Organic Chemistry 5 th Edition L G Wade















- Slides: 32

Organic Chemistry, 5 th Edition L. G. Wade, Jr. Chapter 14 Ethers, Epoxides, and Sulfides Jo Blackburn Richland College, Dallas, TX Dallas County Community College District Chapter 14 ã 2003, Prentice Hall

Introduction • Formula R-O-R where R is alkyl or aryl. • Symmetrical or unsymmetrical • Examples: => Chapter 14 2

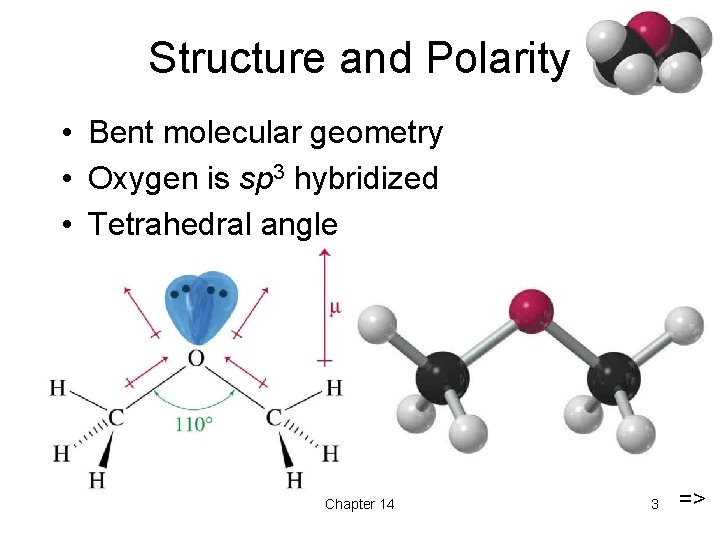
Structure and Polarity • Bent molecular geometry • Oxygen is sp 3 hybridized • Tetrahedral angle Chapter 14 3 =>

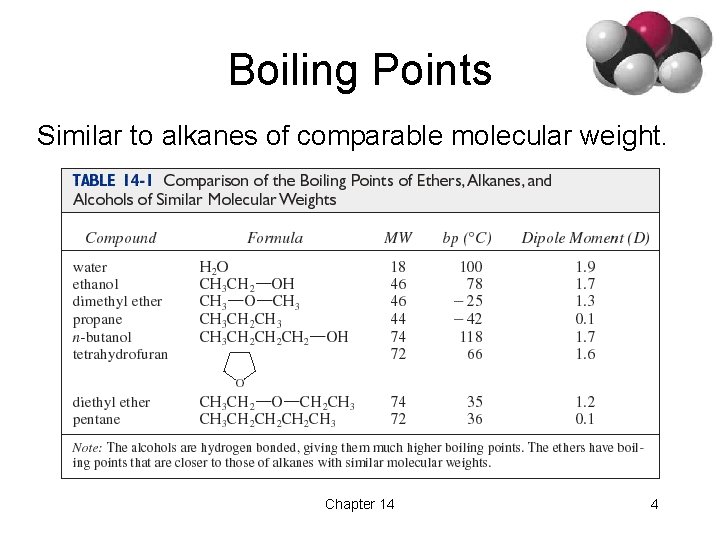
Boiling Points Similar to alkanes of comparable molecular weight. Chapter 14 4

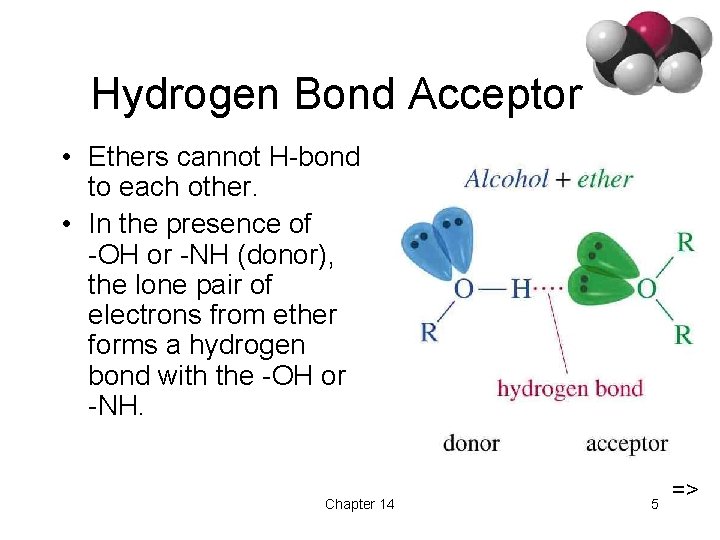
Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. Chapter 14 5 =>

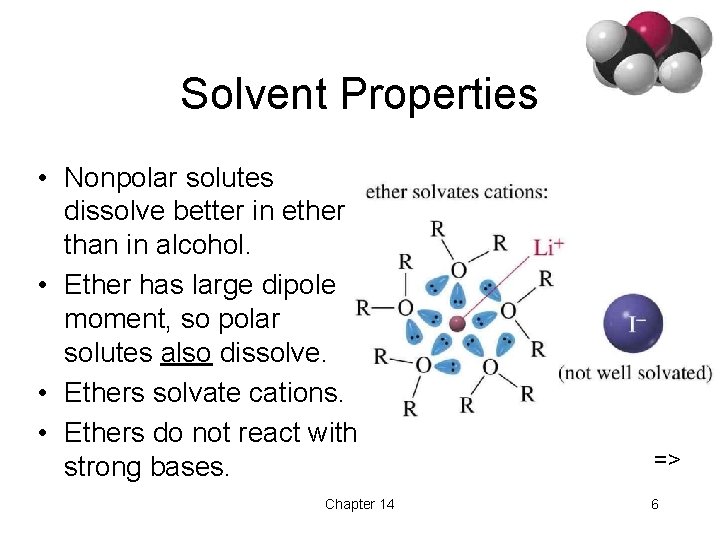
Solvent Properties • Nonpolar solutes dissolve better in ether than in alcohol. • Ether has large dipole moment, so polar solutes also dissolve. • Ethers solvate cations. • Ethers do not react with strong bases. Chapter 14 => 6

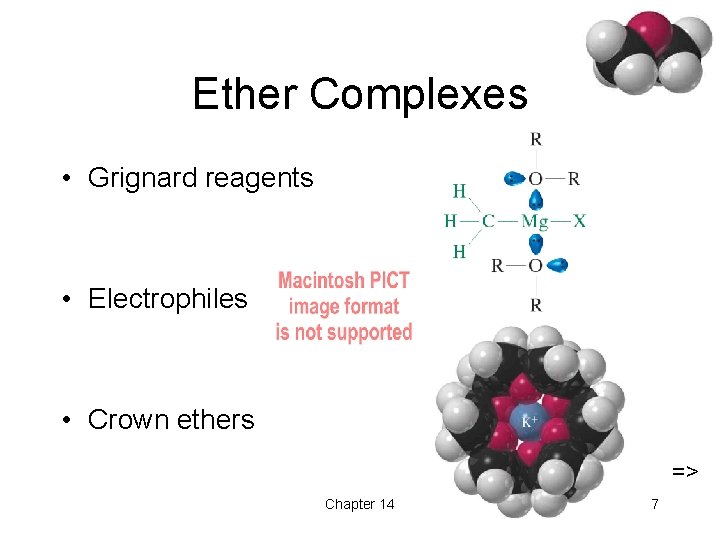
Ether Complexes • Grignard reagents • Electrophiles • Crown ethers => Chapter 14 7

Common Names of Ethers • • • Alkyl alkyl ether Current rule: alphabetical order Old rule: order of increasing complexity Symmetrical: use dialkyl, or just alkyl. Examples: diethyl ether or ethyl ether Chapter 14 t-butyl methyl ether or methyl t-butyl ether 8 =>

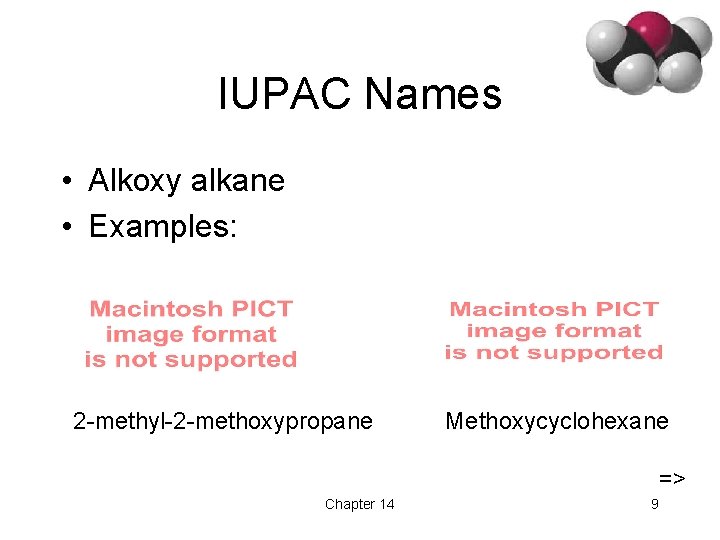
IUPAC Names • Alkoxy alkane • Examples: 2 -methyl-2 -methoxypropane Methoxycyclohexane => Chapter 14 9

Cyclic Ethers • Heterocyclic: oxygen is in ring. • Epoxides (oxiranes) • Oxetanes • Furans (Oxolanes • Pyrans (Oxanes • Dioxanes ) ) => Chapter 14 10

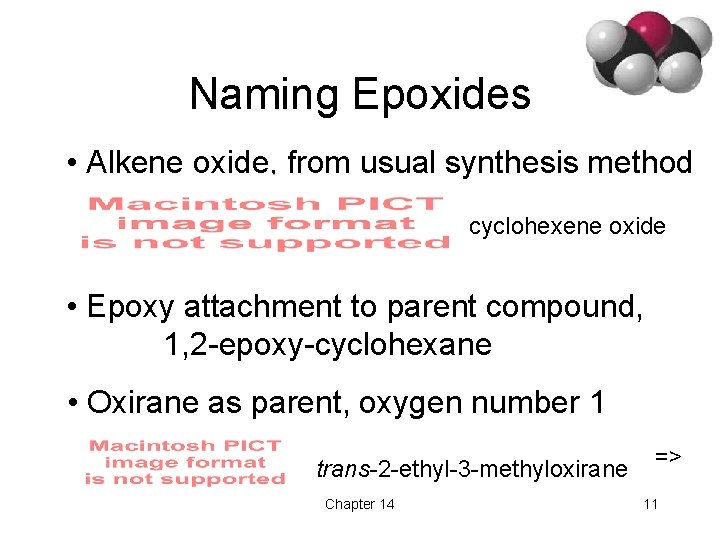
Naming Epoxides • Alkene oxide, from usual synthesis method cyclohexene oxide • Epoxy attachment to parent compound, 1, 2 -epoxy-cyclohexane • Oxirane as parent, oxygen number 1 trans-2 -ethyl-3 -methyloxirane Chapter 14 => 11

Spectroscopy of Ethers • IR: Compound contains oxygen, but O-H and C=O stretches are absent. • MS: -cleavage to form oxonium ion, or loss of either alkyl group. • NMR: 13 C-O signal between 65 - 90, 1 H-C-O signal between 3. 5 - 4. => Chapter 14 12

Williamson Synthesis • Alkoxide ion + 1 alkyl bromide (or tosylate) • Example: Chapter 14 => 13

Phenyl Ethers • Phenoxide ions are easily produced for use in the Williamson synthesis. • Phenyl halides or tosylates cannot be used in this synthesis method. => Chapter 14 14

Alkoxymercuration. Demercuration Use mercuric acetate with an alcohol to add RO-H to a double bond and form the Markovnikov product. => Chapter 14 15

Bimolecular Dehydration of Alcohols • Industrial method, not good lab synthesis. • If temperature is too high, alkene forms. 140°C => Chapter 14 16

Cleavage of Ethers • Ethers are unreactive toward base, but protonated ethers can undergo substitution reactions with strong acids. • Alcohol leaving group is replaced by a halide. • Reactivity: HI > HBr >> HCl => Chapter 14 17

Mechanism for Cleavage • Ether is protonated. • Alcohol leaves as halide attacks. • Alcohol is protonated, halide attacks, and another molecule of alkyl bromide is formed. => Chapter 14 18

Phenyl Ether Cleavage • Phenol cannot react further to become halide. • Example: Chapter 14 => 19

Autoxidation of Ethers • In the presence of atmospheric oxygen, ethers slowly oxidize to hydroperoxides and dialkyl peroxides. • Both are highly explosive. • Precautions: ØDo not distill to dryness. ØStore in full bottles with tight caps. => Chapter 14 20

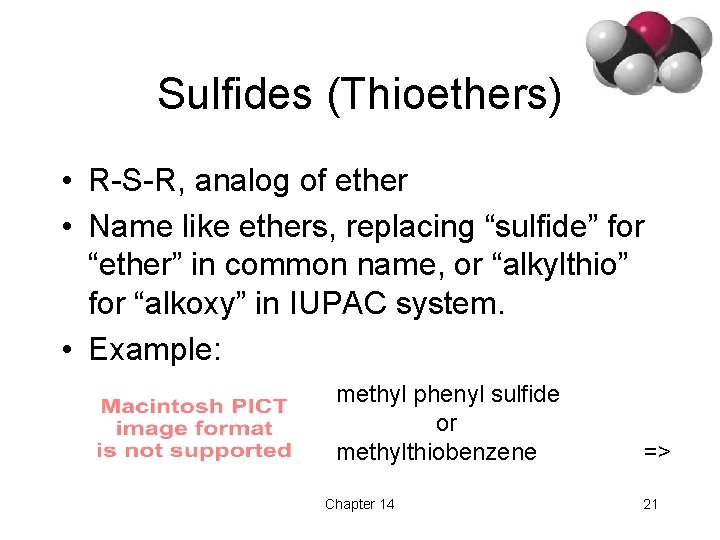
Sulfides (Thioethers) • R-S-R, analog of ether • Name like ethers, replacing “sulfide” for “ether” in common name, or “alkylthio” for “alkoxy” in IUPAC system. • Example: methyl phenyl sulfide or methylthiobenzene Chapter 14 => 21

Thiols and Thiolates • R-SH about same acidity as phenols. • Thiolates are better nucleophiles, weaker bases, than alkoxides. 2 halide Substitution product Chapter 14 => 22

Sulfide Reactions • Sulfides are easily oxidized to sulfoxides and sulfones. • Sulfides react with unhindered alkyl halides to give sulfonium salts. => Chapter 14 23

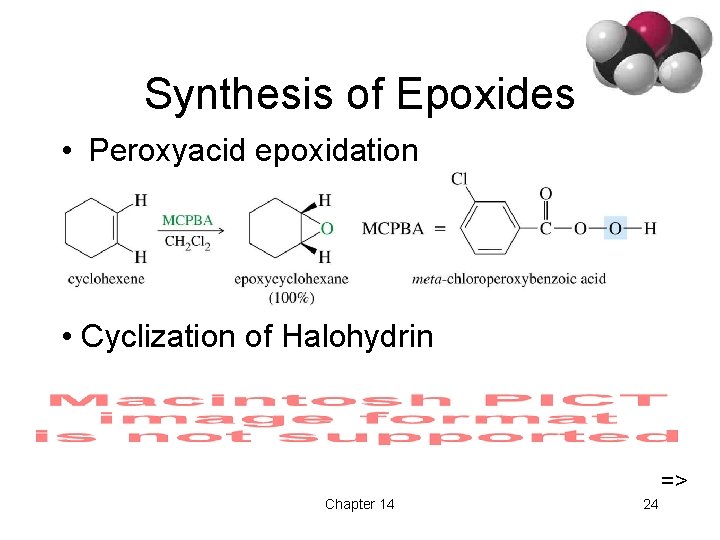
Synthesis of Epoxides • Peroxyacid epoxidation • Cyclization of Halohydrin => Chapter 14 24

Ring Opening in Acid • Trans diol formed in water solvent. • Alkoxy alcohol formed in alcohol solvent. • 1, 2 -Dihalide formed with HI or HBr. Chapter 14 => 25

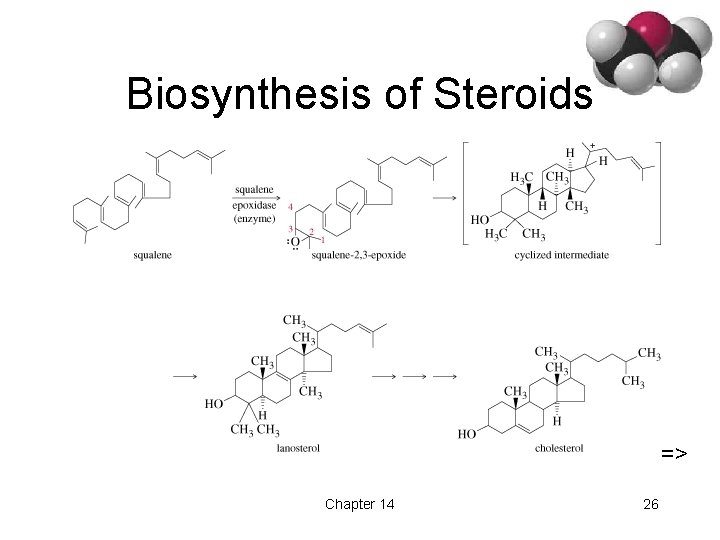
Biosynthesis of Steroids => Chapter 14 26

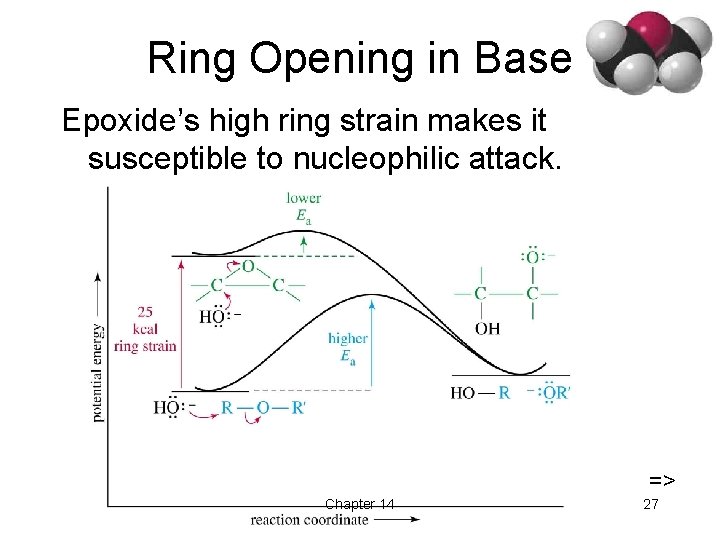
Ring Opening in Base Epoxide’s high ring strain makes it susceptible to nucleophilic attack. => Chapter 14 27

Epoxide Opening in Base • With aqueous hydroxide, a trans 1, 2 -diol is formed. • With alkoxide in alcohol, a trans 1, 2 alkoxy alcohol is formed. • These are the same products that were formed in acid. • Different products are formed in acid and base if epoxide is unsymmetrical. => Chapter 14 28

Orientation of Epoxide Opening • Base attacks the least hindered carbon. • In acid, the nucleophile attacks the protonated epoxide at the most substituted carbon. => Chapter 14 29

Reaction with Grignard and R-Li • Strong base opens the epoxide ring by attacking the less hindered carbon. • Example: => Chapter 14 30

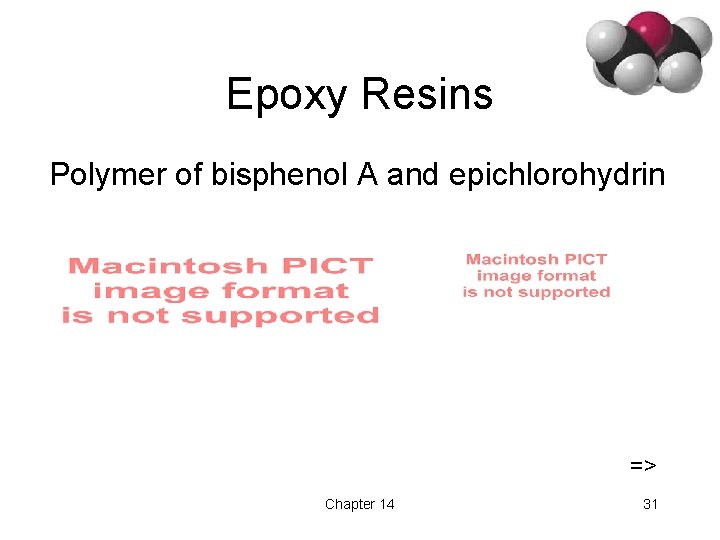
Epoxy Resins Polymer of bisphenol A and epichlorohydrin => Chapter 14 31

End of Chapter 14 32
 Kiliani fischer synthesis
Kiliani fischer synthesis Thermodynamic control
Thermodynamic control Rearranged most stable carbocation is
Rearranged most stable carbocation is David klein organic chemistry
David klein organic chemistry Organic chemistry
Organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Organic chemistry third edition david klein
Organic chemistry third edition david klein Is alkane an organic compound
Is alkane an organic compound Halohydrin
Halohydrin Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Hammonds postulate
Hammonds postulate -oate functional group
-oate functional group Percentage yield practice questions
Percentage yield practice questions Organic chemistry
Organic chemistry What is organic chemistry like
What is organic chemistry like Mass spec of chlorine
Mass spec of chlorine Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? Eth meth prop but pent
Eth meth prop but pent Chemistry mind map
Chemistry mind map Organic chemistry lab report format
Organic chemistry lab report format Organic chemistry
Organic chemistry Organic chemistry william h brown
Organic chemistry william h brown Brooklyn college organic chemistry
Brooklyn college organic chemistry Nonene
Nonene Iodine test for starch
Iodine test for starch A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Nbs
Nbs Father of organic chemistry
Father of organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry
Organic chemistry Organic chemistry introduction
Organic chemistry introduction