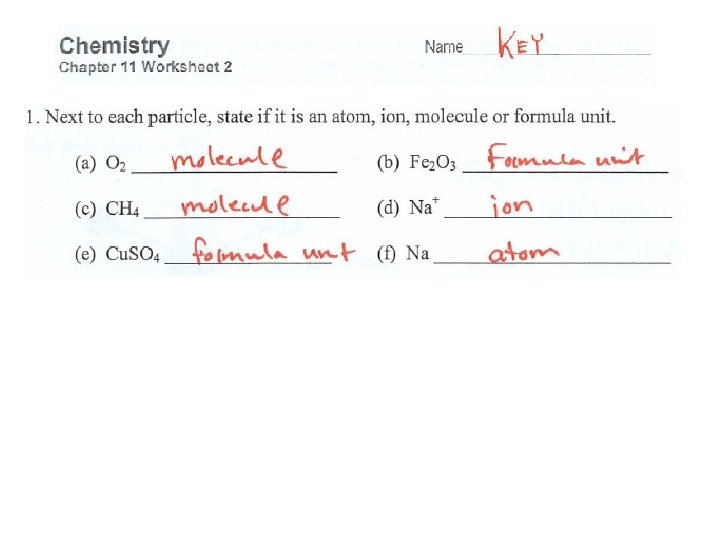
Can you do these? a) Al + b) C 3 H 8 c) Fe. SO 4 d) F 2 + + Na. Cl. O 3 O 2 → Al. F 3 → CO 2 + H 2 O Ba(OH)2 → Fe(OH)2 + Ba. SO 4 → + Na. Cl O 2
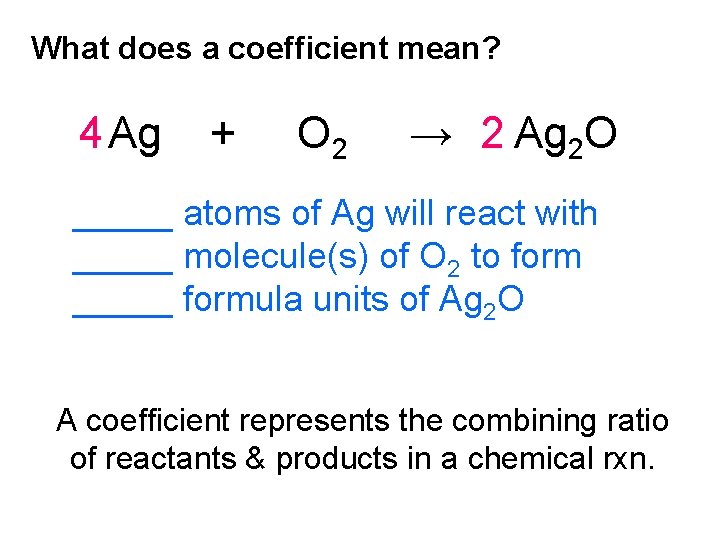
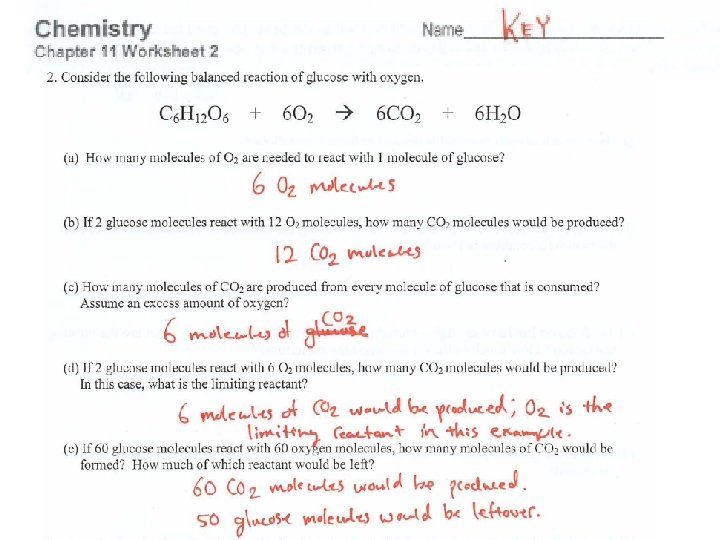
What does a coefficient mean? 4 Ag + O 2 → 2 Ag 2 O _____ atoms of Ag will react with _____ molecule(s) of O 2 to form _____ formula units of Ag 2 O A coefficient represents the combining ratio of reactants & products in a chemical rxn.
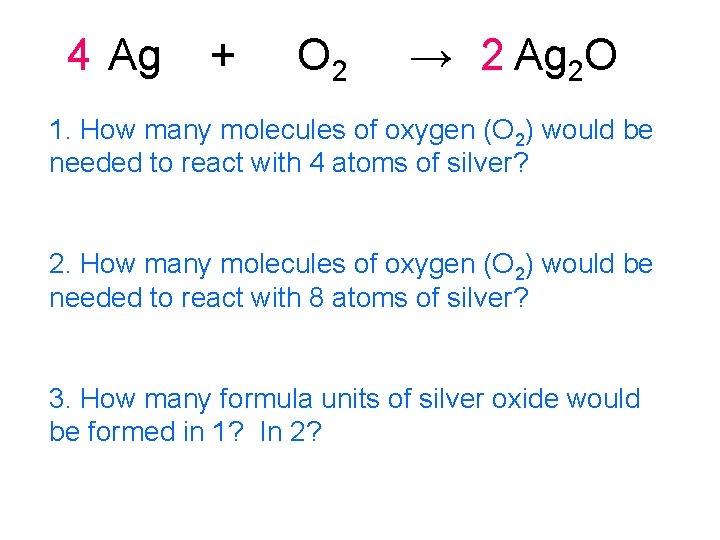
4 Ag + O 2 → 2 Ag 2 O 1. How many molecules of oxygen (O 2) would be needed to react with 4 atoms of silver? 2. How many molecules of oxygen (O 2) would be needed to react with 8 atoms of silver? 3. How many formula units of silver oxide would be formed in 1? In 2?
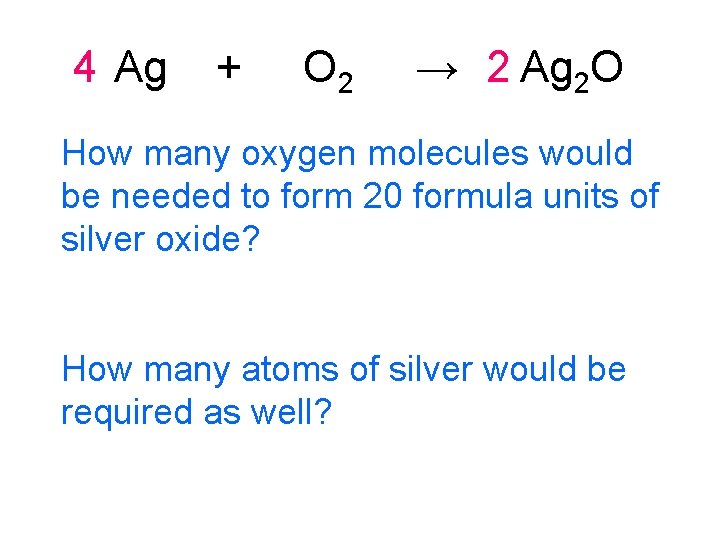
4 Ag + O 2 → 2 Ag 2 O How many oxygen molecules would be needed to form 20 formula units of silver oxide? How many atoms of silver would be required as well?
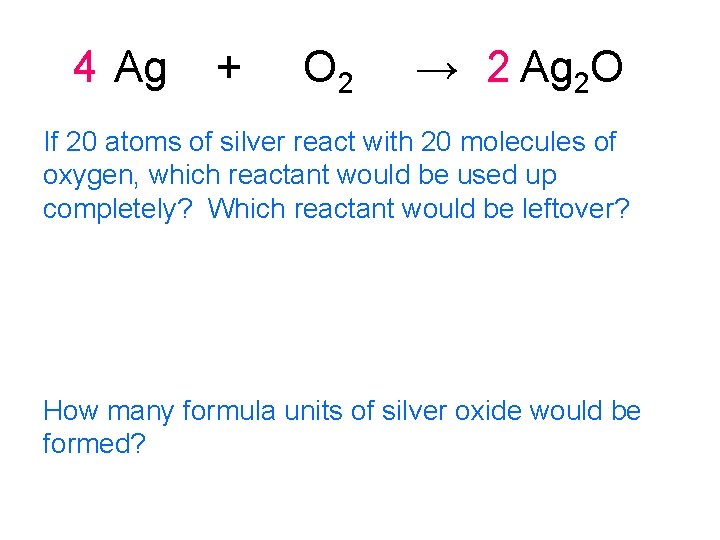
4 Ag + O 2 → 2 Ag 2 O If 20 atoms of silver react with 20 molecules of oxygen, which reactant would be used up completely? Which reactant would be leftover? How many formula units of silver oxide would be formed?

Limiting Reactant that is completely used up; limits the amount of product that can be produced. Excess Reactant that remains un-reacted; is not completely used up.
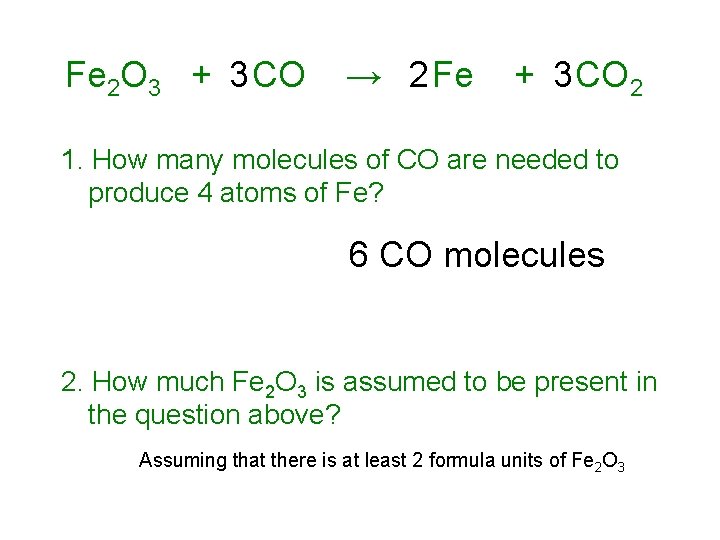
Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2 1. How many molecules of CO are needed to produce 4 atoms of Fe? 6 CO molecules 2. How much Fe 2 O 3 is assumed to be present in the question above? Assuming that there is at least 2 formula units of Fe 2 O 3

ASSIGNMENT: Chapter 11 Worksheet #2

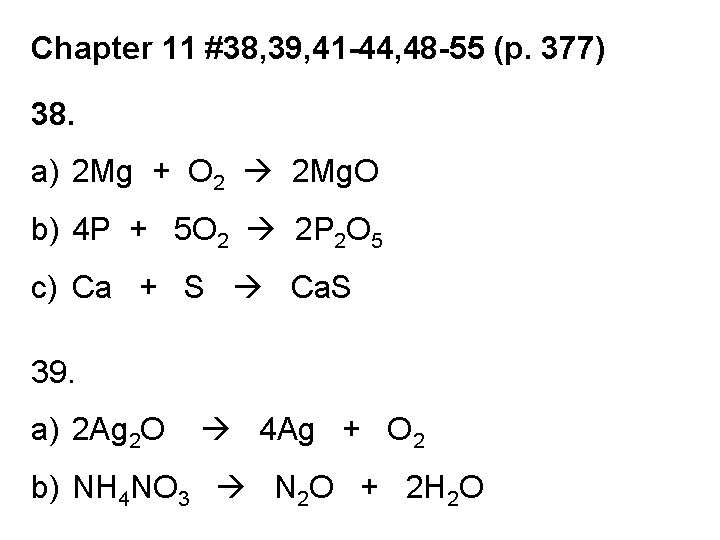
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 38. a) 2 Mg + O 2 2 Mg. O b) 4 P + 5 O 2 2 P 2 O 5 c) Ca + S Ca. S 39. a) 2 Ag 2 O 4 Ag + O 2 b) NH 4 NO 3 N 2 O + 2 H 2 O
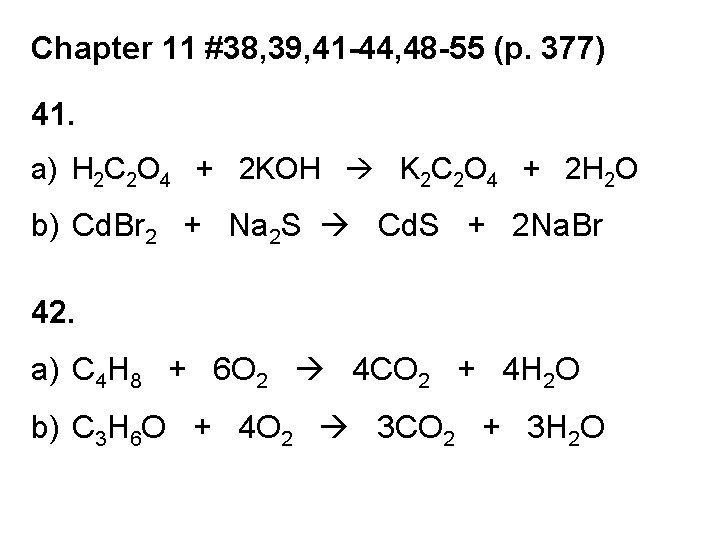
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 41. a) H 2 C 2 O 4 + 2 KOH K 2 C 2 O 4 + 2 H 2 O b) Cd. Br 2 + Na 2 S Cd. S + 2 Na. Br 42. a) C 4 H 8 + 6 O 2 4 CO 2 + 4 H 2 O b) C 3 H 6 O + 4 O 2 3 CO 2 + 3 H 2 O
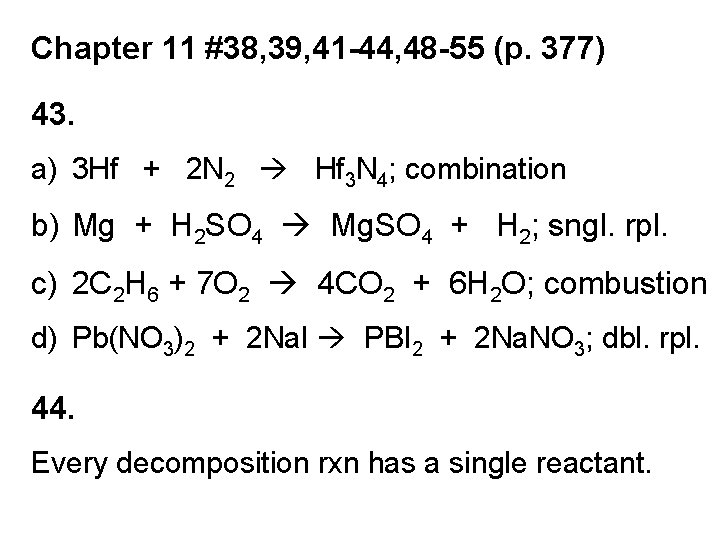
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 43. a) 3 Hf + 2 N 2 Hf 3 N 4; combination b) Mg + H 2 SO 4 Mg. SO 4 + H 2; sngl. rpl. c) 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O; combustion d) Pb(NO 3)2 + 2 Na. I PBI 2 + 2 Na. NO 3; dbl. rpl. 44. Every decomposition rxn has a single reactant.

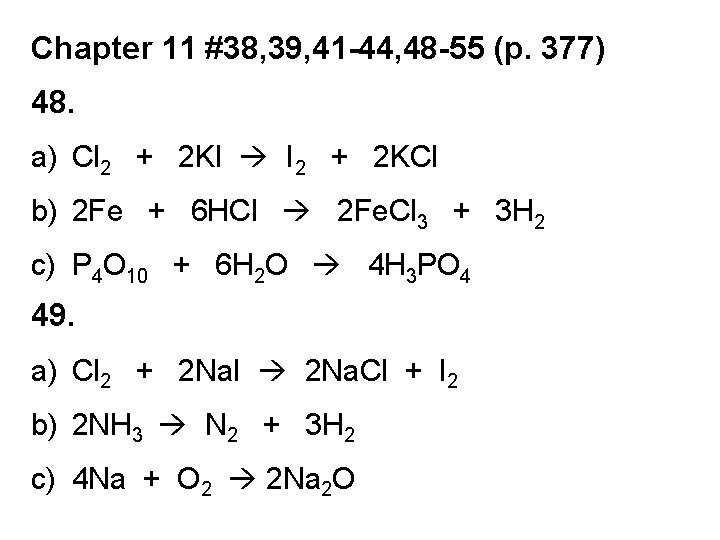
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 48. a) Cl 2 + 2 KI I 2 + 2 KCl b) 2 Fe + 6 HCl 2 Fe. Cl 3 + 3 H 2 c) P 4 O 10 + 6 H 2 O 4 H 3 PO 4 49. a) Cl 2 + 2 Na. I 2 Na. Cl + I 2 b) 2 NH 3 N 2 + 3 H 2 c) 4 Na + O 2 2 Na 2 O

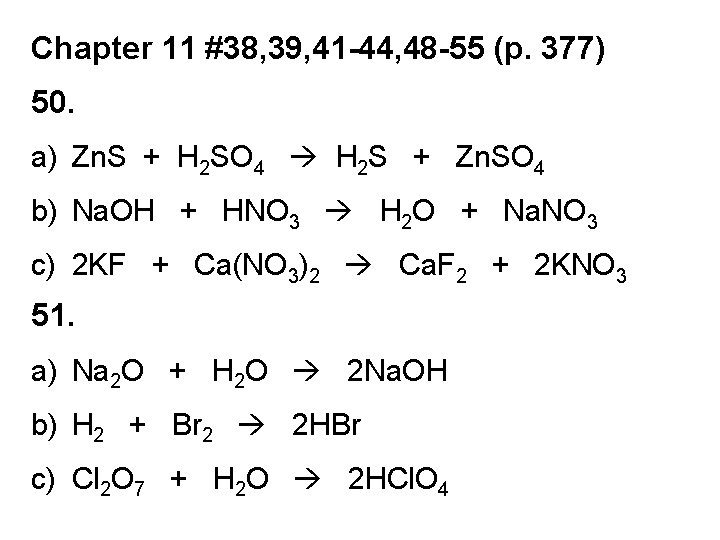
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 50. a) Zn. S + H 2 SO 4 H 2 S + Zn. SO 4 b) Na. OH + HNO 3 H 2 O + Na. NO 3 c) 2 KF + Ca(NO 3)2 Ca. F 2 + 2 KNO 3 51. a) Na 2 O + H 2 O 2 Na. OH b) H 2 + Br 2 2 HBr c) Cl 2 O 7 + H 2 O 2 HCl. O 4

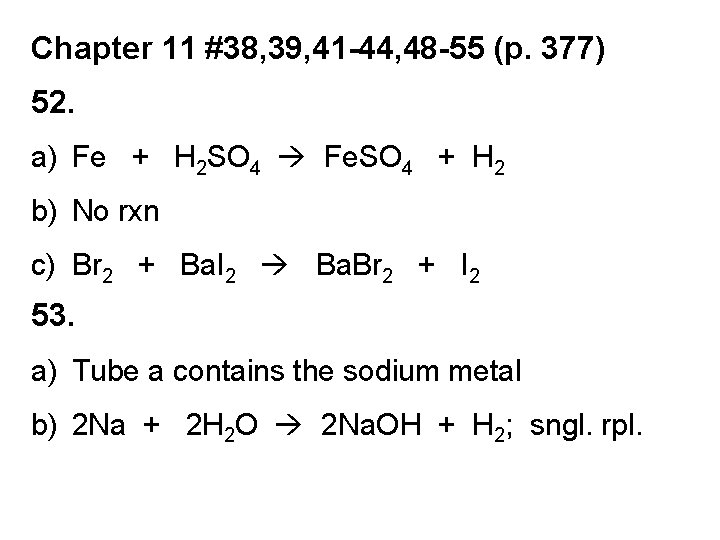
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 52. a) Fe + H 2 SO 4 Fe. SO 4 + H 2 b) No rxn c) Br 2 + Ba. I 2 Ba. Br 2 + I 2 53. a) Tube a contains the sodium metal b) 2 Na + 2 H 2 O 2 Na. OH + H 2; sngl. rpl.

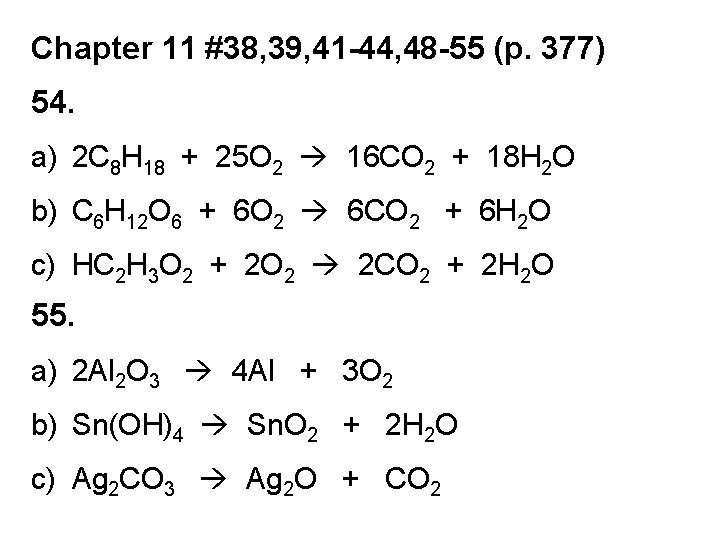
Chapter 11 #38, 39, 41 -44, 48 -55 (p. 377) 54. a) 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O b) C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O c) HC 2 H 3 O 2 + 2 O 2 2 CO 2 + 2 H 2 O 55. a) 2 Al 2 O 3 4 Al + 3 O 2 b) Sn(OH)4 Sn. O 2 + 2 H 2 O c) Ag 2 CO 3 Ag 2 O + CO 2



