Organic Chemistry Second Edition David Klein Chapter 6




![6. 4 Equilibria • In any reaction, collisions are necessary • As [A] and 6. 4 Equilibria • In any reaction, collisions are necessary • As [A] and](https://slidetodoc.com/presentation_image_h/b6fc616354dabf825ca2718a4232c9c5/image-22.jpg)






















- Slides: 86

Organic Chemistry Second Edition David Klein Chapter 6 Chemical Reactivity and Mechanisms Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

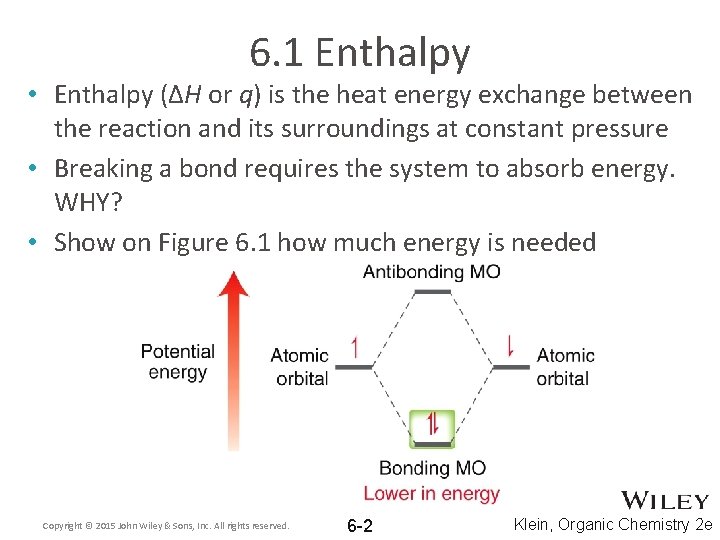
6. 1 Enthalpy • Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure • Breaking a bond requires the system to absorb energy. WHY? • Show on Figure 6. 1 how much energy is needed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -2 Klein, Organic Chemistry 2 e

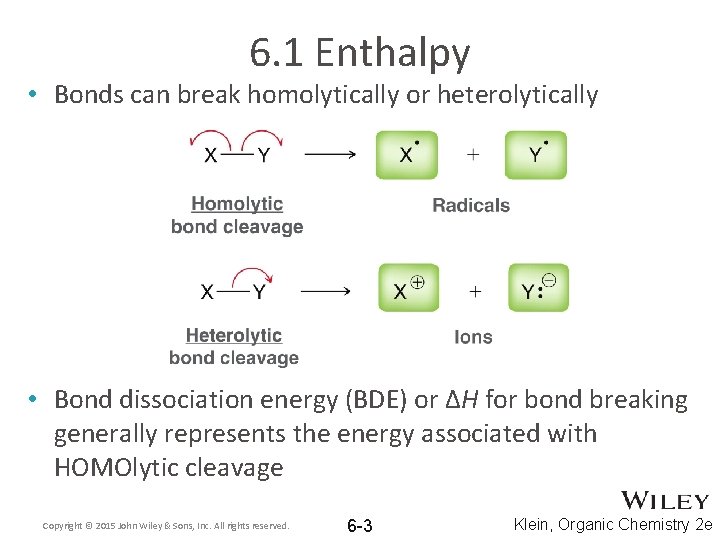
6. 1 Enthalpy • Bonds can break homolytically or heterolytically • Bond dissociation energy (BDE) or ΔH for bond breaking generally represents the energy associated with HOMOlytic cleavage Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -3 Klein, Organic Chemistry 2 e

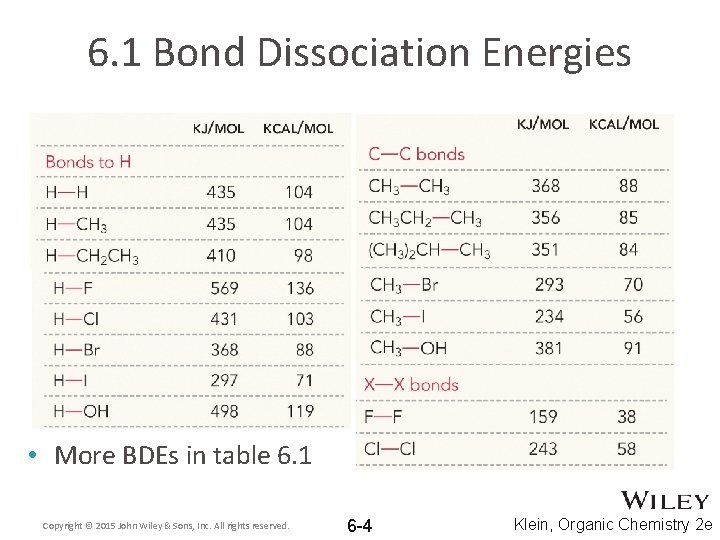
6. 1 Bond Dissociation Energies • More BDEs in table 6. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -4 Klein, Organic Chemistry 2 e

6. 1 BDEs • Explain how heat energy is exchanged between the reaction (system) and the solution (surroundings) for each scenario below 1. H • and F • free radicals come together to form bonds 2. A C–Br bond is broken 3. A strong bond is broken and a weak bond is formed 4. A weak bond is broken and a strong bond is formed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -5 Klein, Organic Chemistry 2 e

6. 1 BDEs • Most reactions involve multiple bonds breaking and forming. The energy associated with each bond that breaks and forms must be considered • If during a chemical reaction the temperature of the reaction solution DECREASES, what can be said about the relative potential energies, stabilities, and bond strengths for the reactants and products • If during a chemical reaction, the temperature of the reaction solution INCREASES, what can be said about the relative potential energies, stabilities, and bond strengths for the reactants and products Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -6 Klein, Organic Chemistry 2 e

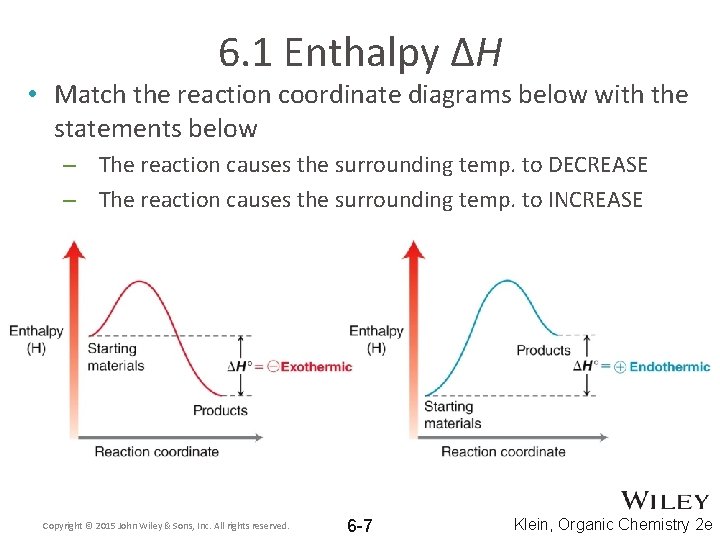
6. 1 Enthalpy ΔH • Match the reaction coordinate diagrams below with the statements below – The reaction causes the surrounding temp. to DECREASE – The reaction causes the surrounding temp. to INCREASE Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -7 Klein, Organic Chemistry 2 e

6. 1 Enthalpy ΔH • For a chemical reaction, why is the sign (+/-) for ΔH important? • If you were performing a chemical reaction in a lab, what experimental considerations might you make if you knew ΔH = + • If you were performing a chemical reaction in a lab, what experimental considerations might you make if you knew ΔH = • Practice with Skill. Builder 6. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -8 Klein, Organic Chemistry 2 e

6. 2 Entropy ΔS • Although most reactions are EXOthermic, there are many ENDOthermic reactions that occur • Enthalphy and entropy must BOTH be considered when predicting whether a reaction will occur • ENTROPY (ΔS) can be though of as molecular disorder, randomness, or freedom • Entropy may most accurately be thought of as the number of states that a molecule’s energy can be distributed over Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -9 Klein, Organic Chemistry 2 e

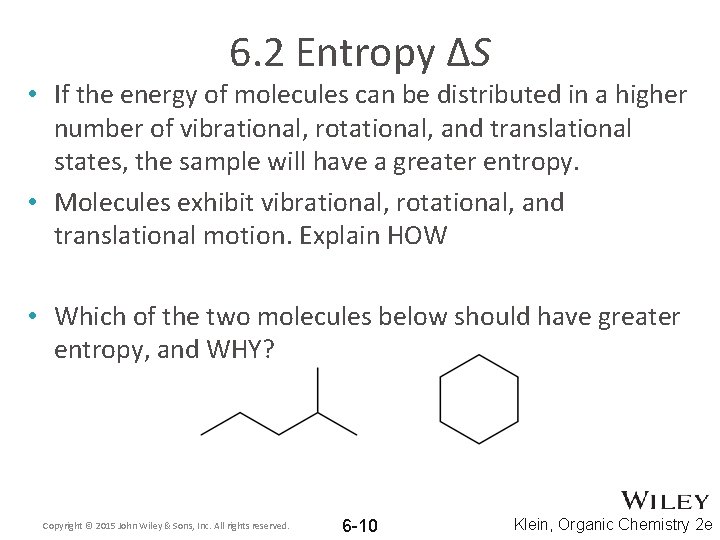
6. 2 Entropy ΔS • If the energy of molecules can be distributed in a higher number of vibrational, rotational, and translational states, the sample will have a greater entropy. • Molecules exhibit vibrational, rotational, and translational motion. Explain HOW • Which of the two molecules below should have greater entropy, and WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -10 Klein, Organic Chemistry 2 e

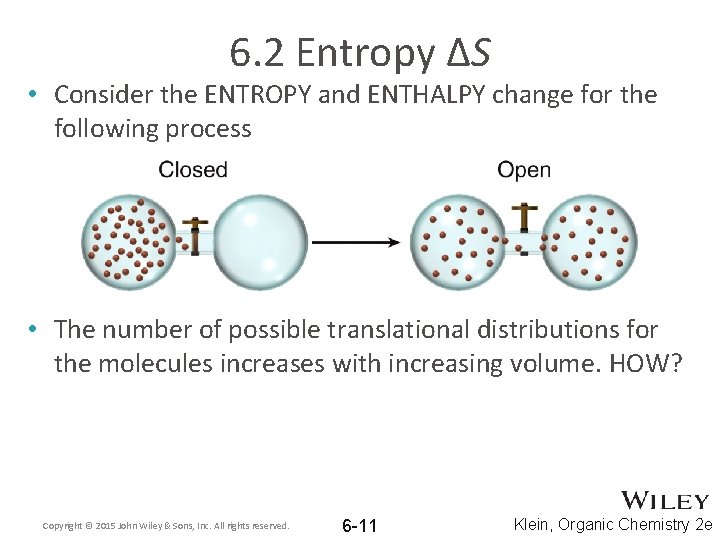
6. 2 Entropy ΔS • Consider the ENTROPY and ENTHALPY change for the following process • The number of possible translational distributions for the molecules increases with increasing volume. HOW? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -11 Klein, Organic Chemistry 2 e

6. 2 Entropy ΔS • The total entropy change will determine whether a process is spontaneous (favors the forward direction) • If ΔStot is positive, the process is spontaneous. What if ΔStot is negative? • When the volume of a gas expands to fill a container, what should ΔSsurr be? • For chemical reactions, we must consider the entropy change for both the system (the reaction) and the surroundings (the solvent usually) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -12 Klein, Organic Chemistry 2 e

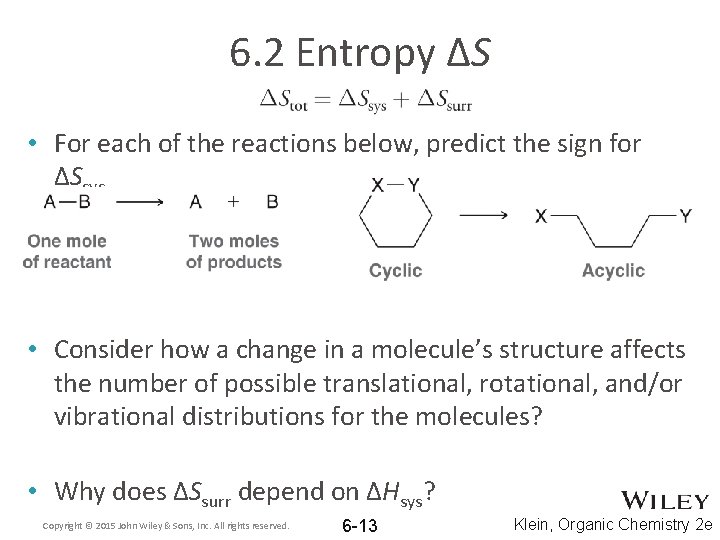
6. 2 Entropy ΔS • For each of the reactions below, predict the sign for ΔSsys • Consider how a change in a molecule’s structure affects the number of possible translational, rotational, and/or vibrational distributions for the molecules? • Why does ΔSsurr depend on ΔHsys? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -13 Klein, Organic Chemistry 2 e

6. 2 Entropy ΔS • How would the following conditions affect spontaneity (the degree to which the reaction is product favored)? 1. The reactions are exothermic 2. The reactions are highly endothermic 3. The reactions are slightly endothermic • Practice with conceptual checkpoint 6. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -14 Klein, Organic Chemistry 2 e

6. 3 Gibbs Free Energy ΔG • We know that the spontaneity of a process depends only on ΔStot • ΔSsys can be measured or estimated • ΔSsurr depends on ΔHsys • Plug in Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -15 Klein, Organic Chemistry 2 e

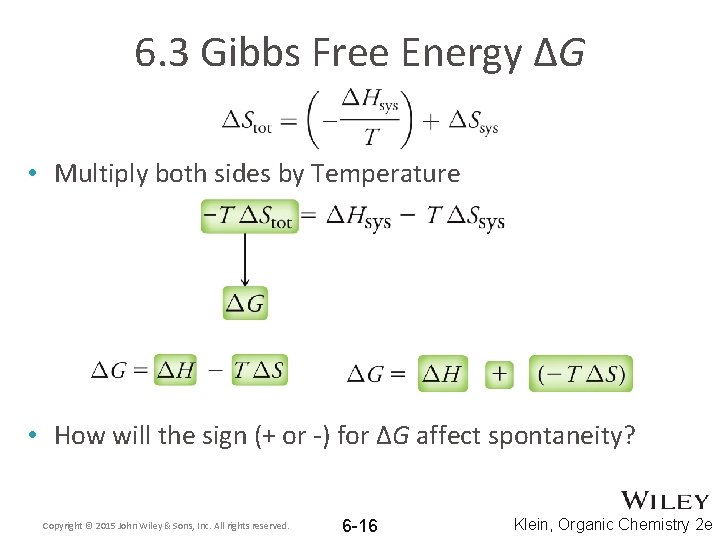
6. 3 Gibbs Free Energy ΔG • Multiply both sides by Temperature » or • How will the sign (+ or -) for ΔG affect spontaneity? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -16 Klein, Organic Chemistry 2 e

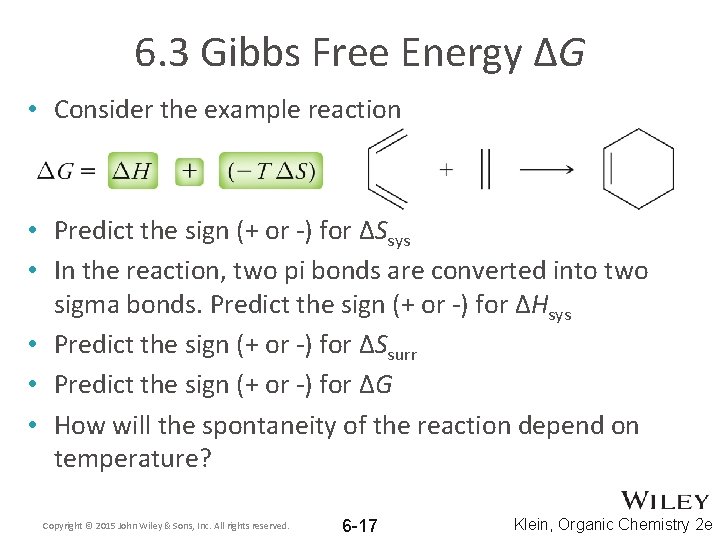
6. 3 Gibbs Free Energy ΔG • Consider the example reaction • Predict the sign (+ or -) for ΔSsys • In the reaction, two pi bonds are converted into two sigma bonds. Predict the sign (+ or -) for ΔHsys • Predict the sign (+ or -) for ΔSsurr • Predict the sign (+ or -) for ΔG • How will the spontaneity of the reaction depend on temperature? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -17 Klein, Organic Chemistry 2 e

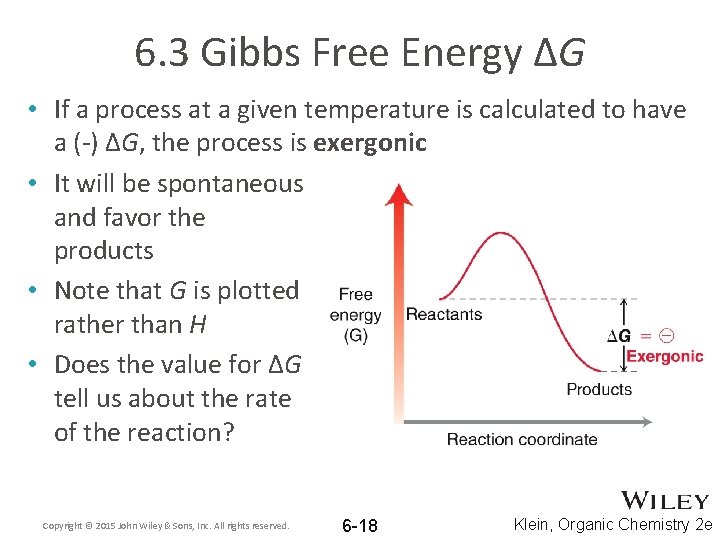
6. 3 Gibbs Free Energy ΔG • If a process at a given temperature is calculated to have a (-) ΔG, the process is exergonic • It will be spontaneous and favor the products • Note that G is plotted rather than H • Does the value for ΔG tell us about the rate of the reaction? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -18 Klein, Organic Chemistry 2 e

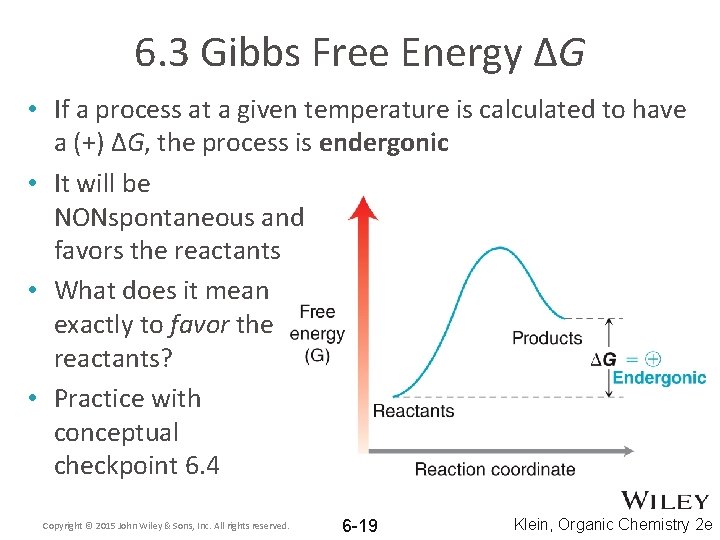
6. 3 Gibbs Free Energy ΔG • If a process at a given temperature is calculated to have a (+) ΔG, the process is endergonic • It will be NONspontaneous and favors the reactants • What does it mean exactly to favor the reactants? • Practice with conceptual checkpoint 6. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -19 Klein, Organic Chemistry 2 e

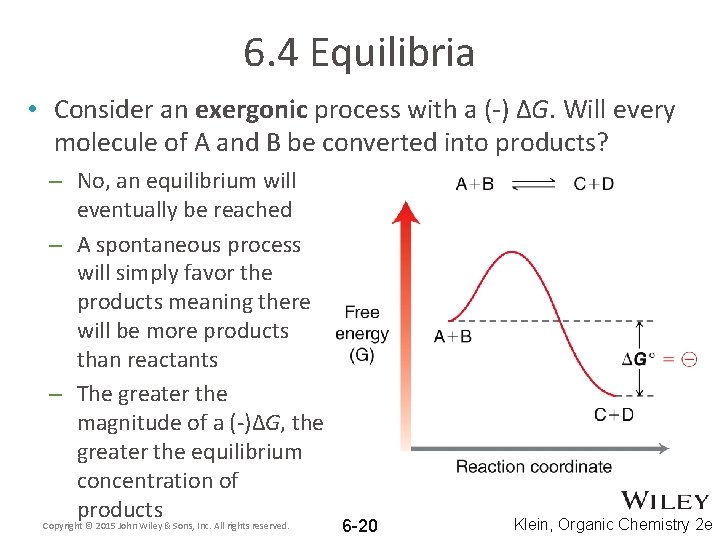
6. 4 Equilibria • Consider an exergonic process with a (-) ΔG. Will every molecule of A and B be converted into products? – No, an equilibrium will eventually be reached – A spontaneous process will simply favor the products meaning there will be more products than reactants – The greater the magnitude of a (-)ΔG, the greater the equilibrium concentration of products Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -20 Klein, Organic Chemistry 2 e

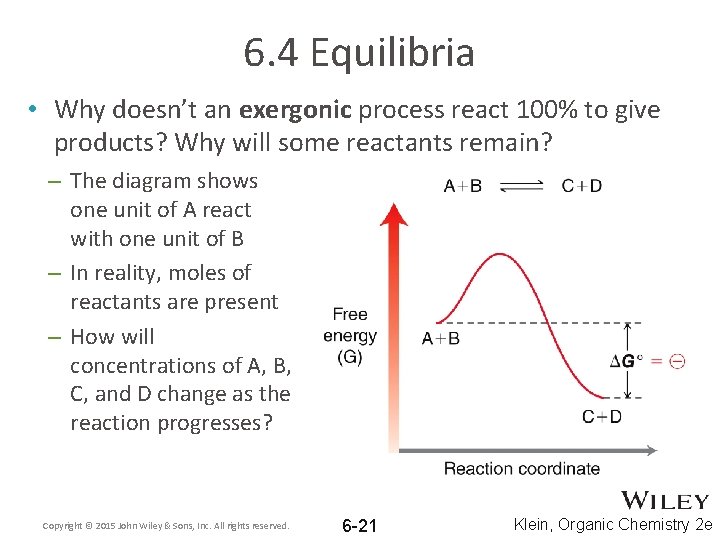
6. 4 Equilibria • Why doesn’t an exergonic process react 100% to give products? Why will some reactants remain? – The diagram shows one unit of A react with one unit of B – In reality, moles of reactants are present – How will concentrations of A, B, C, and D change as the reaction progresses? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -21 Klein, Organic Chemistry 2 e
![6 4 Equilibria In any reaction collisions are necessary As A and 6. 4 Equilibria • In any reaction, collisions are necessary • As [A] and](https://slidetodoc.com/presentation_image_h/b6fc616354dabf825ca2718a4232c9c5/image-22.jpg)
6. 4 Equilibria • In any reaction, collisions are necessary • As [A] and [B] decrease collisions between A and B will occur less often • As [C] and [D] increase, collisions between C and D will occur more often – The more often C and D collide, the more often collisions will occur with enough free energy for the reverse reaction to take place • Recall that equilibrium is dynamic and occurs when the forward and reverse reaction rates are equal Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -22 Klein, Organic Chemistry 2 e

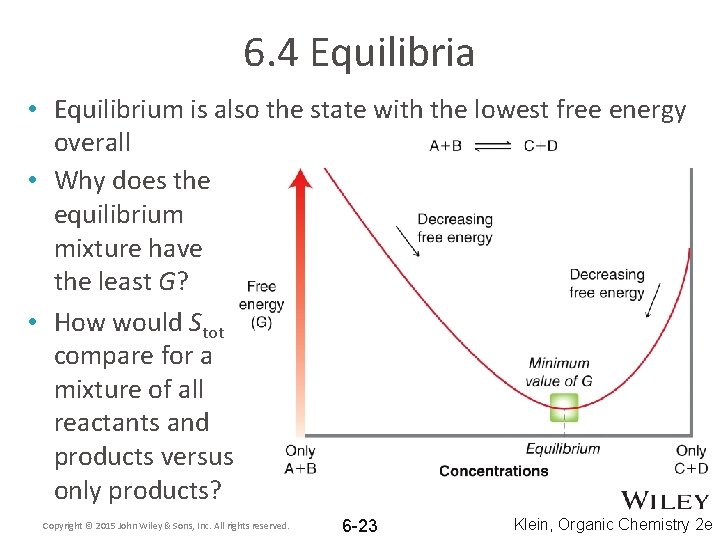
6. 4 Equilibria • Equilibrium is also the state with the lowest free energy overall • Why does the equilibrium mixture have the least G? • How would Stot compare for a mixture of all reactants and products versus only products? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -23 Klein, Organic Chemistry 2 e

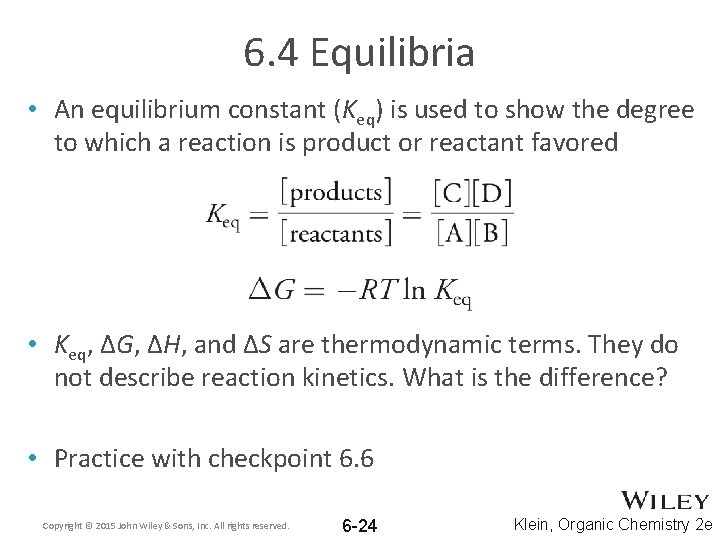
6. 4 Equilibria • An equilibrium constant (Keq) is used to show the degree to which a reaction is product or reactant favored • Keq, ΔG, ΔH, and ΔS are thermodynamic terms. They do not describe reaction kinetics. What is the difference? • Practice with checkpoint 6. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -24 Klein, Organic Chemistry 2 e

6. 4 Equilibria • What trends do you notice in table 6. 2? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -25 Klein, Organic Chemistry 2 e

6. 5 Kinetics • Recall that a (-) sign for ΔG tells us a process is product favored (spontaneous) • That does NOT tell us anything about the RATE or kinetics for the process • Some spontaneous processes are fast such as explosions. Can you think of other examples? • Some spontaneous processes are slow such as C (diamond) C (graphite). Can you think of other examples? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -26 Klein, Organic Chemistry 2 e

6. 5 Kinetics • The rate of a reaction tells us how many molecules are reacting in a given period of time • Give some examples for typical reaction rate units • Recall that for reactions to take place, reactants must collide with sufficient force. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -27 Klein, Organic Chemistry 2 e

6. 5 Kinetics • The reaction rate (the number of collisions that will result in production in a given period of time) is affected by multiple factors 1. 2. 3. 4. 5. The concentrations of the reactants The Activation Energy The Temperature Geometry and Sterics The presence of a catalyst • How will an increase in [reactant] generally affect the reaction rate? WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -28 Klein, Organic Chemistry 2 e

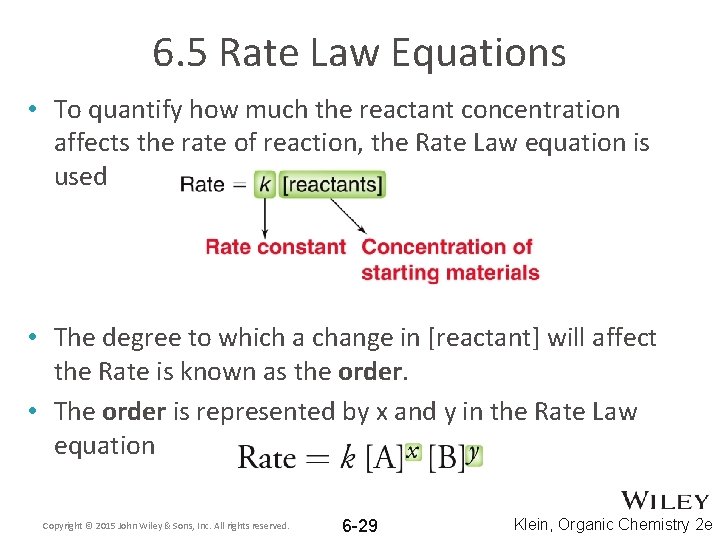
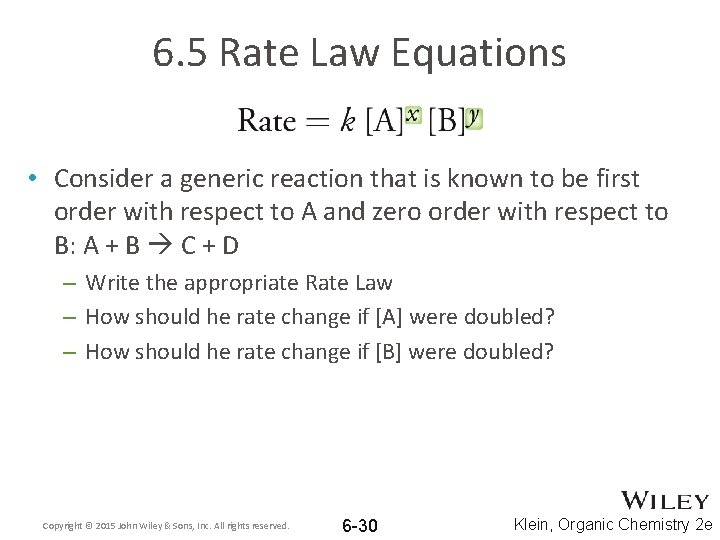
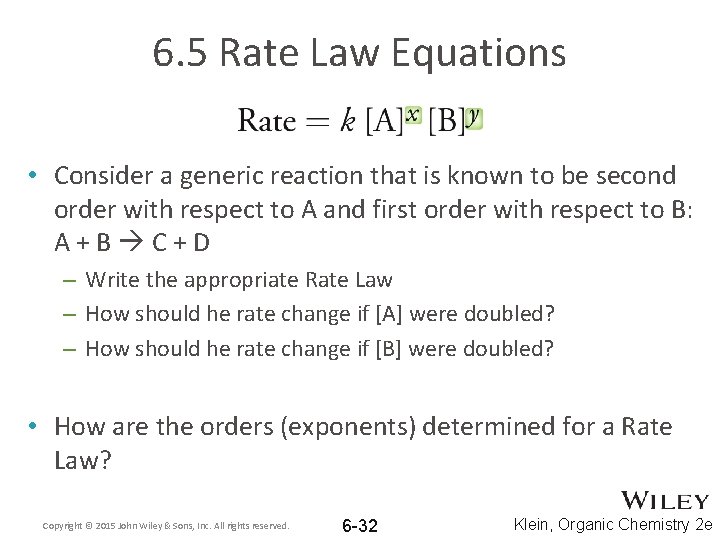
6. 5 Rate Law Equations • To quantify how much the reactant concentration affects the rate of reaction, the Rate Law equation is used • The degree to which a change in [reactant] will affect the Rate is known as the order. • The order is represented by x and y in the Rate Law equation Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -29 Klein, Organic Chemistry 2 e

6. 5 Rate Law Equations • Consider a generic reaction that is known to be first order with respect to A and zero order with respect to B: A + B C + D – Write the appropriate Rate Law – How should he rate change if [A] were doubled? – How should he rate change if [B] were doubled? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -30 Klein, Organic Chemistry 2 e

6. 5 Rate Law Equations • Consider a generic reaction that is known to be first order with respect to A and first order with respect to B: A+B C+D – Write the appropriate Rate Law – How should he rate change if [A] were doubled? – How should he rate change if [B] were doubled? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -31 Klein, Organic Chemistry 2 e

6. 5 Rate Law Equations • Consider a generic reaction that is known to be second order with respect to A and first order with respect to B: A+B C+D – Write the appropriate Rate Law – How should he rate change if [A] were doubled? – How should he rate change if [B] were doubled? • How are the orders (exponents) determined for a Rate Law? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -32 Klein, Organic Chemistry 2 e

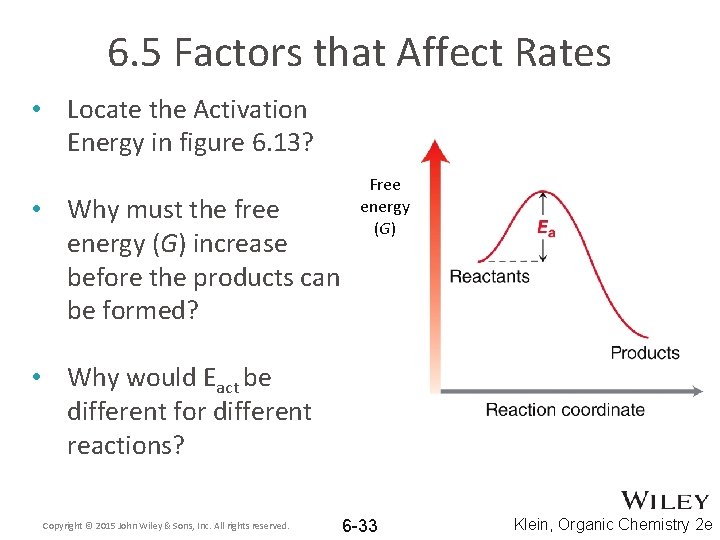
6. 5 Factors that Affect Rates • Locate the Activation Energy in figure 6. 13? • Why must the free energy (G) increase before the products can be formed? Free energy (G) • Why would Eact be different for different reactions? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -33 Klein, Organic Chemistry 2 e

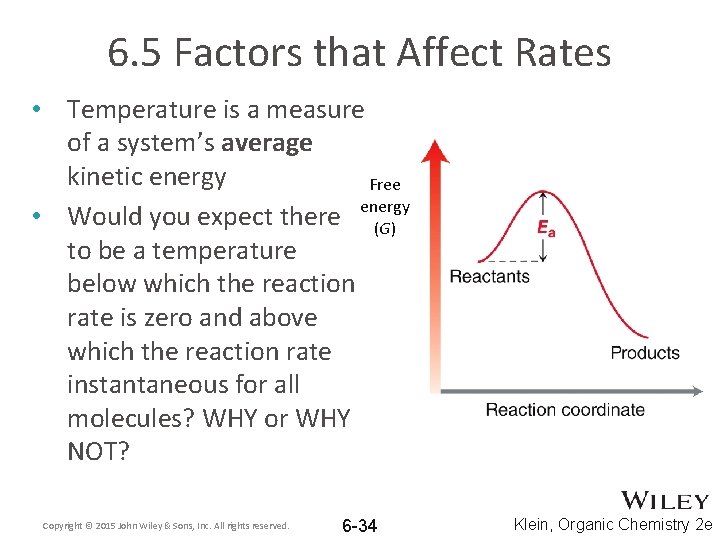
6. 5 Factors that Affect Rates • Temperature is a measure of a system’s average kinetic energy Free • Would you expect there energy (G) to be a temperature below which the reaction rate is zero and above which the reaction rate instantaneous for all molecules? WHY or WHY NOT? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -34 Klein, Organic Chemistry 2 e

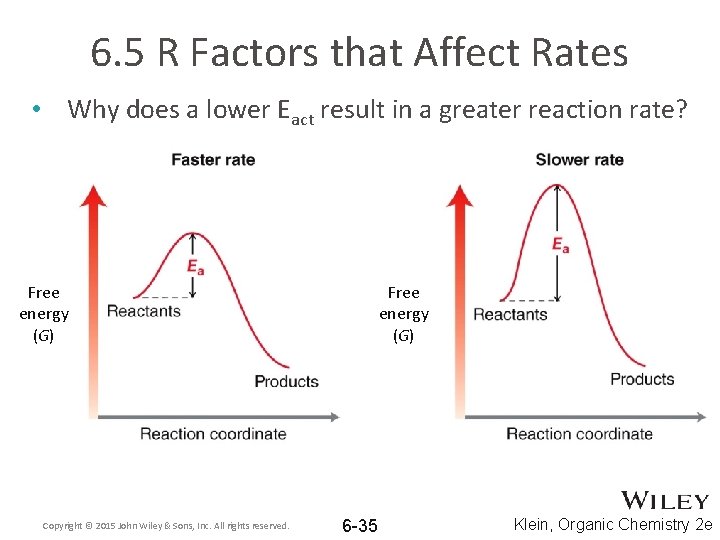
6. 5 R Factors that Affect Rates • Why does a lower Eact result in a greater reaction rate? Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Free energy (G) 6 -35 Klein, Organic Chemistry 2 e

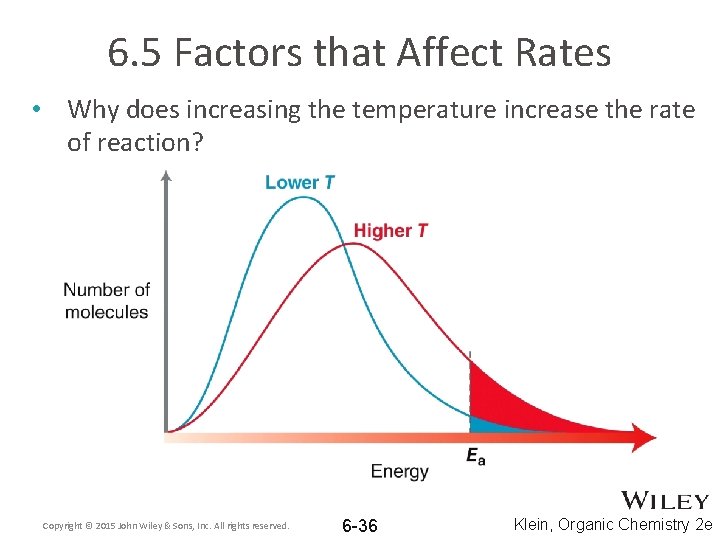
6. 5 Factors that Affect Rates • Why does increasing the temperature increase the rate of reaction? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -36 Klein, Organic Chemistry 2 e

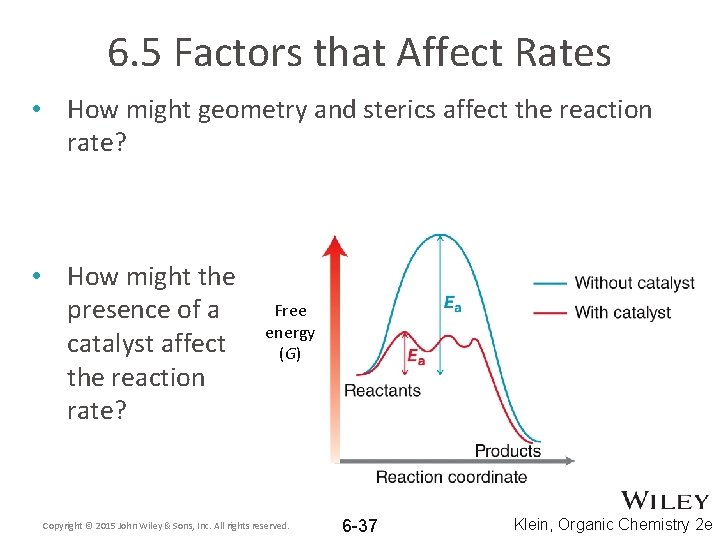
6. 5 Factors that Affect Rates • How might geometry and sterics affect the reaction rate? • How might the presence of a catalyst affect the reaction rate? Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -37 Klein, Organic Chemistry 2 e

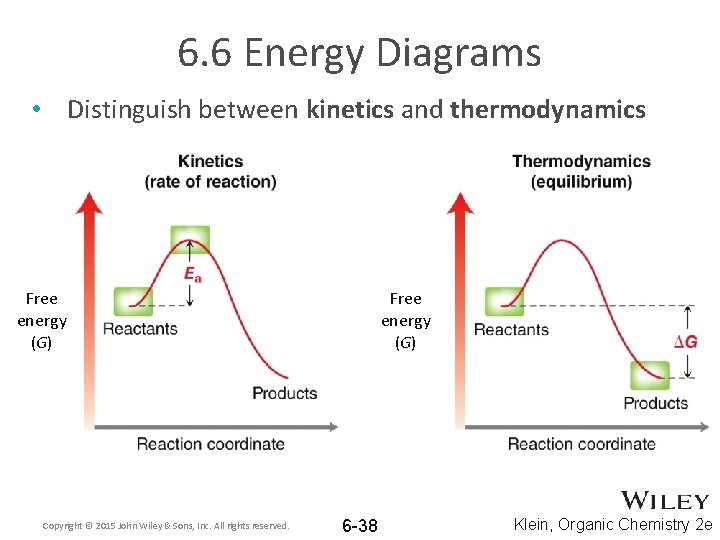
6. 6 Energy Diagrams • Distinguish between kinetics and thermodynamics Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Free energy (G) 6 -38 Klein, Organic Chemistry 2 e

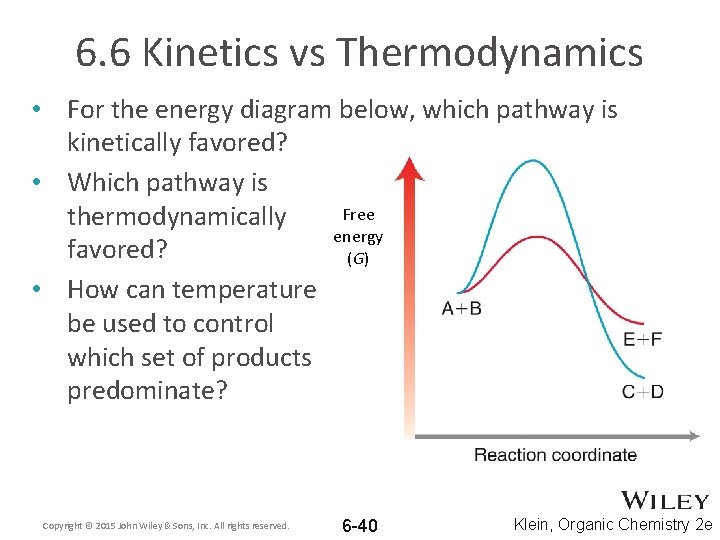
6. 6 Kinetics vs Thermodynamics • For the energy diagram below, which pathway do you think is favored? WHY? • Will a decrease in Free temperature affect energy which pathway is (G) favored? • Will an increase in temperature affect which pathway is favored? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -39 Klein, Organic Chemistry 2 e

6. 6 Kinetics vs Thermodynamics • For the energy diagram below, which pathway is kinetically favored? • Which pathway is Free thermodynamically energy favored? (G) • How can temperature be used to control which set of products predominate? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -40 Klein, Organic Chemistry 2 e

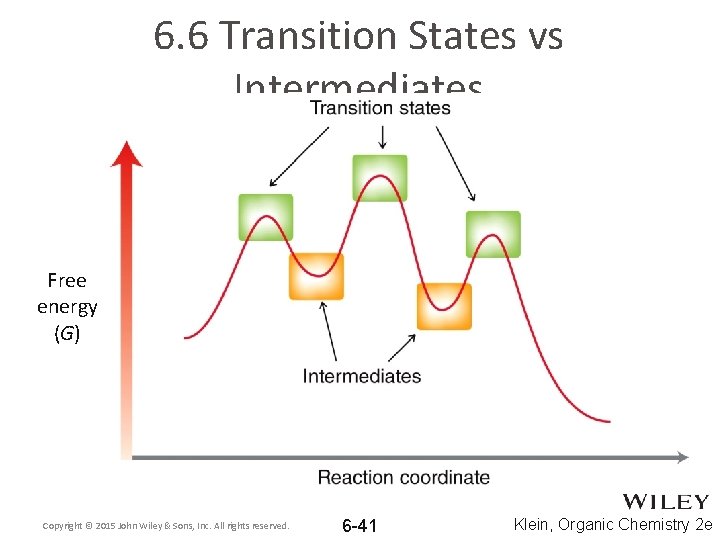
6. 6 Transition States vs Intermediates Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -41 Klein, Organic Chemistry 2 e

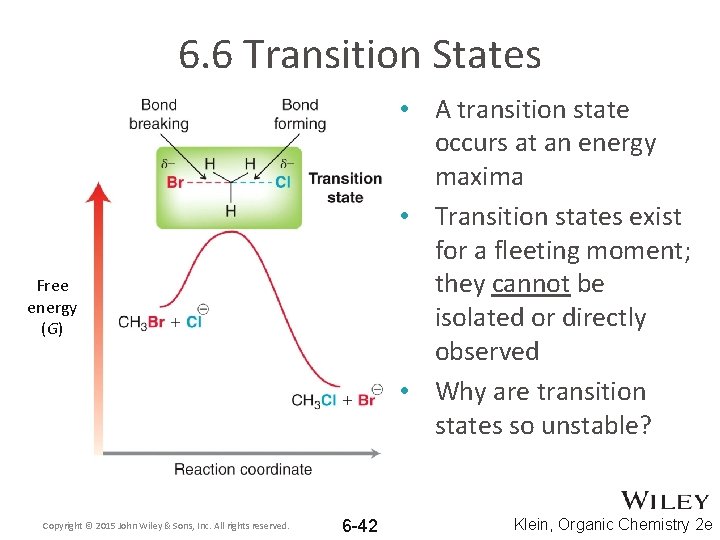
6. 6 Transition States • A transition state occurs at an energy maxima • Transition states exist for a fleeting moment; they cannot be isolated or directly observed • Why are transition states so unstable? Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -42 Klein, Organic Chemistry 2 e

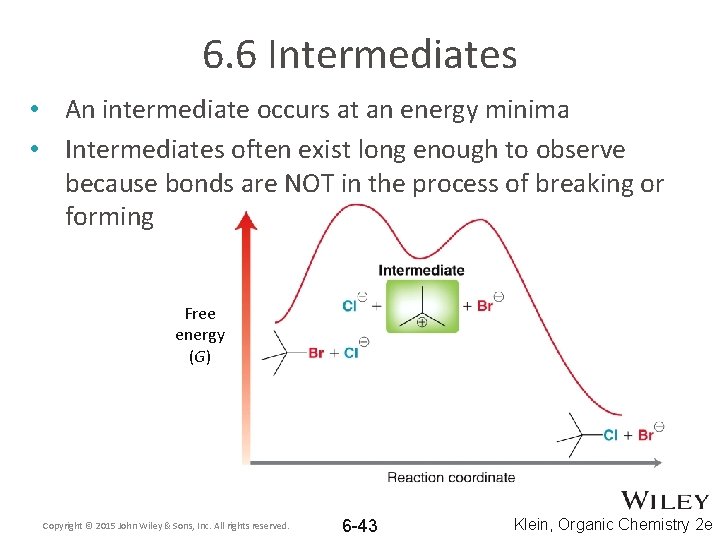
6. 6 Intermediates • An intermediate occurs at an energy minima • Intermediates often exist long enough to observe because bonds are NOT in the process of breaking or forming Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -43 Klein, Organic Chemistry 2 e

6. 6 The Hammond Postulate • Two points on an energy diagram that are close in energy should be similar in structure Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -44 Klein, Organic Chemistry 2 e

6. 6 The Hammond Postulate • For each of the diagrams below, will the transition state structure look more like the reactants or the products? Free energy (G) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Free energy (G) 6 -45 Klein, Organic Chemistry 2 e

6. 6 The Hammond Postulate • Draw a reaction coordinate diagram for the generic exergonic reaction sequence below. Label the axes, reactants, products, intermediates, and transition states A B+C C+D E Net reaction: A + D B + E • How would the diagram look different if it were endergonic? • Practice with conceptual checkpoint 6. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -46 Klein, Organic Chemistry 2 e

6. 7 Nucleophiles and Electrophiles • A major focus in this course is on predicting reaction products for ionic reactions and explaining HOW such reactions work • Ionic or polar reactions result from the force of attraction between opposite charges • Ionic reactions are also guided by the octet rule • Consider how methyl chloride and methyl lithium might react Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -47 Klein, Organic Chemistry 2 e

6. 7 Nucleophiles • When an atom carries a formal or partial negative charge and an available pair of electrons, it is considered a nucleophile • It will love to attack a nucleus. WHY? • Explain how the molecules below are nucleophiles • What is the difference between a nucleophile and a Lewis Base? NOTHING! Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -48 Klein, Organic Chemistry 2 e

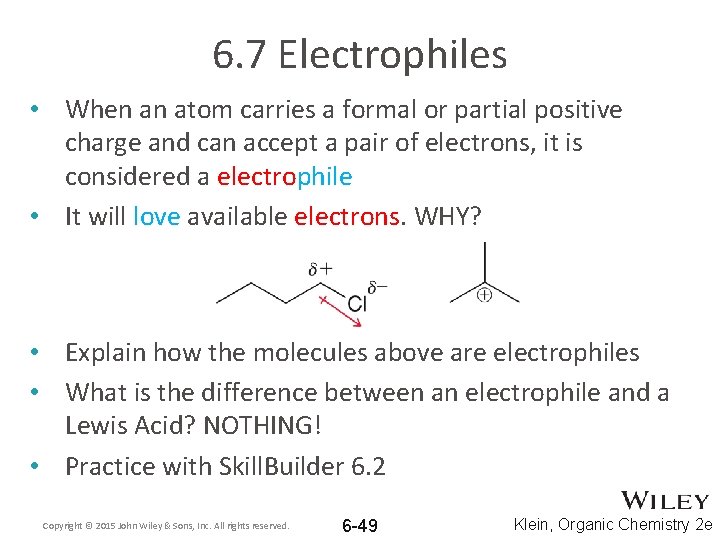
6. 7 Electrophiles • When an atom carries a formal or partial positive charge and can accept a pair of electrons, it is considered a electrophile • It will love available electrons. WHY? • Explain how the molecules above are electrophiles • What is the difference between an electrophile and a Lewis Acid? NOTHING! • Practice with Skill. Builder 6. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -49 Klein, Organic Chemistry 2 e

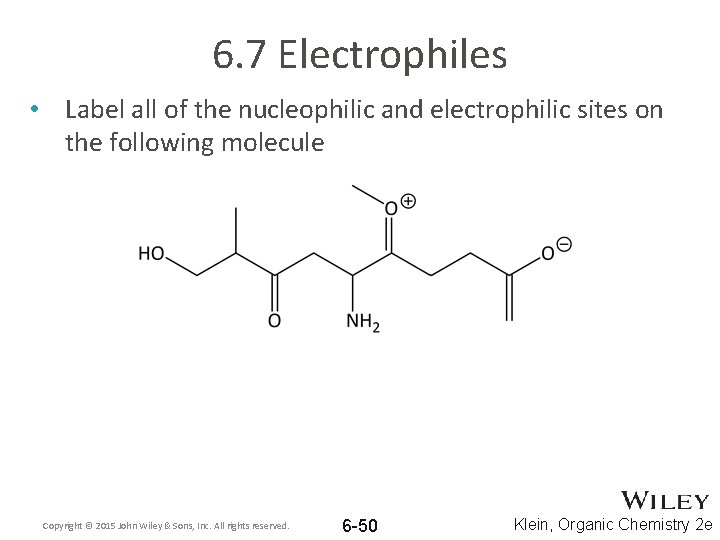
6. 7 Electrophiles • Label all of the nucleophilic and electrophilic sites on the following molecule Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -50 Klein, Organic Chemistry 2 e

6. 8 Mechanisms and Arrow Pushing • We use arrows to show electrons move when bonds break and form • It will be a huge benefit in this course to master the skill of arrow pushing • There are four main ways that electrons move in ionic reactions 1. 2. 3. 4. Nucleophilic Attack Loss of a Leaving Group Proton Transfers (Acid/Base) Rearrangements Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -51 Klein, Organic Chemistry 2 e

6. 8 Nucleophilic Attack • When you identify a nucleophilic site and an electrophilic site, the arrow shows the nucleophile attacking • The tail of the arrow starts on the electrons (- charge) • The head of the arrow ends on a nucleus (+ charge) • The electrons end up being sharing rather than transferred Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -52 Klein, Organic Chemistry 2 e

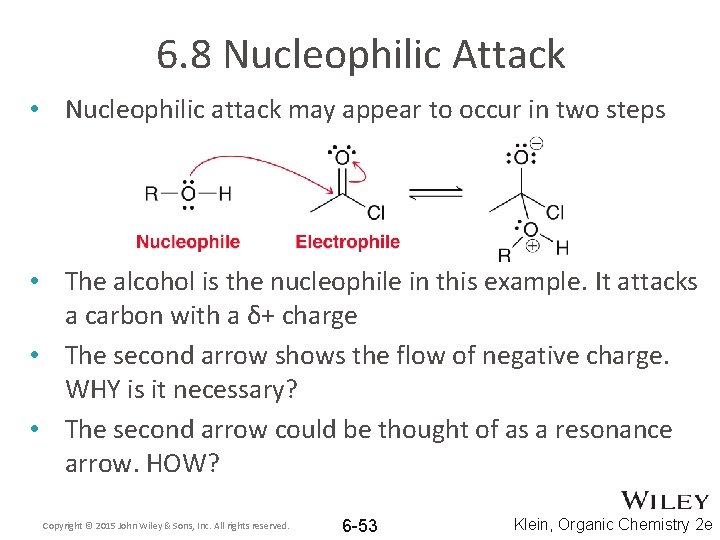
6. 8 Nucleophilic Attack • Nucleophilic attack may appear to occur in two steps • The alcohol is the nucleophile in this example. It attacks a carbon with a δ+ charge • The second arrow shows the flow of negative charge. WHY is it necessary? • The second arrow could be thought of as a resonance arrow. HOW? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -53 Klein, Organic Chemistry 2 e

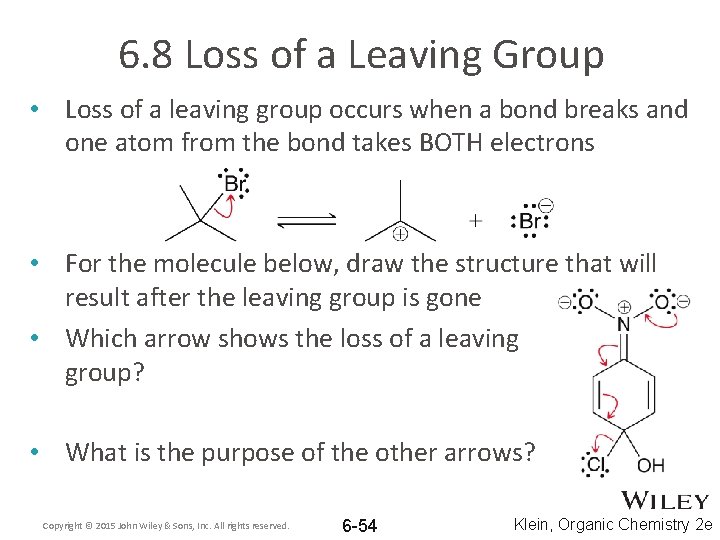
6. 8 Loss of a Leaving Group • Loss of a leaving group occurs when a bond breaks and one atom from the bond takes BOTH electrons • For the molecule below, draw the structure that will result after the leaving group is gone • Which arrow shows the loss of a leaving group? • What is the purpose of the other arrows? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -54 Klein, Organic Chemistry 2 e

6. 8 Proton Transfers • Recall from Chapter 3 that a base is protonated when it uses a pair of electrons to take an H+ from the acid. • The acid retains its electron pair • A group can also be deprotonated (sometimes shown by writing –H+ over the reaction arrow) or Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -55 Klein, Organic Chemistry 2 e

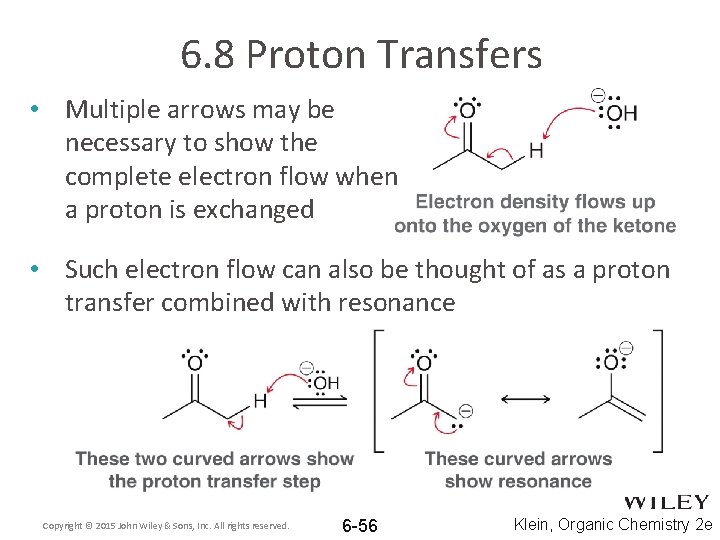
6. 8 Proton Transfers • Multiple arrows may be necessary to show the complete electron flow when a proton is exchanged • Such electron flow can also be thought of as a proton transfer combined with resonance Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -56 Klein, Organic Chemistry 2 e

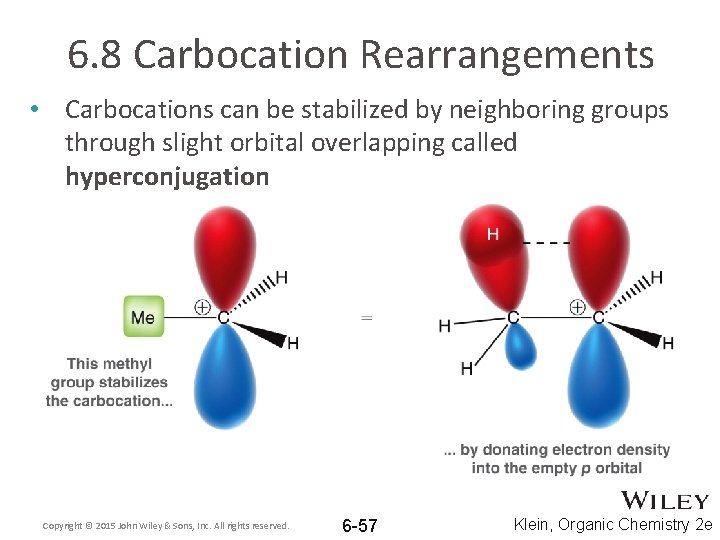
6. 8 Carbocation Rearrangements • Carbocations can be stabilized by neighboring groups through slight orbital overlapping called hyperconjugation Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -57 Klein, Organic Chemistry 2 e

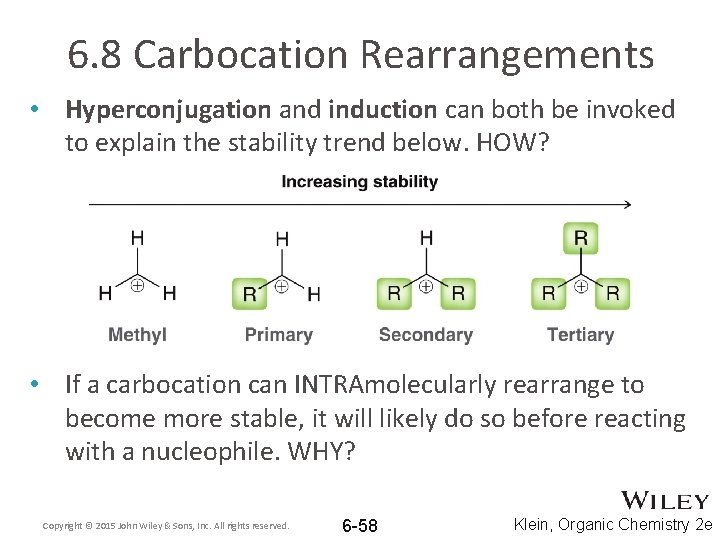
6. 8 Carbocation Rearrangements • Hyperconjugation and induction can both be invoked to explain the stability trend below. HOW? • If a carbocation can INTRAmolecularly rearrange to become more stable, it will likely do so before reacting with a nucleophile. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -58 Klein, Organic Chemistry 2 e

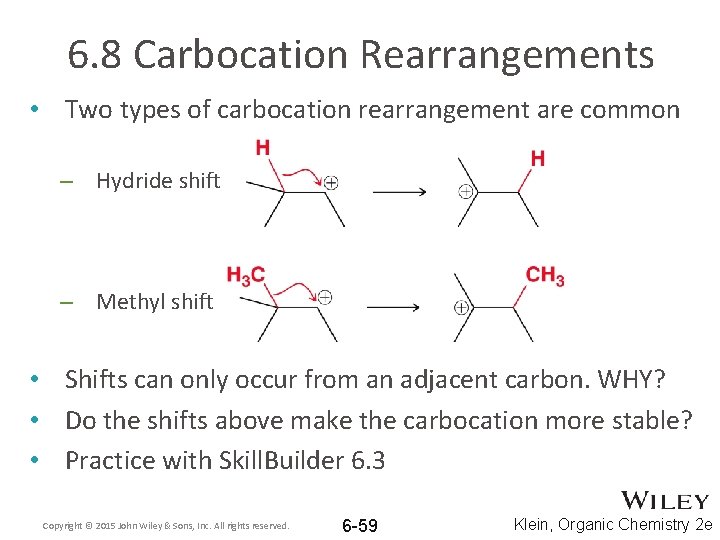
6. 8 Carbocation Rearrangements • Two types of carbocation rearrangement are common – Hydride shift – Methyl shift • Shifts can only occur from an adjacent carbon. WHY? • Do the shifts above make the carbocation more stable? • Practice with Skill. Builder 6. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -59 Klein, Organic Chemistry 2 e

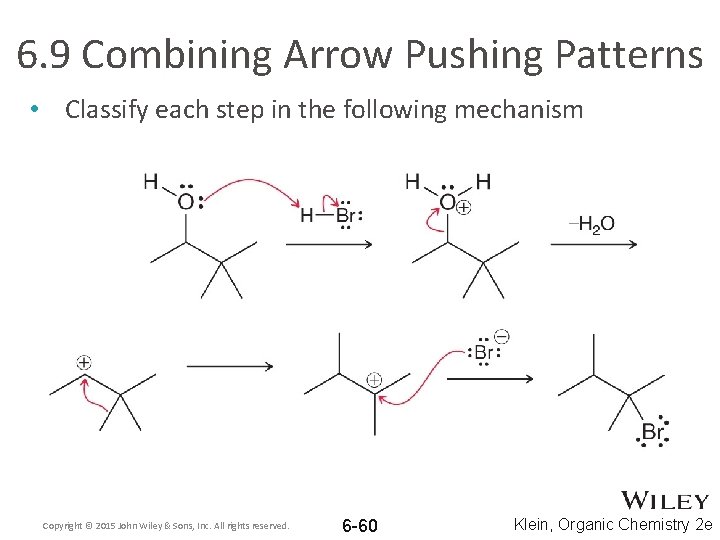
6. 9 Combining Arrow Pushing Patterns • Classify each step in the following mechanism Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -60 Klein, Organic Chemistry 2 e

6. 9 Combining Arrow Pushing Patterns • Many times a single step in a mechanism will include more than one arrow pushing pattern • Identify the patterns below • There are hundreds of mechanisms that involve these key patterns • Practice with Skill. Builder 6. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -61 Klein, Organic Chemistry 2 e

6. 10 Arrow Pushing Rules • To draw reasonable mechanisms, a few key rules should be followed 1. The arrow starts ON A PAIR OF ELECTRONS (a bonded pair or a lone pair) – Don’t make the mistake of starting an arrow on a nucleus! Both arrows below are incorrect. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -62 Klein, Organic Chemistry 2 e

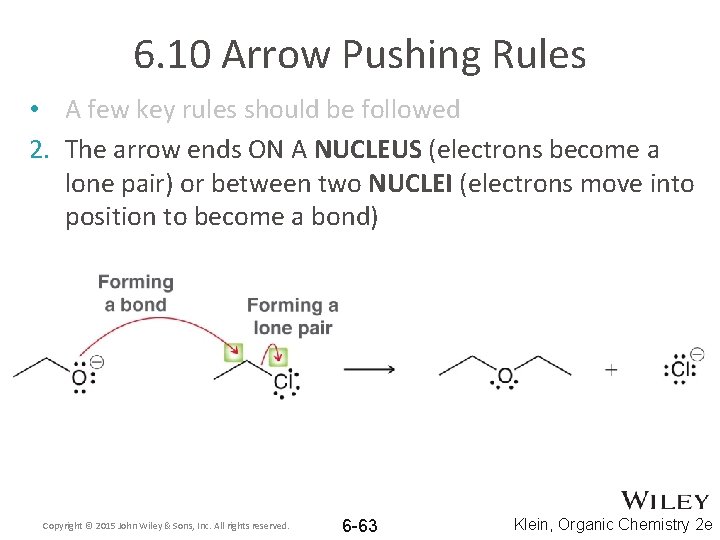
6. 10 Arrow Pushing Rules • A few key rules should be followed 2. The arrow ends ON A NUCLEUS (electrons become a lone pair) or between two NUCLEI (electrons move into position to become a bond) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -63 Klein, Organic Chemistry 2 e

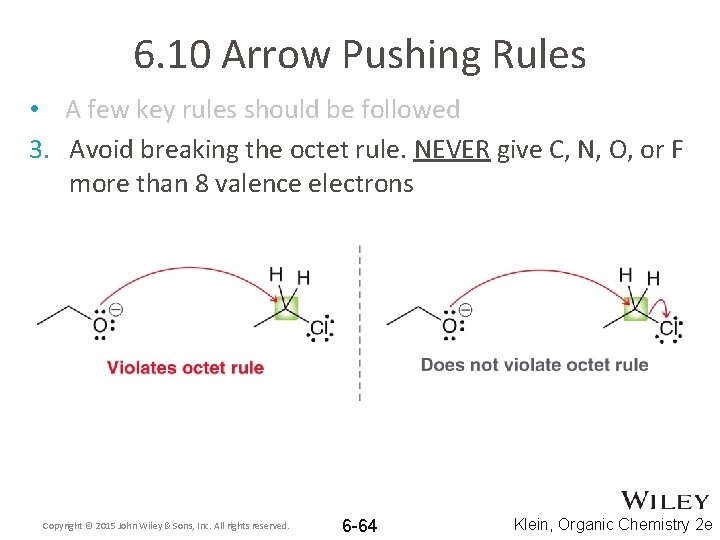
6. 10 Arrow Pushing Rules • A few key rules should be followed 3. Avoid breaking the octet rule. NEVER give C, N, O, or F more than 8 valence electrons Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -64 Klein, Organic Chemistry 2 e

6. 10 Arrow Pushing Rules • A few key rules should be followed 4. Draw arrows that follow the 4 key patterns we outlined – • The arrow below is unreasonable. WHY? Practice with Skill. Builder 6 -65 6. 5 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

6. 10 Arrow Pushing Rules • Fill in necessary arrows for the reaction below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -66 Klein, Organic Chemistry 2 e

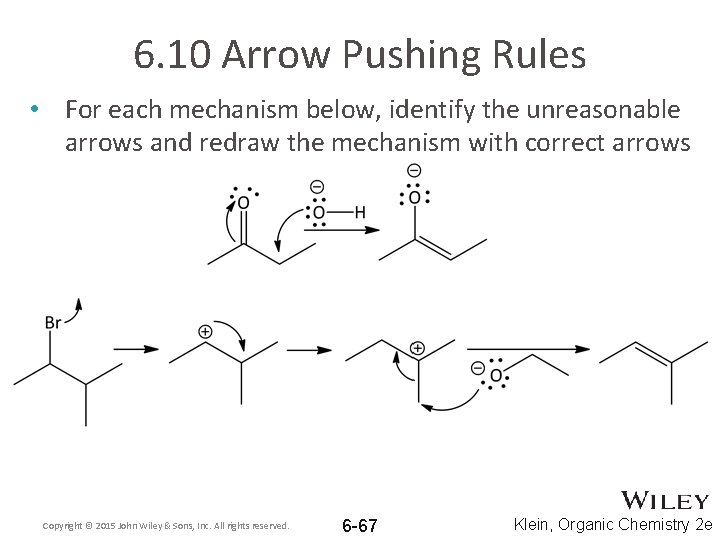
6. 10 Arrow Pushing Rules • For each mechanism below, identify the unreasonable arrows and redraw the mechanism with correct arrows Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -67 Klein, Organic Chemistry 2 e

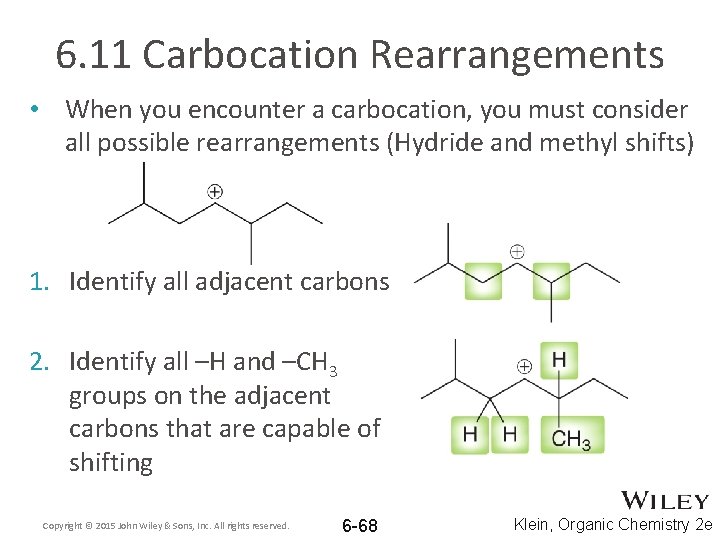
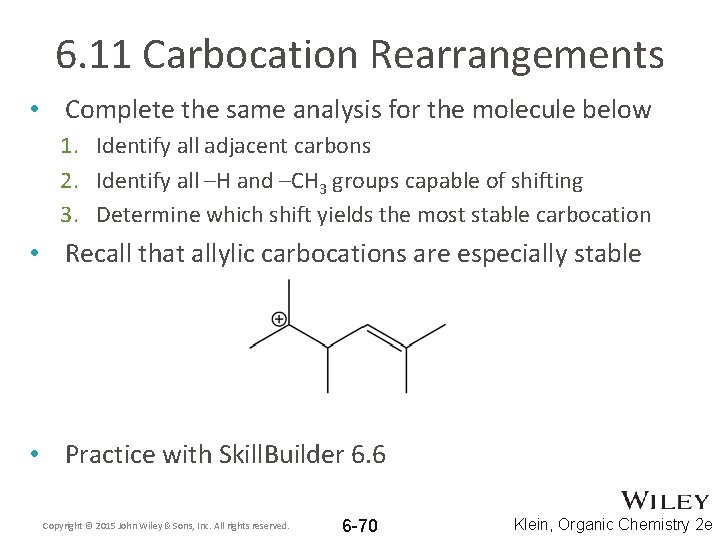
6. 11 Carbocation Rearrangements • When you encounter a carbocation, you must consider all possible rearrangements (Hydride and methyl shifts) 1. Identify all adjacent carbons 2. Identify all –H and –CH 3 groups on the adjacent carbons that are capable of shifting Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -68 Klein, Organic Chemistry 2 e

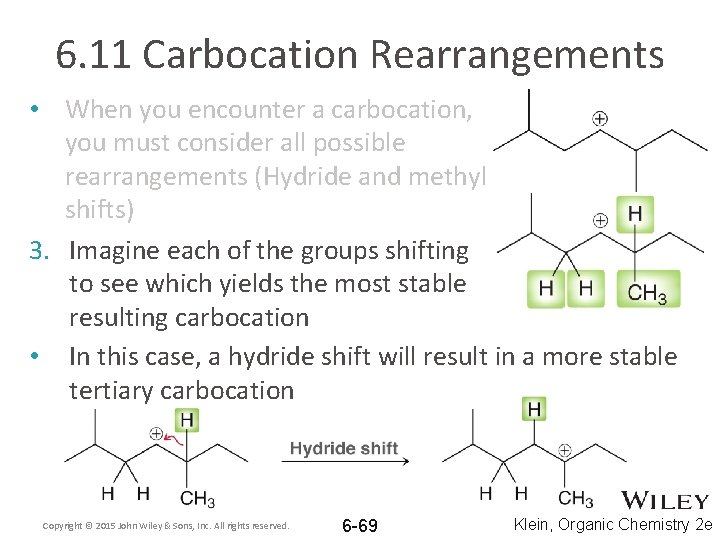
6. 11 Carbocation Rearrangements • When you encounter a carbocation, you must consider all possible rearrangements (Hydride and methyl shifts) 3. Imagine each of the groups shifting to see which yields the most stable resulting carbocation • In this case, a hydride shift will result in a more stable tertiary carbocation Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -69 Klein, Organic Chemistry 2 e

6. 11 Carbocation Rearrangements • Complete the same analysis for the molecule below 1. Identify all adjacent carbons 2. Identify all –H and –CH 3 groups capable of shifting 3. Determine which shift yields the most stable carbocation • Recall that allylic carbocations are especially stable • Practice with Skill. Builder 6. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -70 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • Why are some reactions are drawn as equilibria and others are essentially irreversible? • The question of reversibility a both kinetic and a thermodynamic question. HOW? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -71 Klein, Organic Chemistry 2 e

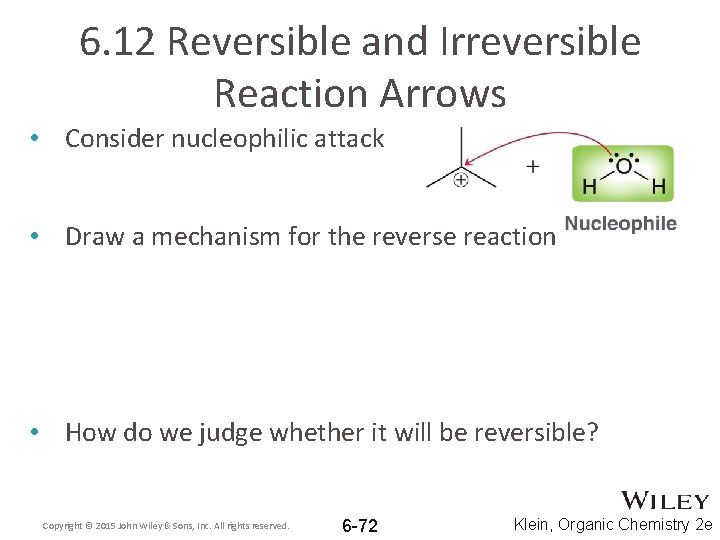
6. 12 Reversible and Irreversible Reaction Arrows • Consider nucleophilic attack • Draw a mechanism for the reverse reaction • How do we judge whether it will be reversible? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -72 Klein, Organic Chemistry 2 e

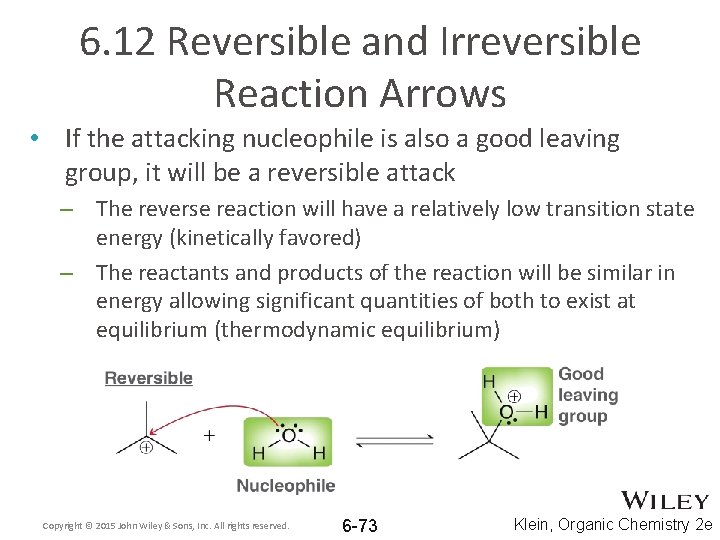
6. 12 Reversible and Irreversible Reaction Arrows • If the attacking nucleophile is also a good leaving group, it will be a reversible attack – The reverse reaction will have a relatively low transition state energy (kinetically favored) – The reactants and products of the reaction will be similar in energy allowing significant quantities of both to exist at equilibrium (thermodynamic equilibrium) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -73 Klein, Organic Chemistry 2 e

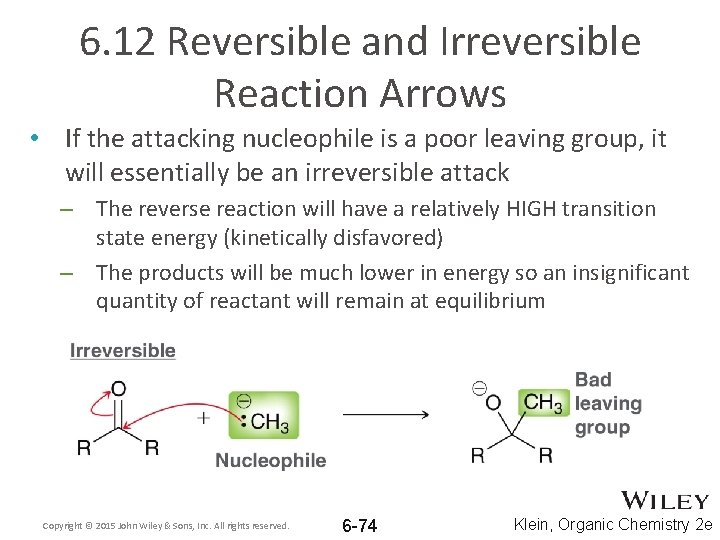
6. 12 Reversible and Irreversible Reaction Arrows • If the attacking nucleophile is a poor leaving group, it will essentially be an irreversible attack – The reverse reaction will have a relatively HIGH transition state energy (kinetically disfavored) – The products will be much lower in energy so an insignificant quantity of reactant will remain at equilibrium Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -74 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • Consider loss or a leaving group • Draw a mechanism for the reverse reaction • How do we judge whether it will be reversible? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -75 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • Consider proton transfer • We analyzed this in detail in chapter 3 using p. Ka values • How do we judge reversiblity? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -76 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • Consider proton transfer • If the p. Ka difference is 10 units or more, it is generally considered irreversible Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -77 Klein, Organic Chemistry 2 e

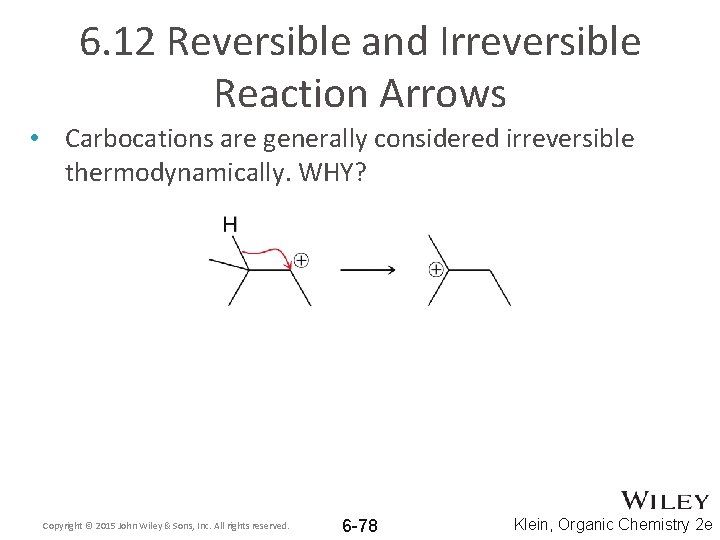
6. 12 Reversible and Irreversible Reaction Arrows • Carbocations are generally considered irreversible thermodynamically. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -78 Klein, Organic Chemistry 2 e

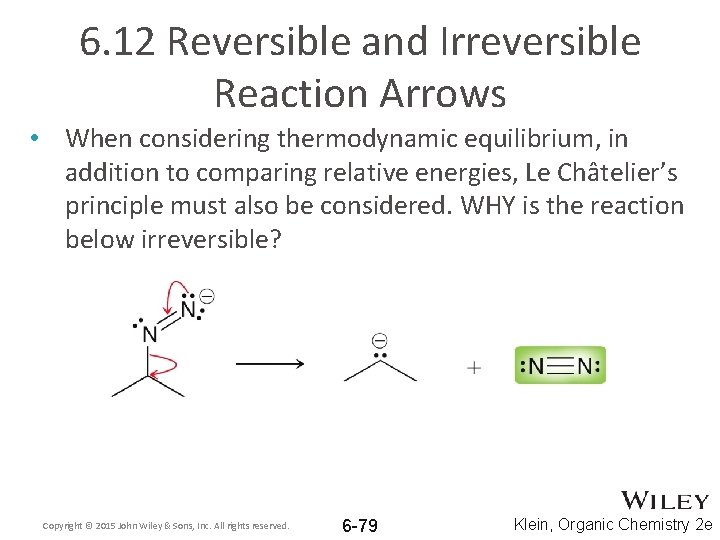
6. 12 Reversible and Irreversible Reaction Arrows • When considering thermodynamic equilibrium, in addition to comparing relative energies, Le Châtelier’s principle must also be considered. WHY is the reaction below irreversible? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -79 Klein, Organic Chemistry 2 e

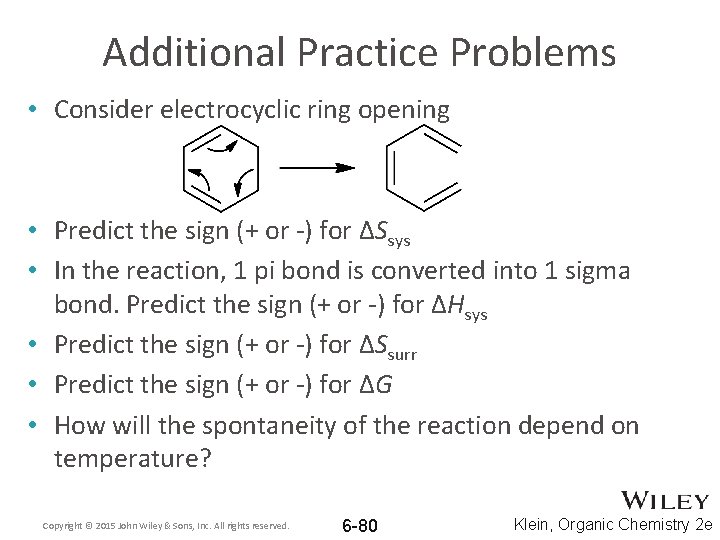
Additional Practice Problems • Consider electrocyclic ring opening • Predict the sign (+ or -) for ΔSsys • In the reaction, 1 pi bond is converted into 1 sigma bond. Predict the sign (+ or -) for ΔHsys • Predict the sign (+ or -) for ΔSsurr • Predict the sign (+ or -) for ΔG • How will the spontaneity of the reaction depend on temperature? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -80 Klein, Organic Chemistry 2 e

Additional Practice Problems • Explain why an equilibrium mixture is always lower in free energy than pure products or pure reactants. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -81 Klein, Organic Chemistry 2 e

Additional Practice Problems • Reactants A and B can react by two different pathways. Pathway 1 is thermodynamically favored, and pathway 2 is kinetically favored. Draw a reaction coordinate diagraph to illustrate the relative energies and explain which pathway will be favored at low temperatures versus higher temperatures. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -82 Klein, Organic Chemistry 2 e

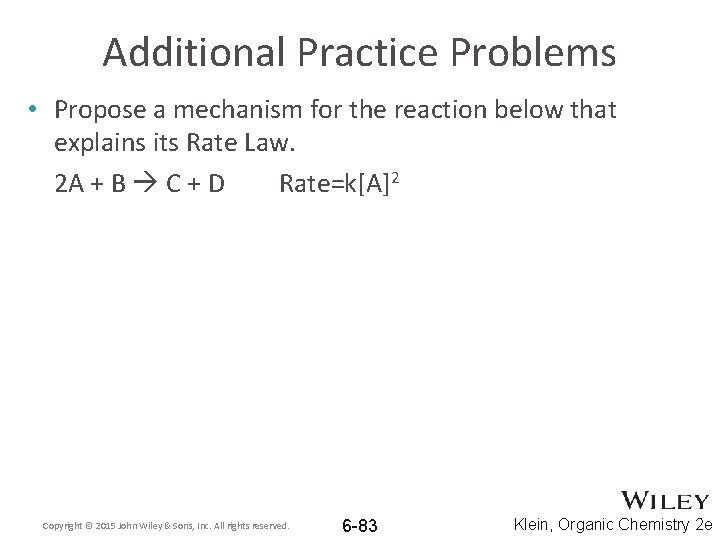
Additional Practice Problems • Propose a mechanism for the reaction below that explains its Rate Law. 2 A + B C + D Rate=k[A]2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -83 Klein, Organic Chemistry 2 e

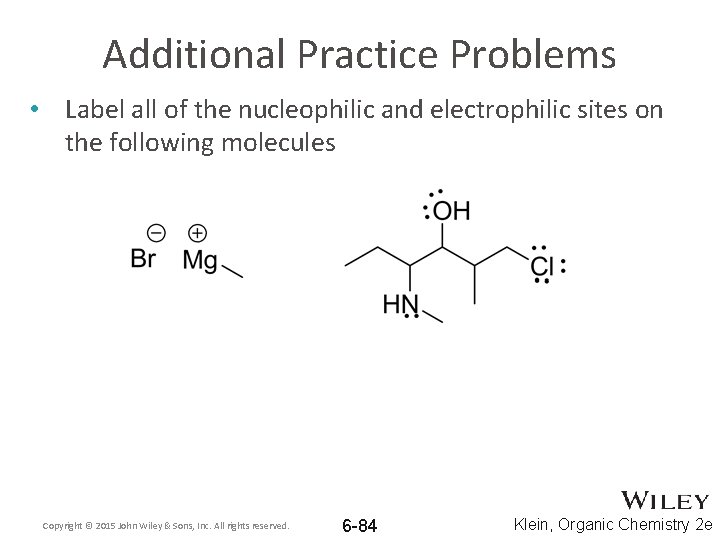
Additional Practice Problems • Label all of the nucleophilic and electrophilic sites on the following molecules Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -84 Klein, Organic Chemistry 2 e

Additional Practice Problems • Draw a mechanism for a generic nucleophilic attack followed by a proton transfer. • Draw a mechanism for a generic loss of leaving group followed by a carbocation rearrangement. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -85 Klein, Organic Chemistry 2 e

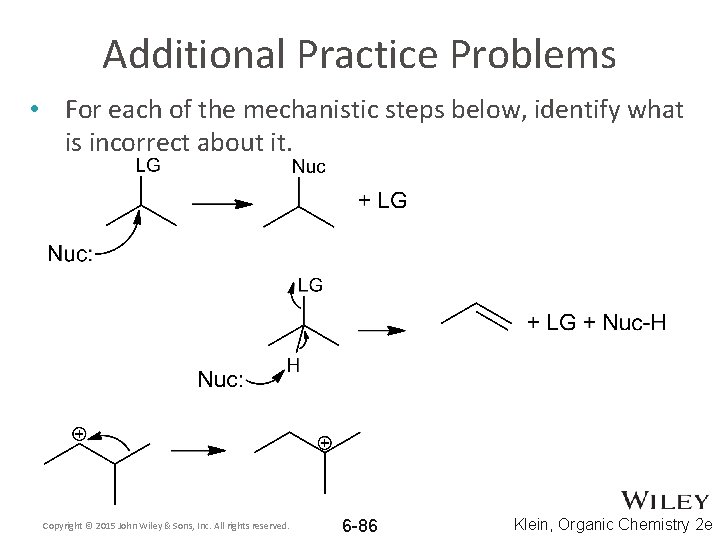
Additional Practice Problems • For each of the mechanistic steps below, identify what is incorrect about it. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 6 -86 Klein, Organic Chemistry 2 e