Organic Chemistry 5 th Edition L G Wade









- Slides: 62

Organic Chemistry, 5 th Edition L. G. Wade, Jr. Chapter 21 Carboxylic Acid Derivatives Jo Blackburn Richland College, Dallas, TX Dallas County Community College District Chapter 21 ã 2003, Prentice Hall

Acid Derivatives • All can be converted to the carboxylic acid by acidic or basic hydrolysis. • Esters and amides common in nature. => Chapter 21 2

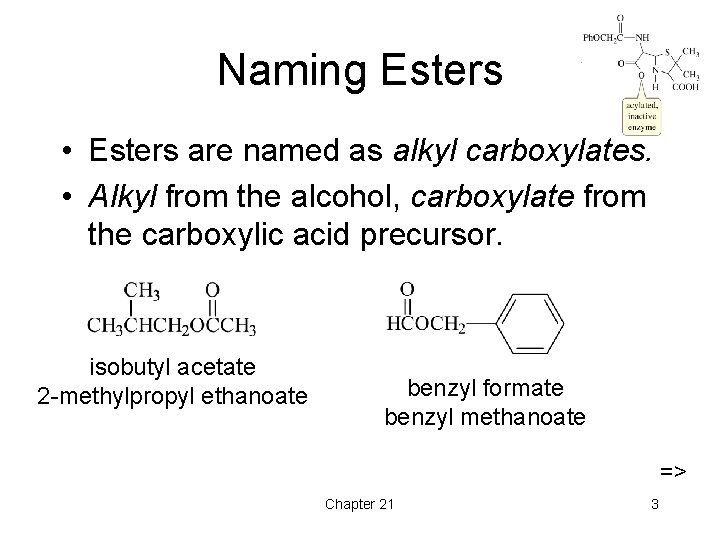
Naming Esters • Esters are named as alkyl carboxylates. • Alkyl from the alcohol, carboxylate from the carboxylic acid precursor. isobutyl acetate 2 -methylpropyl ethanoate benzyl formate benzyl methanoate => Chapter 21 3

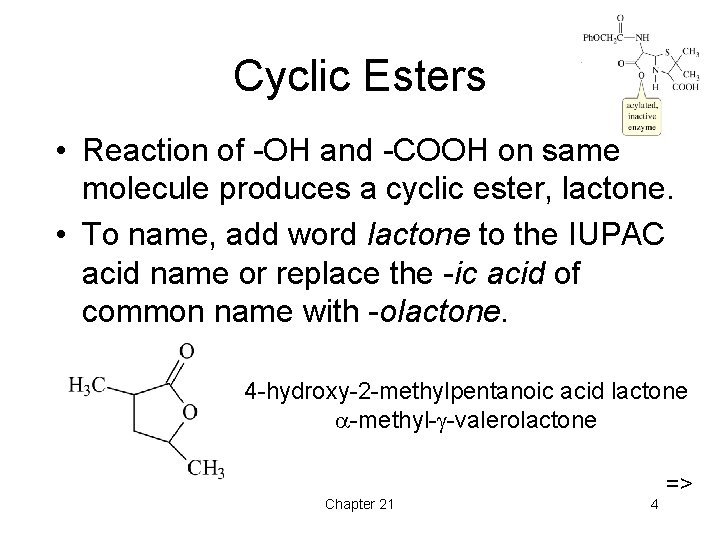
Cyclic Esters • Reaction of -OH and -COOH on same molecule produces a cyclic ester, lactone. • To name, add word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. 4 -hydroxy-2 -methylpentanoic acid lactone -methyl- -valerolactone Chapter 21 4 =>

Amides • Product of the reaction of a carboxylic acid and ammonia or an amine. • Not basic because the lone pair on nitrogen is delocalized by resonance. Chapter 21 Bond angles around N are close to 120. 5 =>

Classes of Amides • 1 amide has one C-N bond (two N-H). • 2 amide or N-substituted amide has two C-N bonds (one N-H). • 3 amide or N, N-disubstituted amide has three C-N bonds (no N-H). => Chapter 21 6

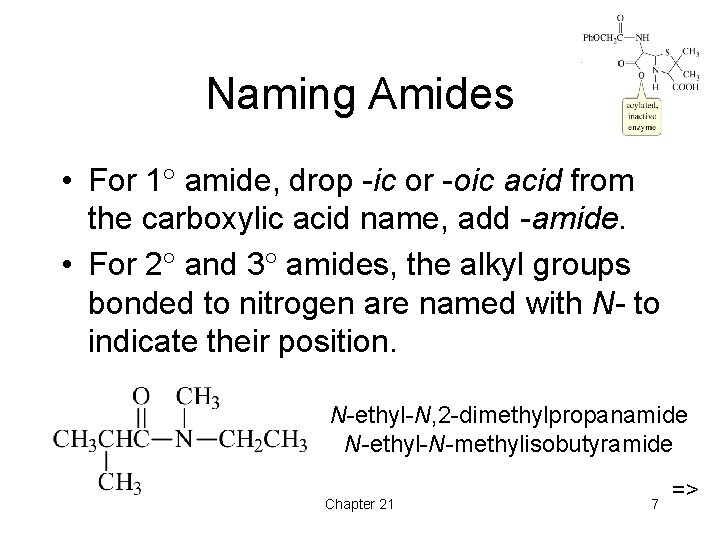
Naming Amides • For 1 amide, drop -ic or -oic acid from the carboxylic acid name, add -amide. • For 2 and 3 amides, the alkyl groups bonded to nitrogen are named with N- to indicate their position. N-ethyl-N, 2 -dimethylpropanamide N-ethyl-N-methylisobutyramide Chapter 21 7 =>

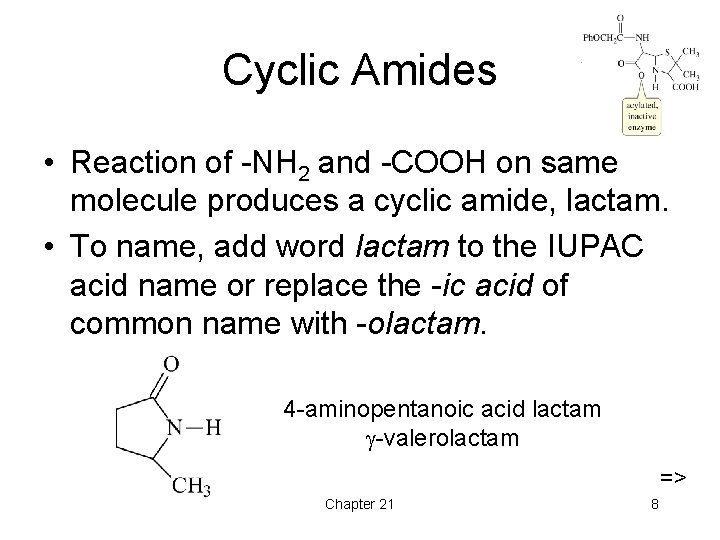
Cyclic Amides • Reaction of -NH 2 and -COOH on same molecule produces a cyclic amide, lactam. • To name, add word lactam to the IUPAC acid name or replace the -ic acid of common name with -olactam. 4 -aminopentanoic acid lactam -valerolactam => Chapter 21 8

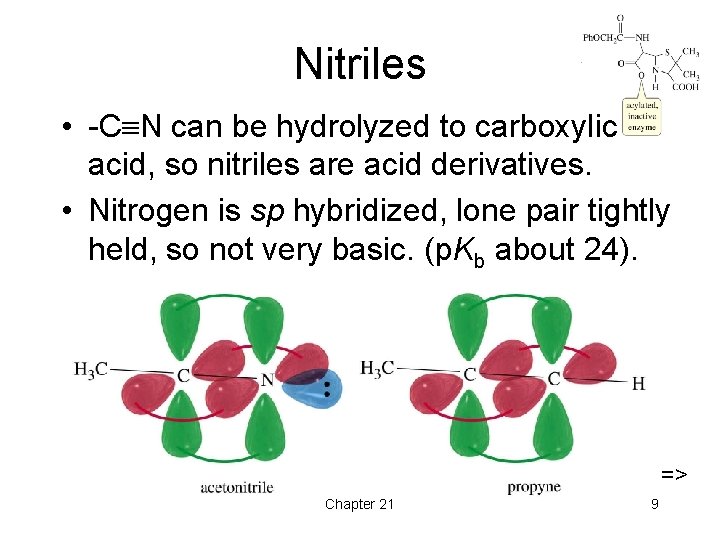
Nitriles • -C N can be hydrolyzed to carboxylic acid, so nitriles are acid derivatives. • Nitrogen is sp hybridized, lone pair tightly held, so not very basic. (p. Kb about 24). => Chapter 21 9

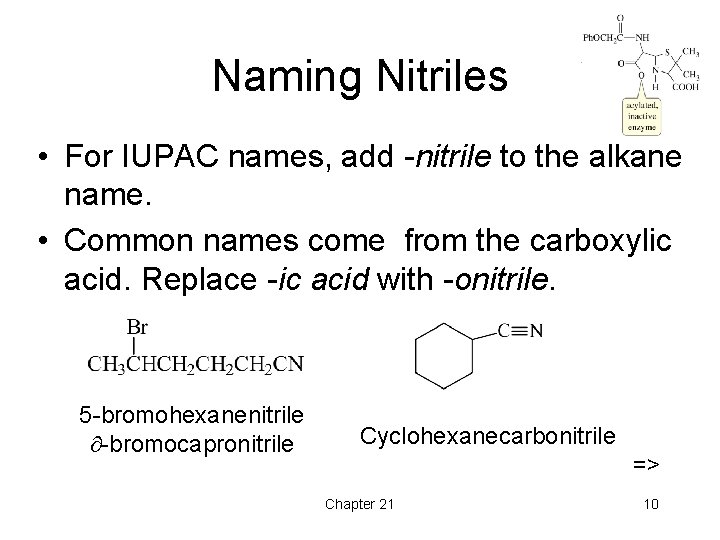
Naming Nitriles • For IUPAC names, add -nitrile to the alkane name. • Common names come from the carboxylic acid. Replace -ic acid with -onitrile. 5 -bromohexanenitrile -bromocapronitrile Cyclohexanecarbonitrile => Chapter 21 10

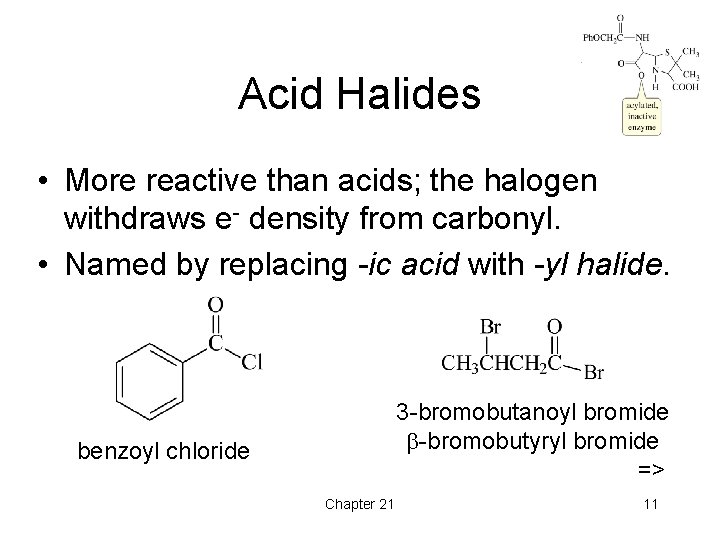
Acid Halides • More reactive than acids; the halogen withdraws e- density from carbonyl. • Named by replacing -ic acid with -yl halide. 3 -bromobutanoyl bromide -bromobutyryl bromide => benzoyl chloride Chapter 21 11

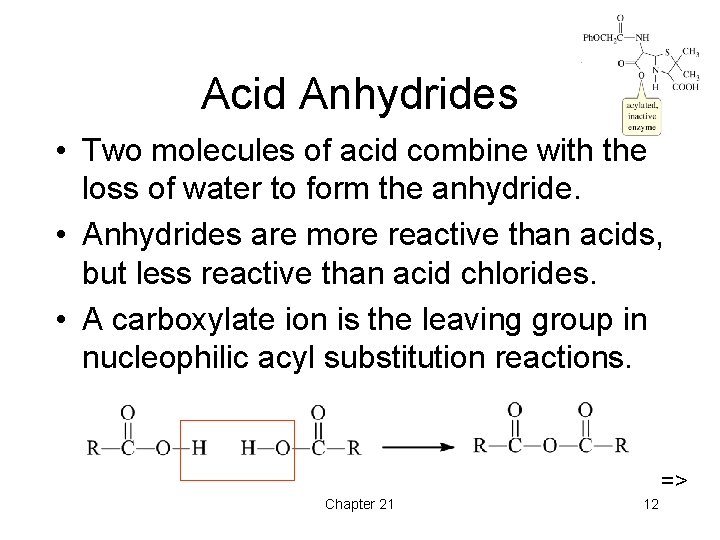
Acid Anhydrides • Two molecules of acid combine with the loss of water to form the anhydride. • Anhydrides are more reactive than acids, but less reactive than acid chlorides. • A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. => Chapter 21 12

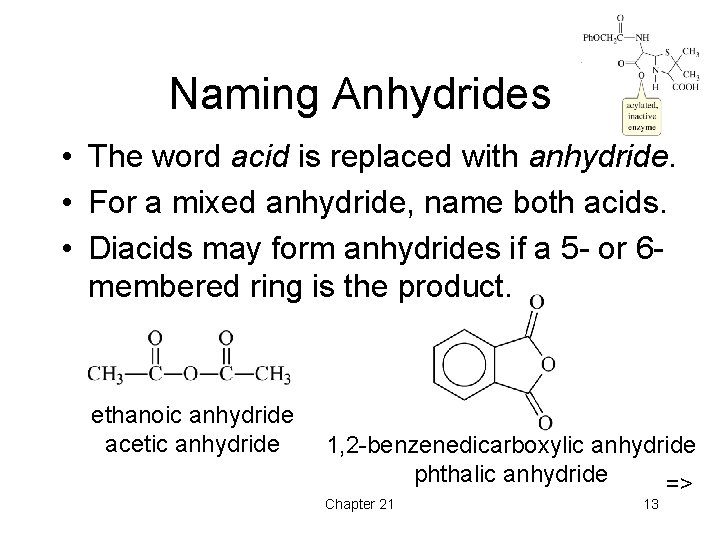
Naming Anhydrides • The word acid is replaced with anhydride. • For a mixed anhydride, name both acids. • Diacids may form anhydrides if a 5 - or 6 membered ring is the product. ethanoic anhydride acetic anhydride 1, 2 -benzenedicarboxylic anhydride phthalic anhydride => Chapter 21 13

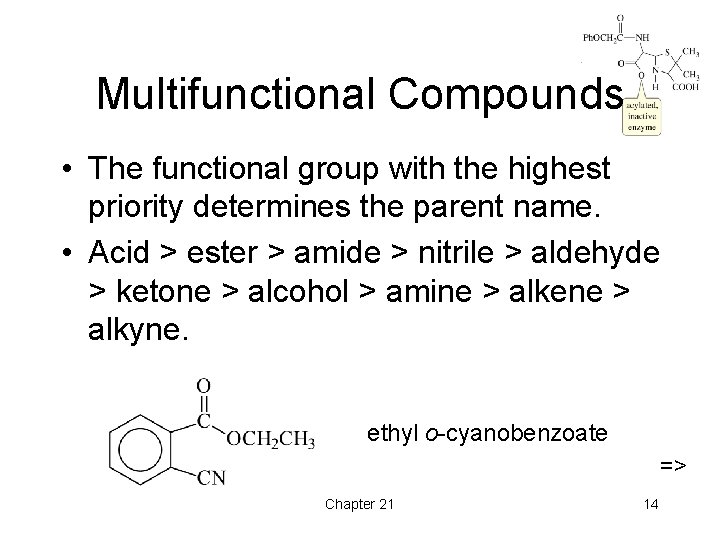
Multifunctional Compounds • The functional group with the highest priority determines the parent name. • Acid > ester > amide > nitrile > aldehyde > ketone > alcohol > amine > alkene > alkyne. ethyl o-cyanobenzoate => Chapter 21 14

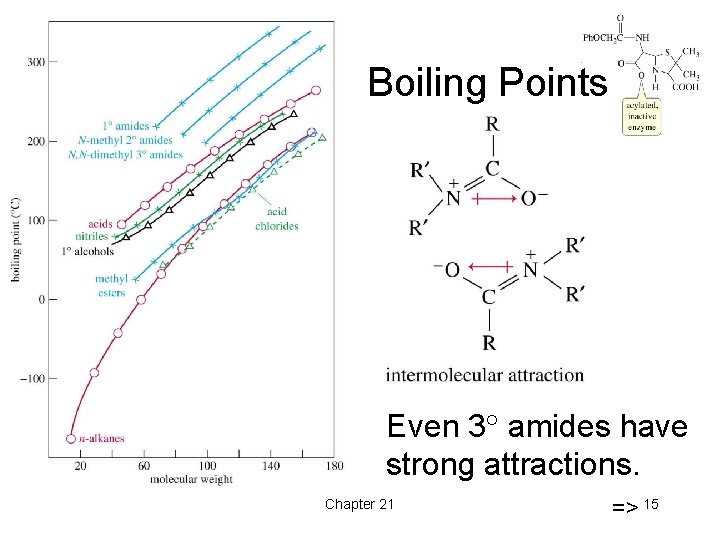
Boiling Points Even 3 amides have strong attractions. Chapter 21 => 15

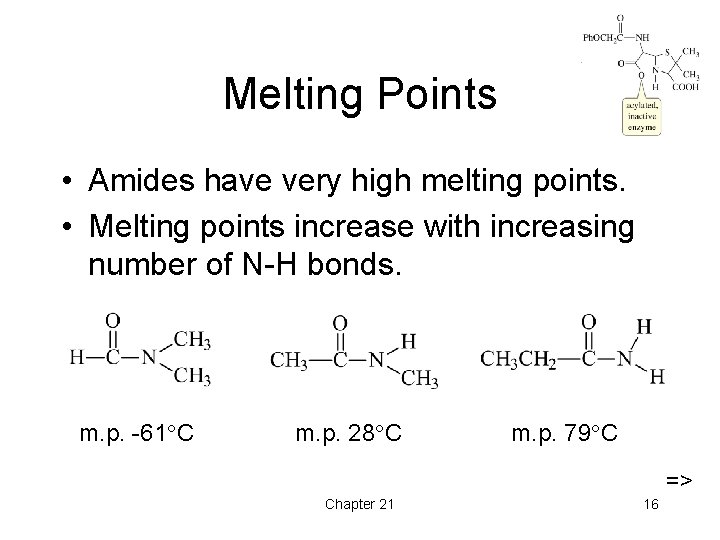
Melting Points • Amides have very high melting points. • Melting points increase with increasing number of N-H bonds. m. p. -61 C m. p. 28 C m. p. 79 C => Chapter 21 16

Solubility • Acid chlorides and anhydrides are too reactive to be used with water or alcohol. • Esters, 3 amides, and nitriles are good polar aprotic solvents. • Solvents commonly used in organic reactions: ØEthyl acetate ØDimethylformamide (DMF) ØAcetonitrile Chapter 21 =>17

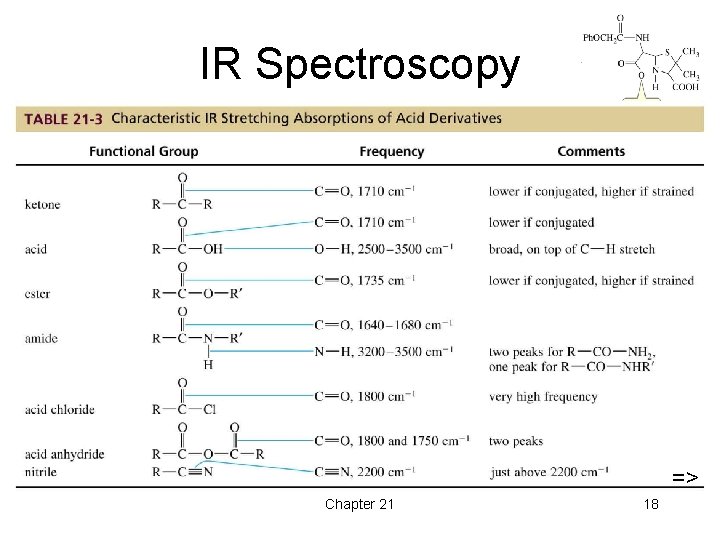
IR Spectroscopy => => Chapter 21 18

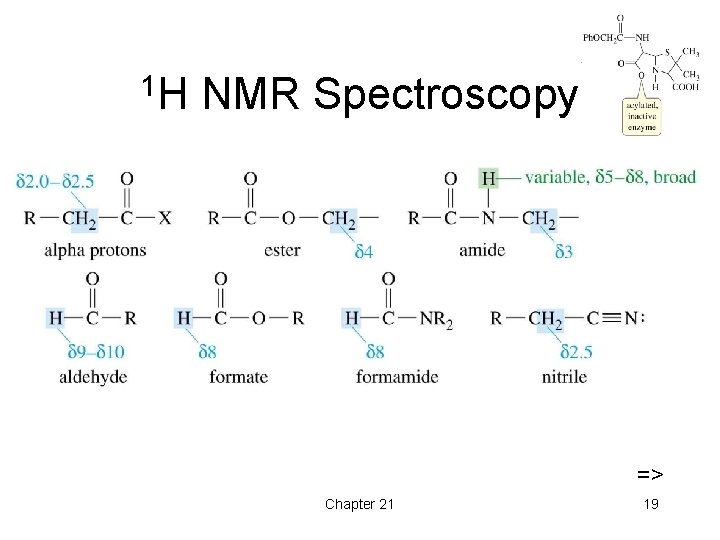
1 H NMR Spectroscopy => Chapter 21 19

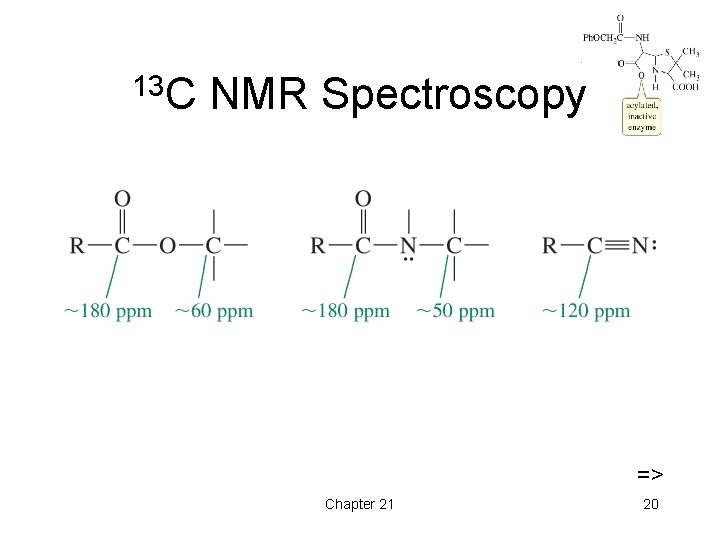
13 C NMR Spectroscopy => Chapter 21 20

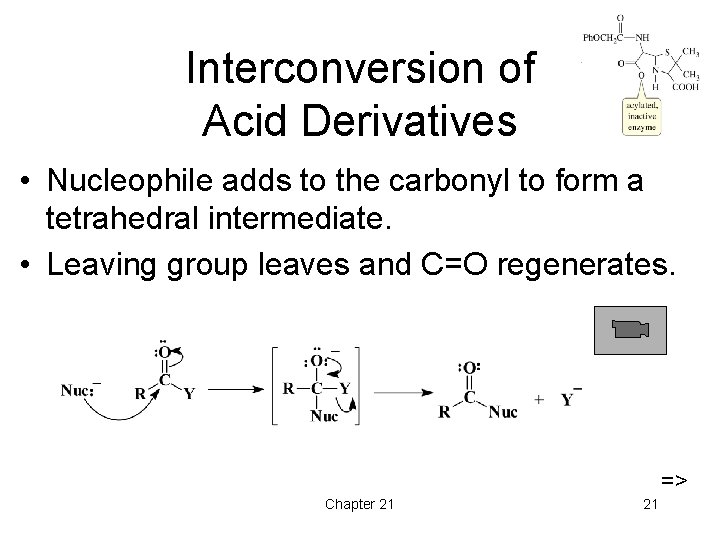
Interconversion of Acid Derivatives • Nucleophile adds to the carbonyl to form a tetrahedral intermediate. • Leaving group leaves and C=O regenerates. => Chapter 21 21

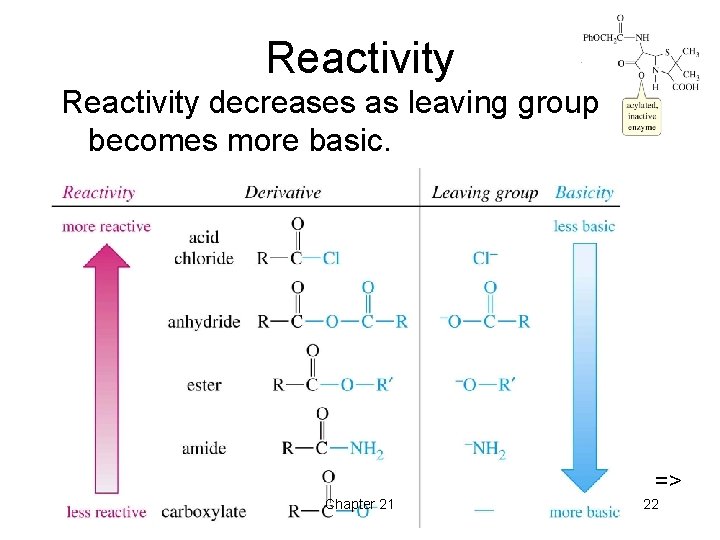
Reactivity decreases as leaving group becomes more basic. => Chapter 21 22

Interconversion of Derivatives More reactive derivatives can be converted to less reactive derivatives. => Chapter 21 23

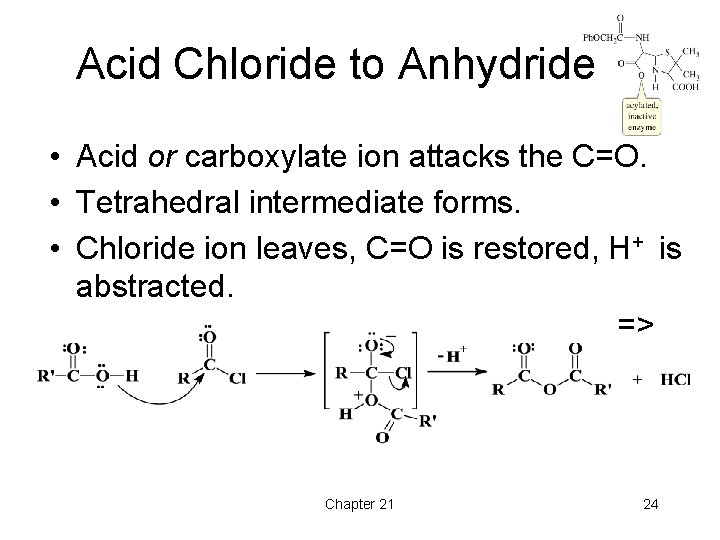
Acid Chloride to Anhydride • Acid or carboxylate ion attacks the C=O. • Tetrahedral intermediate forms. • Chloride ion leaves, C=O is restored, H+ is abstracted. => Chapter 21 24

Acid Chloride to Ester • Alcohol attacks the C=O. • Tetrahedral intermediate forms. • Chloride ion leaves, C=O is restored, H+ is abstracted. => Chapter 21 25

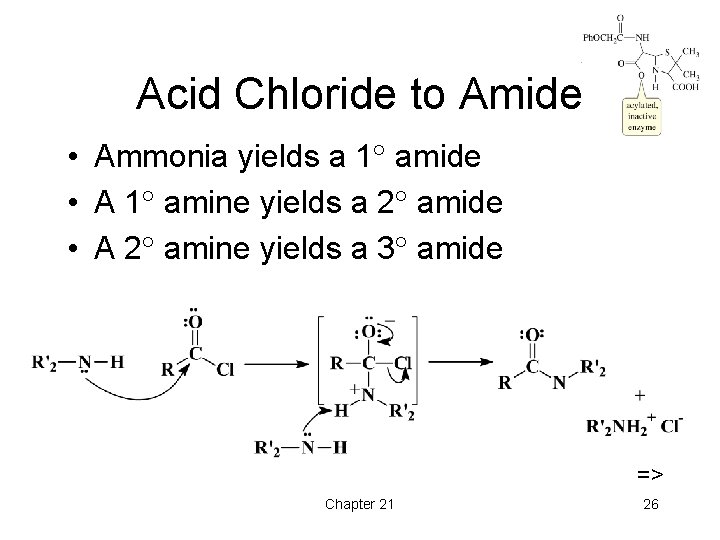
Acid Chloride to Amide • Ammonia yields a 1 amide • A 1 amine yields a 2 amide • A 2 amine yields a 3 amide => Chapter 21 26

Anhydride to Ester • Alcohol attacks one C=O of anhydride. • Tetrahedral intermediate forms. • Carboxylate ion leaves, C=O is restored, H+ is abstracted. => Chapter 21 27

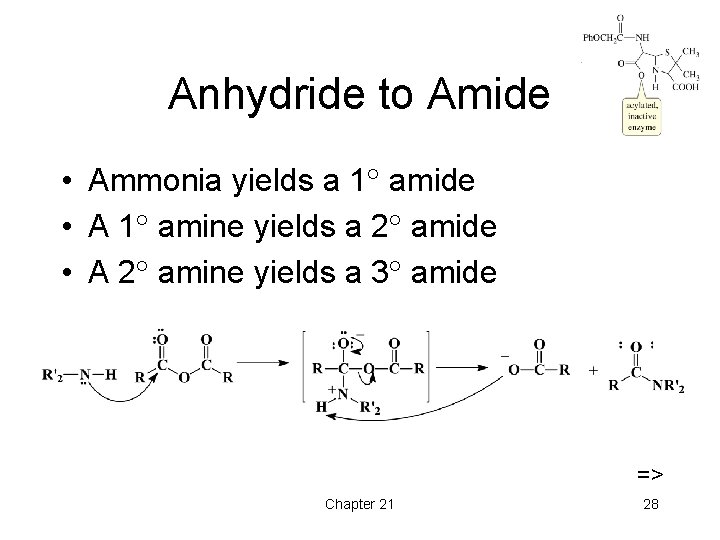
Anhydride to Amide • Ammonia yields a 1 amide • A 1 amine yields a 2 amide • A 2 amine yields a 3 amide => Chapter 21 28

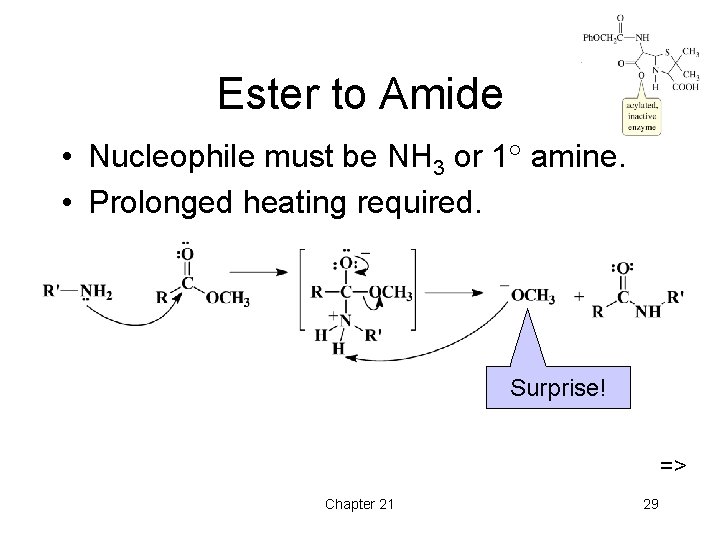
Ester to Amide • Nucleophile must be NH 3 or 1 amine. • Prolonged heating required. Surprise! => Chapter 21 29

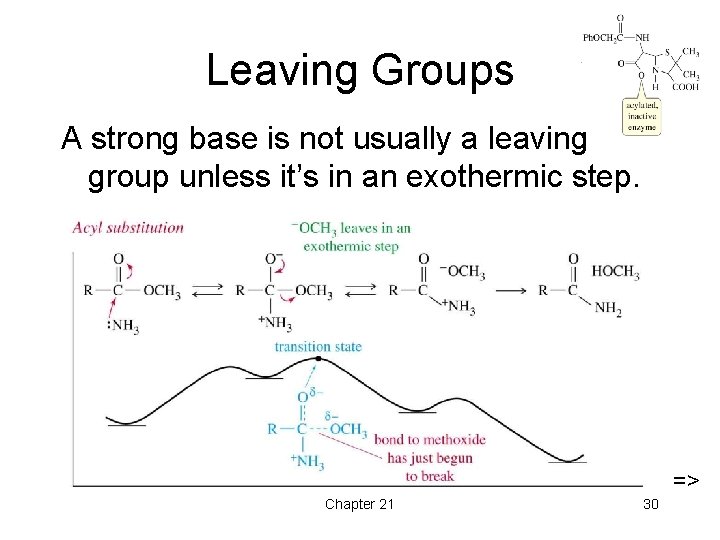
Leaving Groups A strong base is not usually a leaving group unless it’s in an exothermic step. => Chapter 21 30

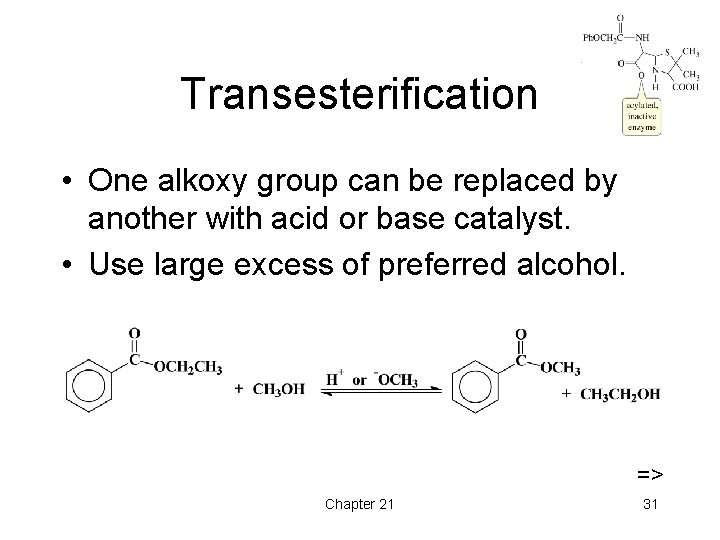
Transesterification • One alkoxy group can be replaced by another with acid or base catalyst. • Use large excess of preferred alcohol. => Chapter 21 31

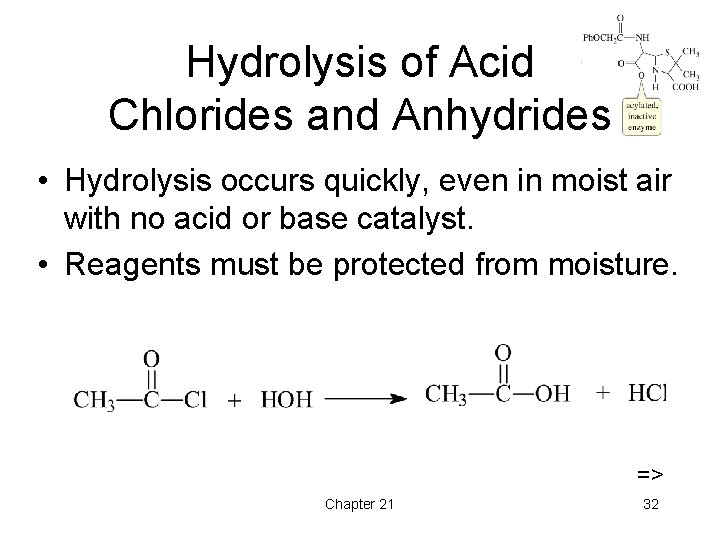
Hydrolysis of Acid Chlorides and Anhydrides • Hydrolysis occurs quickly, even in moist air with no acid or base catalyst. • Reagents must be protected from moisture. => Chapter 21 32

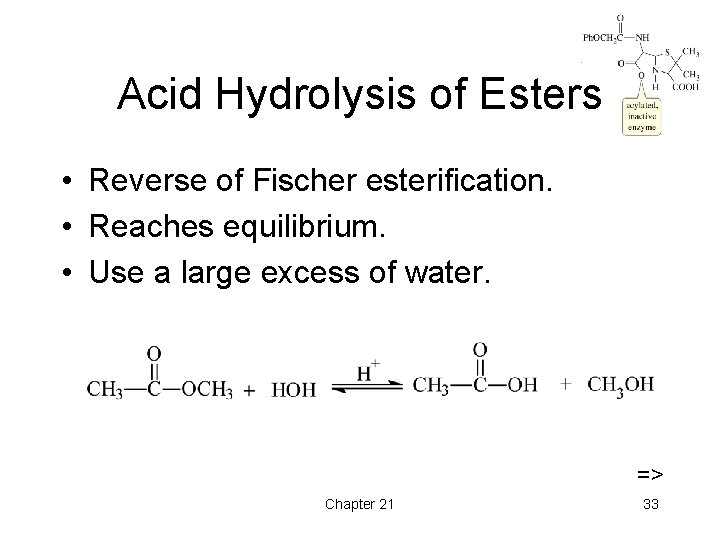
Acid Hydrolysis of Esters • Reverse of Fischer esterification. • Reaches equilibrium. • Use a large excess of water. => Chapter 21 33

Saponification • Base-catalyzed hydrolysis of ester. • “Saponification” means “soap-making. ” • Soaps are made by heating Na. OH with a fat (triester of glycerol) to produce the sodium salt of a fatty acid - a soap. • One example of a soap is sodium stearate, Na+ -OOC(CH 2)16 CH 3. => Chapter 21 34

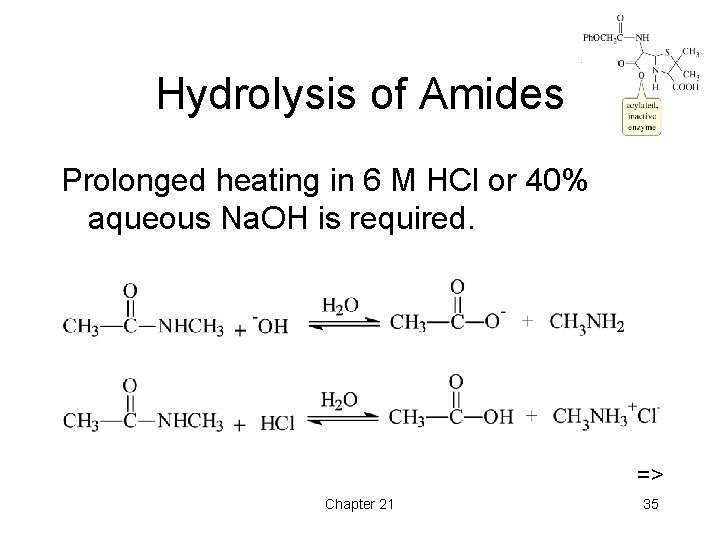
Hydrolysis of Amides Prolonged heating in 6 M HCl or 40% aqueous Na. OH is required. => Chapter 21 35

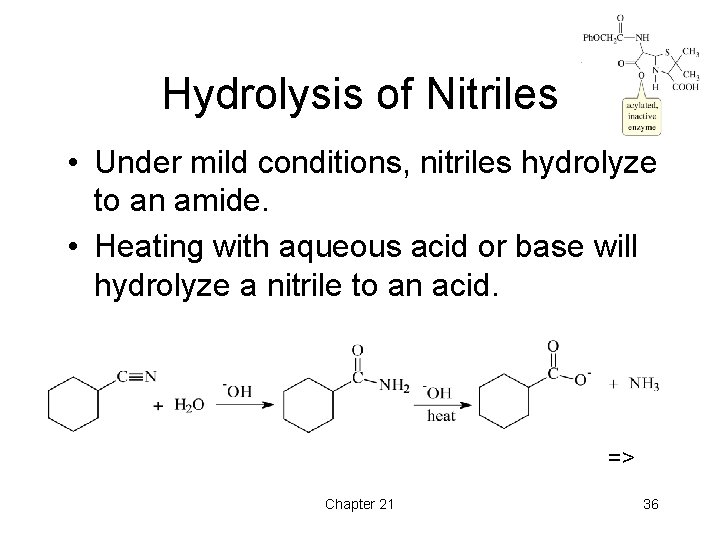
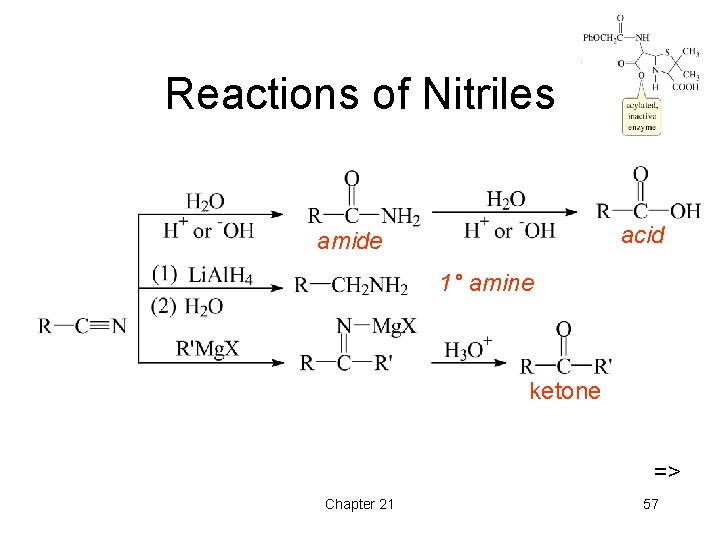
Hydrolysis of Nitriles • Under mild conditions, nitriles hydrolyze to an amide. • Heating with aqueous acid or base will hydrolyze a nitrile to an acid. => Chapter 21 36

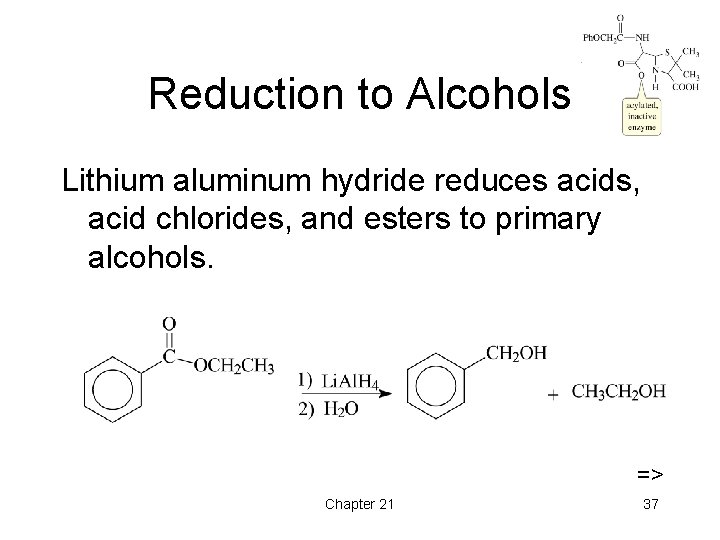
Reduction to Alcohols Lithium aluminum hydride reduces acids, acid chlorides, and esters to primary alcohols. => Chapter 21 37

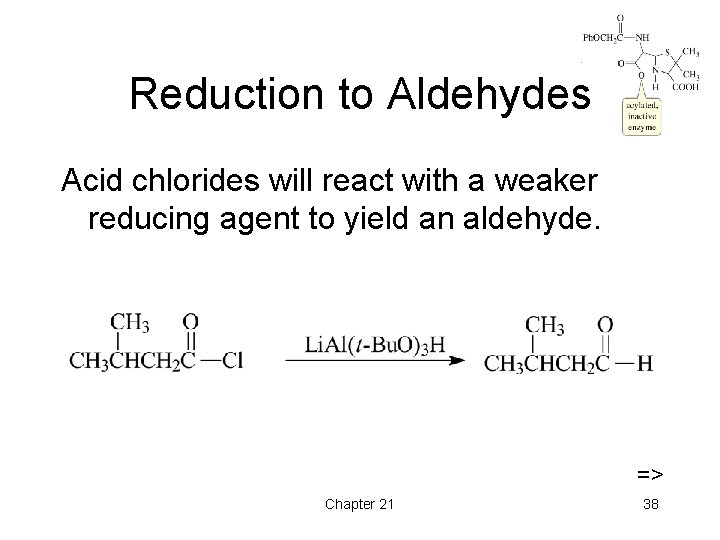
Reduction to Aldehydes Acid chlorides will react with a weaker reducing agent to yield an aldehyde. => Chapter 21 38

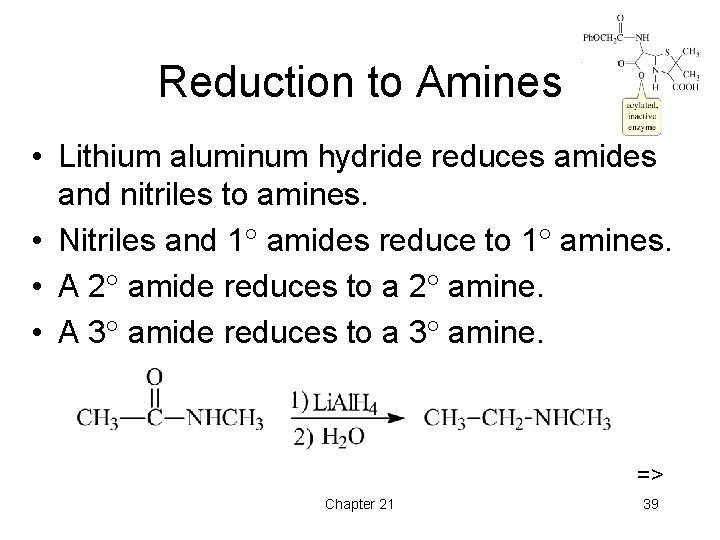
Reduction to Amines • Lithium aluminum hydride reduces amides and nitriles to amines. • Nitriles and 1 amides reduce to 1 amines. • A 2 amide reduces to a 2 amine. • A 3 amide reduces to a 3 amine. => Chapter 21 39

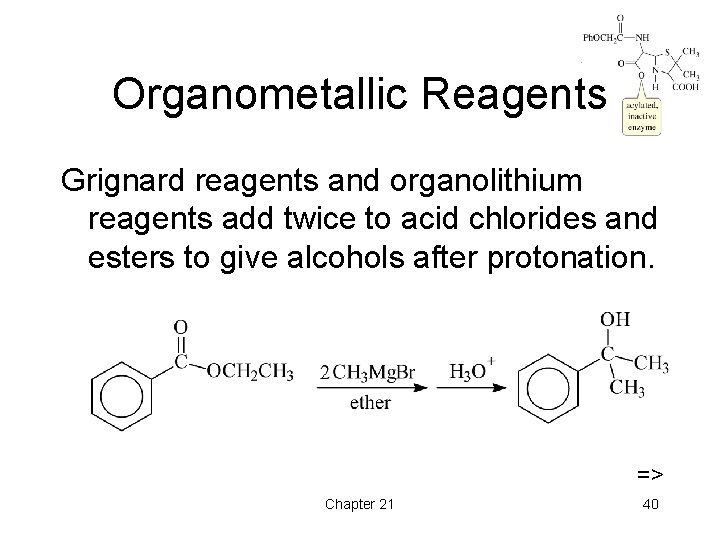
Organometallic Reagents Grignard reagents and organolithium reagents add twice to acid chlorides and esters to give alcohols after protonation. => Chapter 21 40

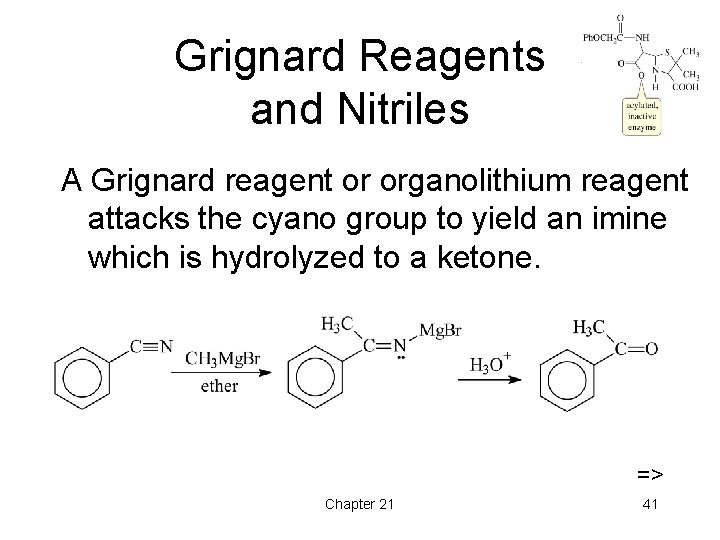
Grignard Reagents and Nitriles A Grignard reagent or organolithium reagent attacks the cyano group to yield an imine which is hydrolyzed to a ketone. => Chapter 21 41

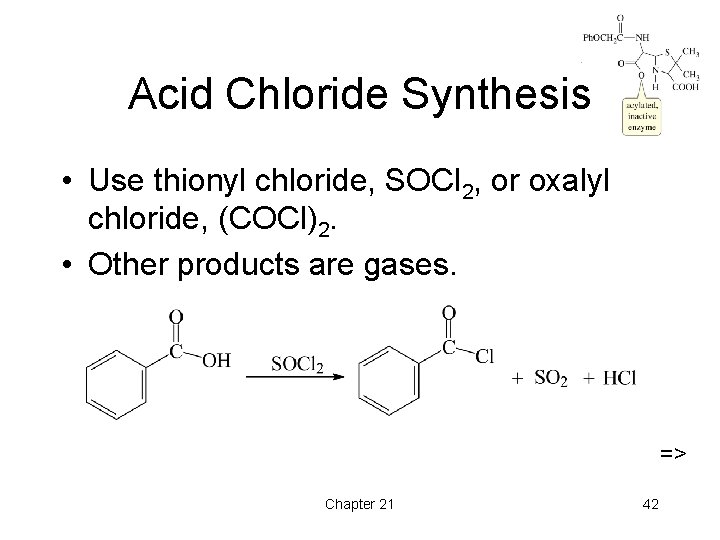
Acid Chloride Synthesis • Use thionyl chloride, SOCl 2, or oxalyl chloride, (COCl)2. • Other products are gases. => Chapter 21 42

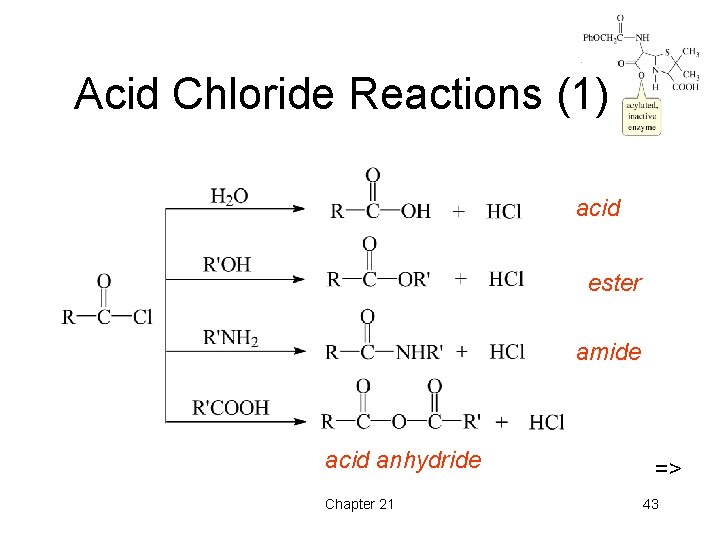
Acid Chloride Reactions (1) acid ester amide acid anhydride Chapter 21 => 43

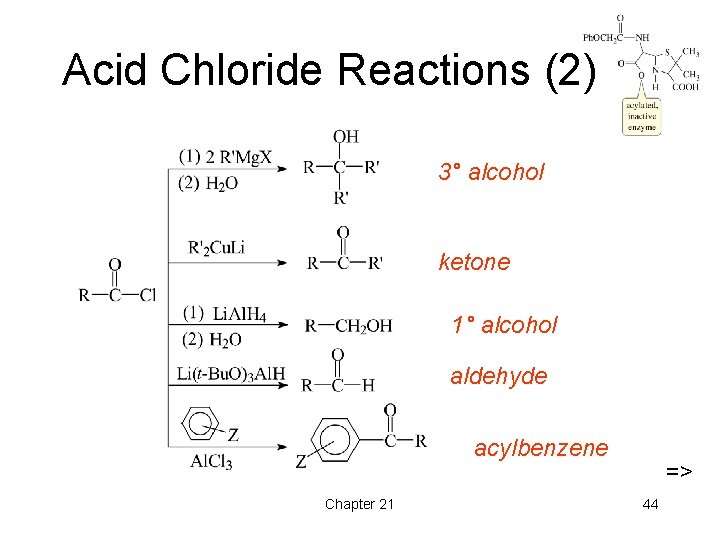
Acid Chloride Reactions (2) 3° alcohol ketone 1° alcohol aldehyde acylbenzene Chapter 21 => 44

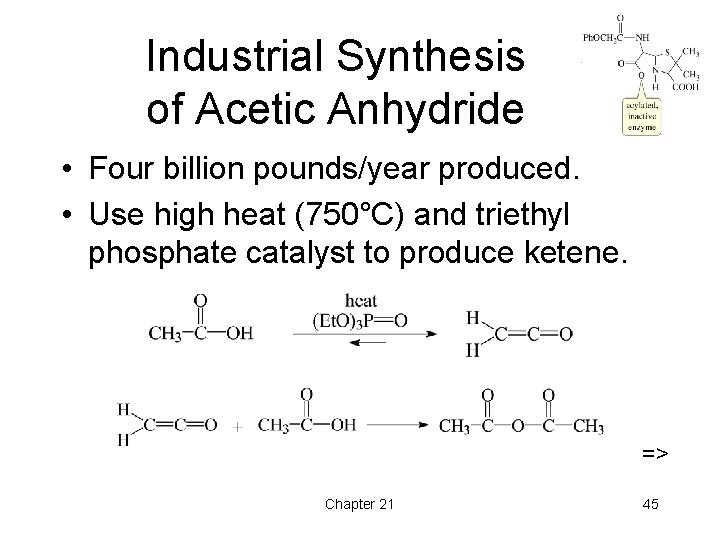
Industrial Synthesis of Acetic Anhydride • Four billion pounds/year produced. • Use high heat (750°C) and triethyl phosphate catalyst to produce ketene. => Chapter 21 45

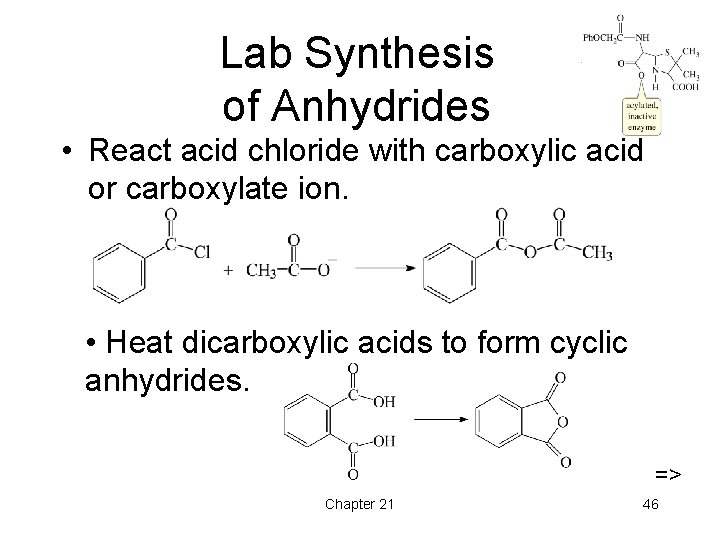
Lab Synthesis of Anhydrides • React acid chloride with carboxylic acid or carboxylate ion. • Heat dicarboxylic acids to form cyclic anhydrides. => Chapter 21 46

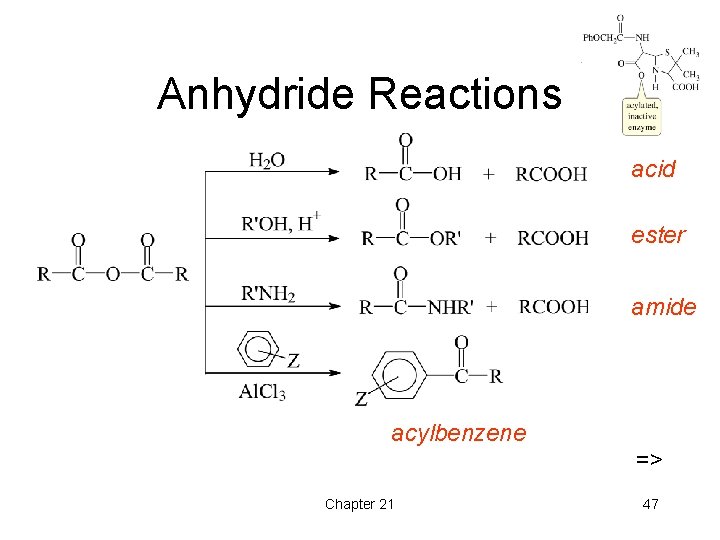
Anhydride Reactions acid ester amide acylbenzene => Chapter 21 47

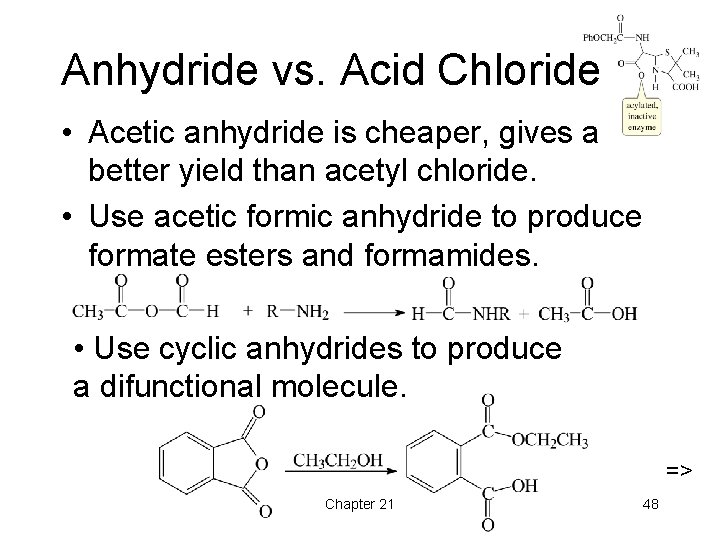
Anhydride vs. Acid Chloride • Acetic anhydride is cheaper, gives a better yield than acetyl chloride. • Use acetic formic anhydride to produce formate esters and formamides. • Use cyclic anhydrides to produce a difunctional molecule. => Chapter 21 48

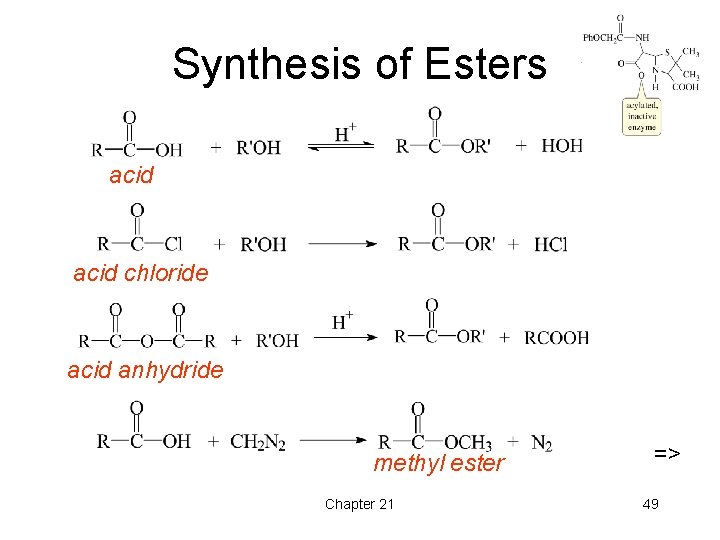
Synthesis of Esters acid chloride acid anhydride methyl ester Chapter 21 => 49

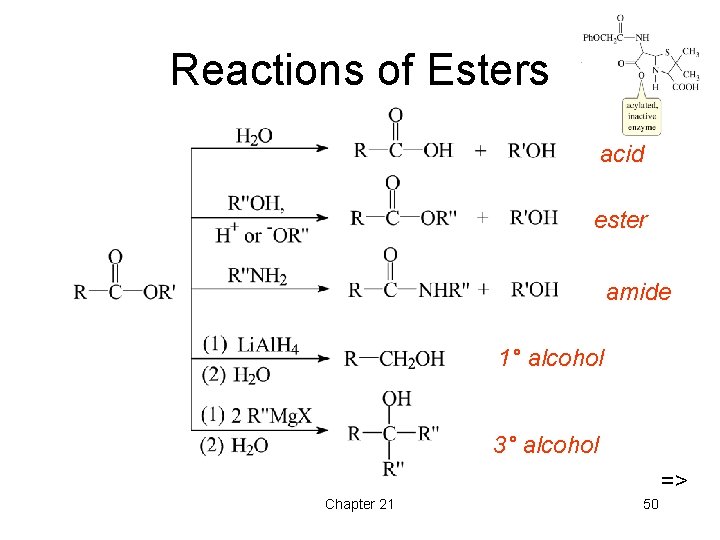
Reactions of Esters acid ester amide 1° alcohol 3° alcohol => Chapter 21 50

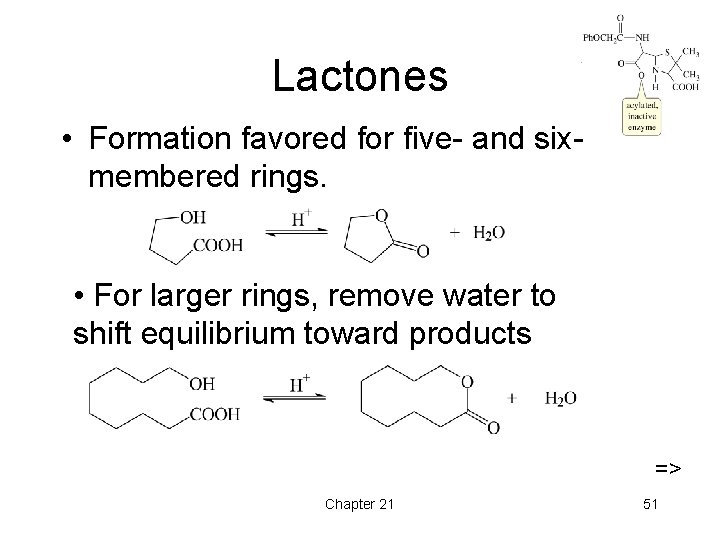
Lactones • Formation favored for five- and sixmembered rings. • For larger rings, remove water to shift equilibrium toward products => Chapter 21 51

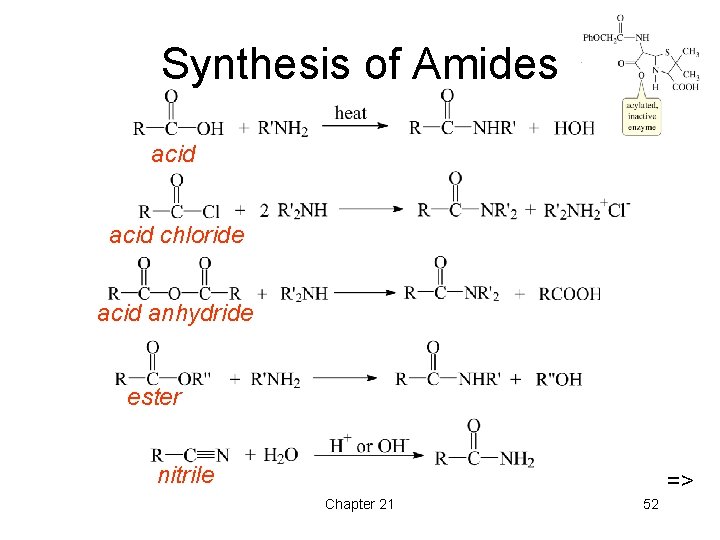
Synthesis of Amides acid chloride acid anhydride ester nitrile => Chapter 21 52

Reactions of Amides acid and amine 1° amine nitrile => Chapter 21 53

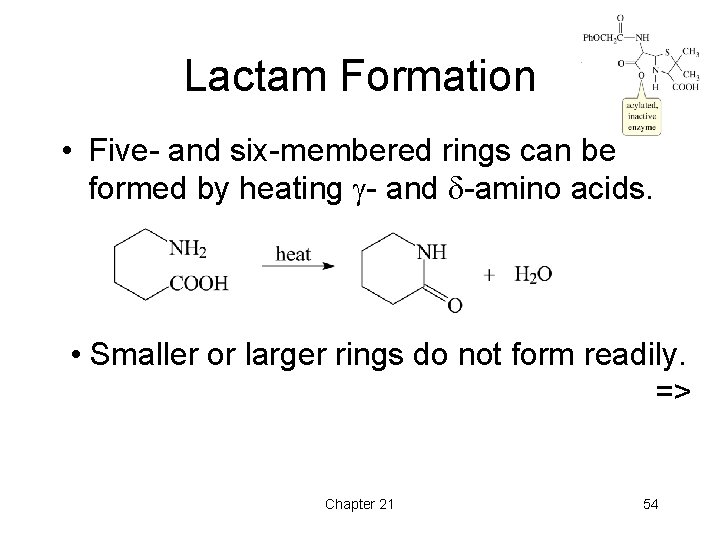
Lactam Formation • Five- and six-membered rings can be formed by heating - and -amino acids. • Smaller or larger rings do not form readily. => Chapter 21 54

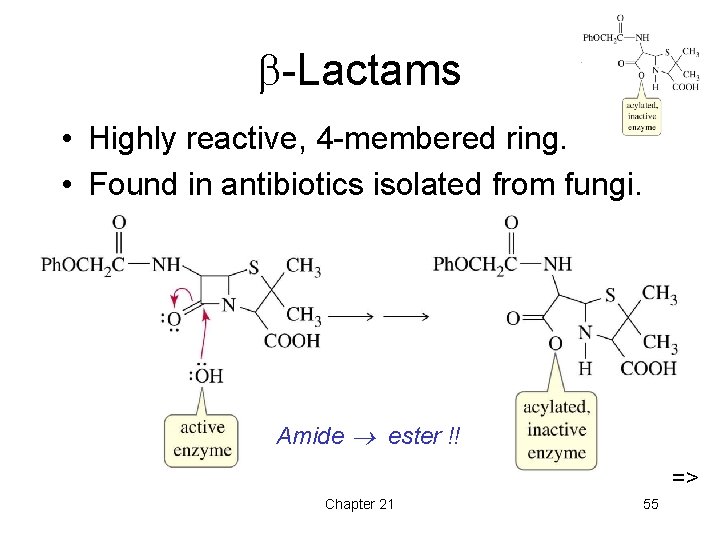
-Lactams • Highly reactive, 4 -membered ring. • Found in antibiotics isolated from fungi. Amide ester !! => Chapter 21 55

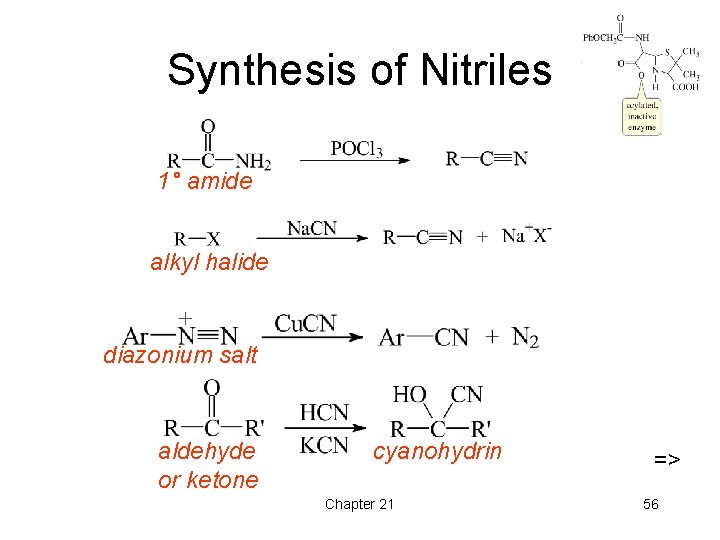
Synthesis of Nitriles 1° amide alkyl halide diazonium salt aldehyde or ketone cyanohydrin Chapter 21 => 56

Reactions of Nitriles acid amide 1° amine ketone => Chapter 21 57

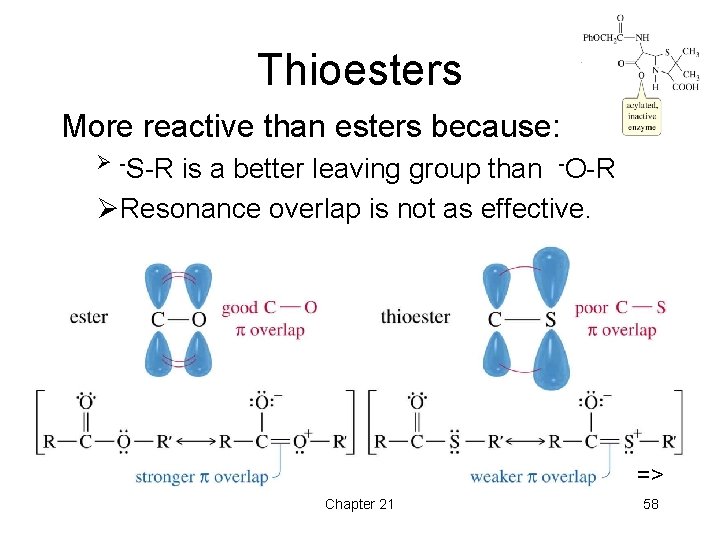
Thioesters More reactive than esters because: Ø -S-R is a better leaving group than -O-R ØResonance overlap is not as effective. => Chapter 21 58

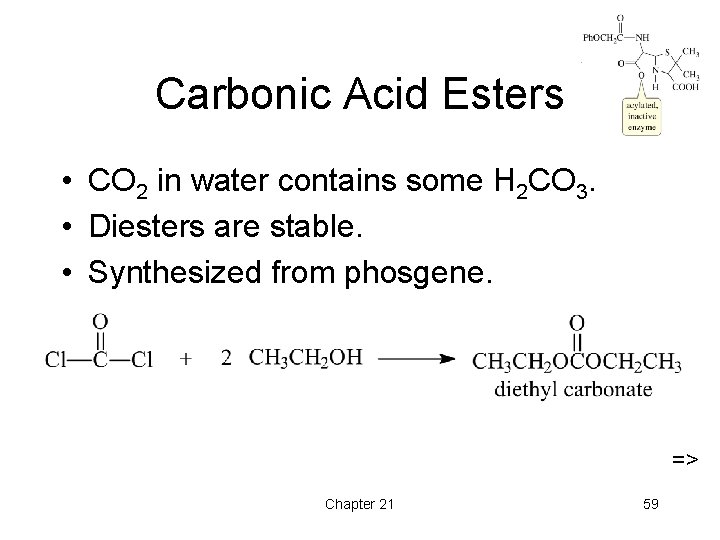
Carbonic Acid Esters • CO 2 in water contains some H 2 CO 3. • Diesters are stable. • Synthesized from phosgene. => Chapter 21 59

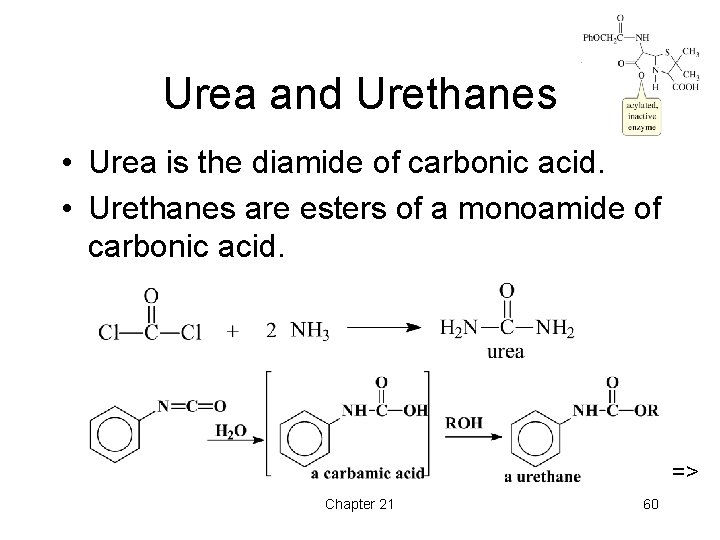
Urea and Urethanes • Urea is the diamide of carbonic acid. • Urethanes are esters of a monoamide of carbonic acid. => Chapter 21 60

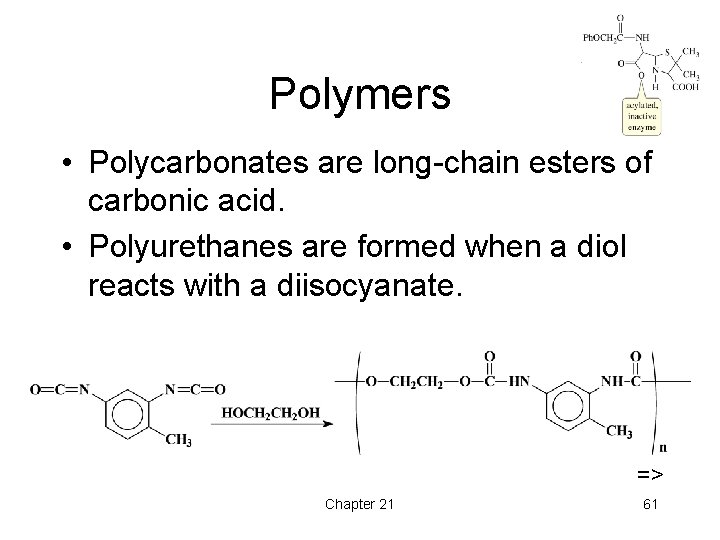
Polymers • Polycarbonates are long-chain esters of carbonic acid. • Polyurethanes are formed when a diol reacts with a diisocyanate. => Chapter 21 61

End of Chapter 21 62
 Organic chemistry wade
Organic chemistry wade Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Transition state energy diagram
Transition state energy diagram David klein organic chemistry
David klein organic chemistry Organic chemistry
Organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry third edition david klein
Organic chemistry third edition david klein Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Halohydrin
Halohydrin Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ib chemistry functional groups
Ib chemistry functional groups Organic chemistry cheat sheet
Organic chemistry cheat sheet Nomenclature chemistry
Nomenclature chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Chapter 7 chemistry review
Chapter 7 chemistry review Sodalime test
Sodalime test Separation scheme of caffeine from vivarin tablets
Separation scheme of caffeine from vivarin tablets Cho
Cho Organic chemistry topic 11
Organic chemistry topic 11 Organic chemistry
Organic chemistry The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms Cyclo organic chemistry
Cyclo organic chemistry Lewis dot structure ch4
Lewis dot structure ch4 Ee organic chemistry
Ee organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Functional group
Functional group How to calculate percent yield
How to calculate percent yield Where is lysine found
Where is lysine found What is organic chemistry like
What is organic chemistry like Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? What is chemistry
What is chemistry Alpha cleavage
Alpha cleavage Mindup mind map
Mindup mind map Organic chemistry lab report example
Organic chemistry lab report example Organic chemistry
Organic chemistry Organic chemistry william h brown
Organic chemistry william h brown Brooklyn college organic chemistry
Brooklyn college organic chemistry Entane
Entane Danswer
Danswer A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Organic chemistry
Organic chemistry Founder of organic chemistry
Founder of organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers David klein
David klein Intro to organic chemistry
Intro to organic chemistry Conjugation organic chemistry
Conjugation organic chemistry Ario organic chemistry
Ario organic chemistry Oxidation of carbohydrates
Oxidation of carbohydrates Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry
Organic chemistry Organic chemistry class 11 notes
Organic chemistry class 11 notes Neon organic or inorganic
Neon organic or inorganic Hono organic chemistry
Hono organic chemistry Organic chemistry naming practice
Organic chemistry naming practice Organic chemistry myanmar
Organic chemistry myanmar Ethos
Ethos Organic chemistry conversion chart
Organic chemistry conversion chart Organic chemistry
Organic chemistry Analytical chemistry chapter 1
Analytical chemistry chapter 1 Conjugation organic chemistry
Conjugation organic chemistry Define compound lipids
Define compound lipids