Chemical Equations Reactions http www unit 5 orgchemistryEquations







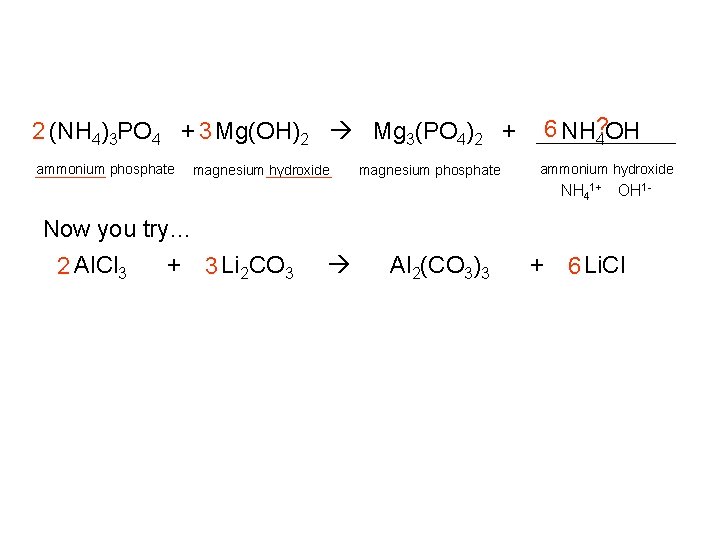





![Double Replacement Reaction aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] Double Replacement Reaction aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)]](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-41.jpg)
![aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-42.jpg)
![Double Replacement Reaction aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] Double Replacement Reaction aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)]](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-43.jpg)
![aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-44.jpg)


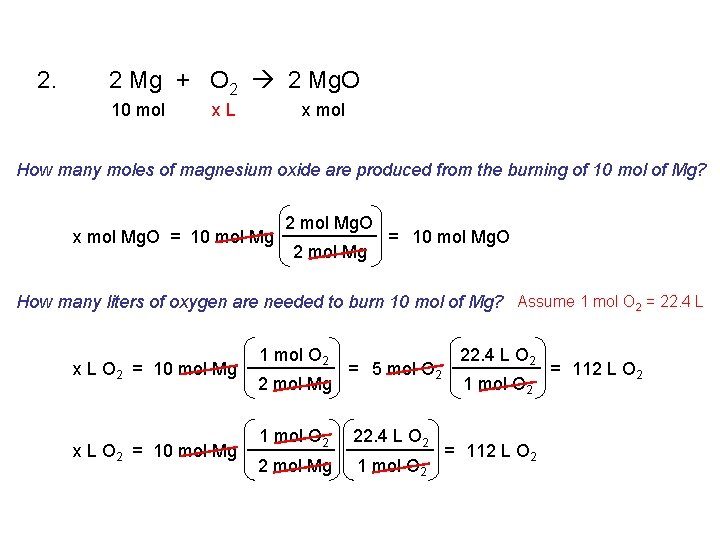

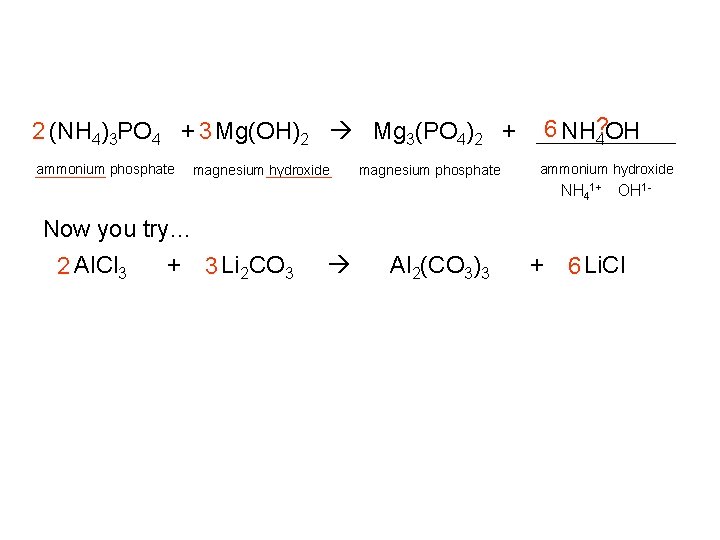

- Slides: 84

Chemical Equations & Reactions http: //www. unit 5. org/chemistry/Equations. html

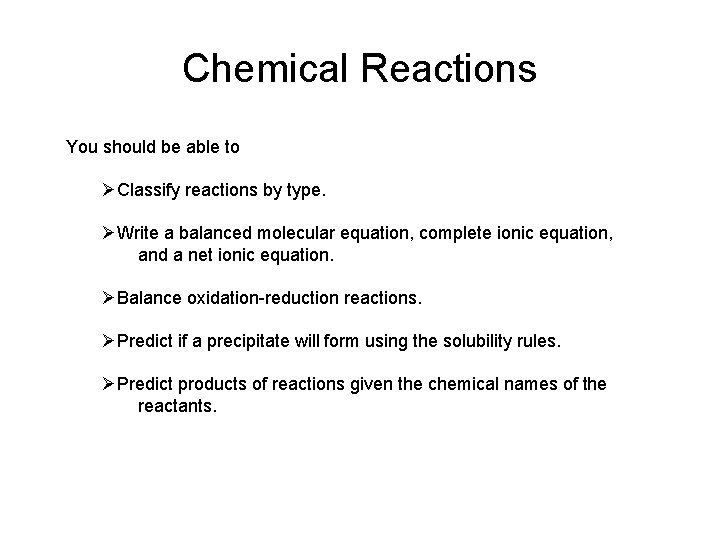
Chemical Reactions You should be able to ØClassify reactions by type. ØWrite a balanced molecular equation, complete ionic equation, and a net ionic equation. ØBalance oxidation-reduction reactions. ØPredict if a precipitate will form using the solubility rules. ØPredict products of reactions given the chemical names of the reactants.

Organize Your Thoughts Chemical reactions Chemical equations • Balancing equations • Predicting products from reactants Packard, Jacobs, Marshall, Chemistry Pearson AGS Globe, page 175 Chemical equations • Synthesis • Decomposition • Single replacement • Double replacement • Combustion

Describing a Chemical Reaction Indications of a Chemical Reaction – Evolution of heat, light, and/or sound – Production of a gas – Formation of a precipitate – Color change

Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse

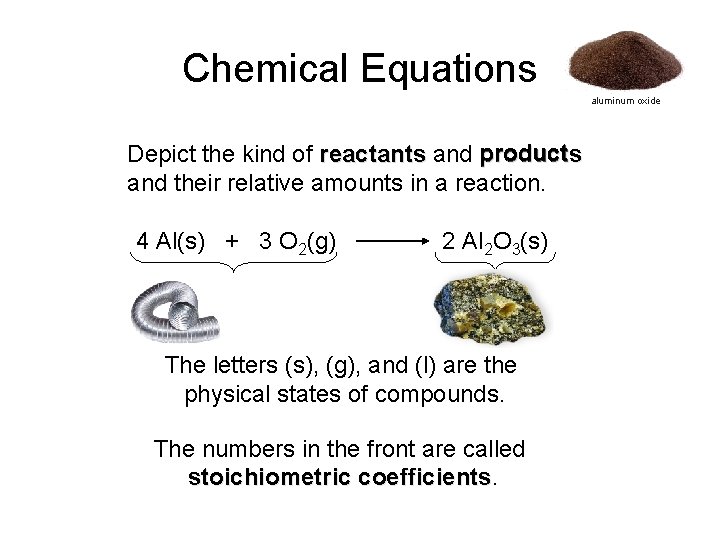
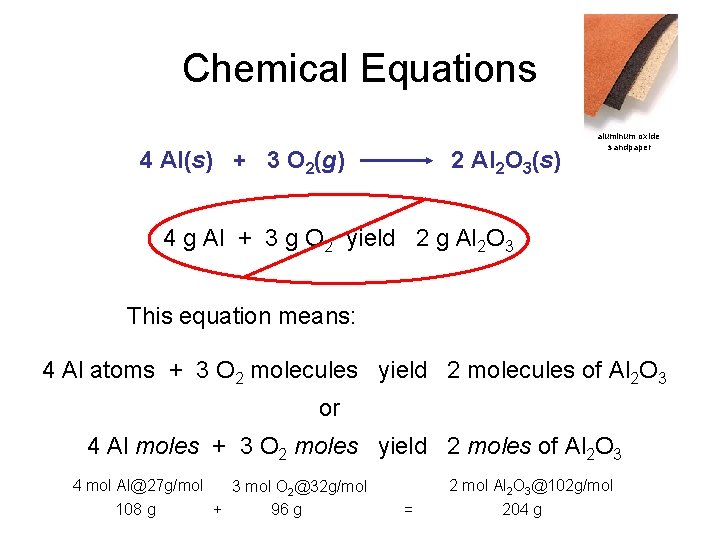
Chemical Equations aluminum oxide Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) The letters (s), (g), and (l) are the physical states of compounds. The numbers in the front are called stoichiometric coefficients

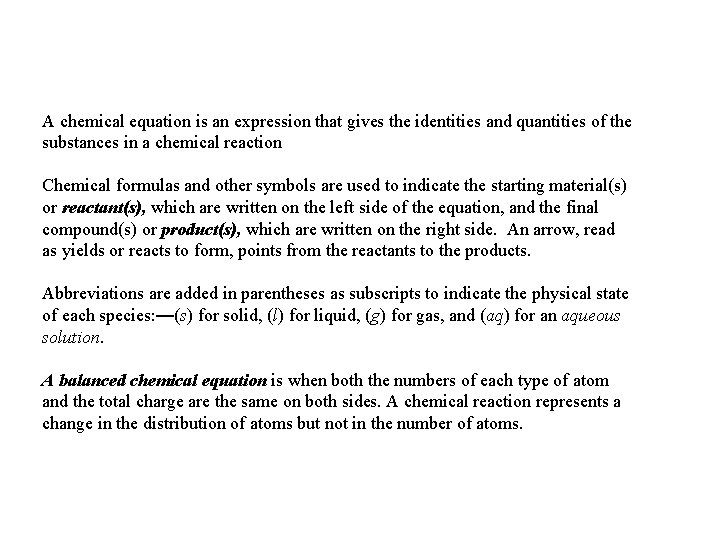
A chemical equation is an expression that gives the identities and quantities of the substances in a chemical reaction Chemical formulas and other symbols are used to indicate the starting material(s) or reactant(s), which are written on the left side of the equation, and the final compound(s) or product(s), which are written on the right side. An arrow, read as yields or reacts to form, points from the reactants to the products. Abbreviations are added in parentheses as subscripts to indicate the physical state of each species: —(s) for solid, (l) for liquid, (g) for gas, and (aq) for an aqueous solution. A balanced chemical equation is when both the numbers of each type of atom and the total charge are the same on both sides. A chemical reaction represents a change in the distribution of atoms but not in the number of atoms.

Chemical Equations 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) aluminum oxide sandpaper 4 g Al + 3 g O 2 yield 2 g Al 2 O 3 This equation means: 4 Al atoms + 3 O 2 molecules yield 2 molecules of Al 2 O 3 or 4 Al moles + 3 O 2 moles yield 2 moles of Al 2 O 3 4 mol Al@27 g/mol 3 mol O 2@32 g/mol 108 g + 96 g 2 mol Al 2 O 3@102 g/mol = 204 g

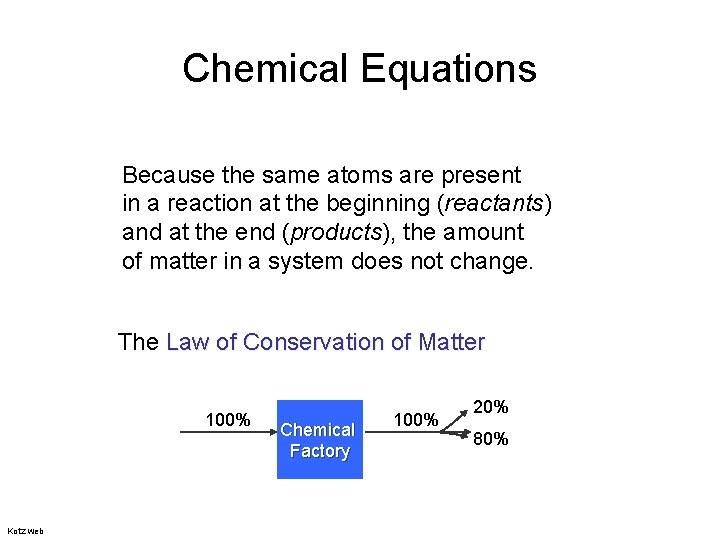
Chemical Equations Because the same atoms are present in a reaction at the beginning (reactants) and at the end (products), the amount of matter in a system does not change. The Law of Conservation of Matter 100% Kotz web Chemical Factory 100% 20% 80%

Chemical Equations Because of the principle of the conservation of matter, matter An equation must be balanced It must have the same number of atoms of the same kind on both sides. Lavoisier, 1788

Characteristics of Chemical Equations • The equation must represent known facts. • The equation must contain the correct formulas for the reactants and products. • The law of conservation of mass must be satisfied.

Chemical Equations • Reactants – the substances that exist before a chemical change (or reaction) takes place. • Products – the new substance(s) that are formed during the chemical changes. • CHEMICAL EQUATION indicates the reactants and products of a reaction. REACTANTS PRODUCTS

Word Equations • A WORD EQUATION describes chemical change using the names of the reactants and products. Write the word equation for the reaction of methane gas with oxygen gas to form carbon dioxide and water. methane + oxygen Reactant CH 4 + 2 O 2 carbon dioxide + water Product CO 2 + 2 H 2 O

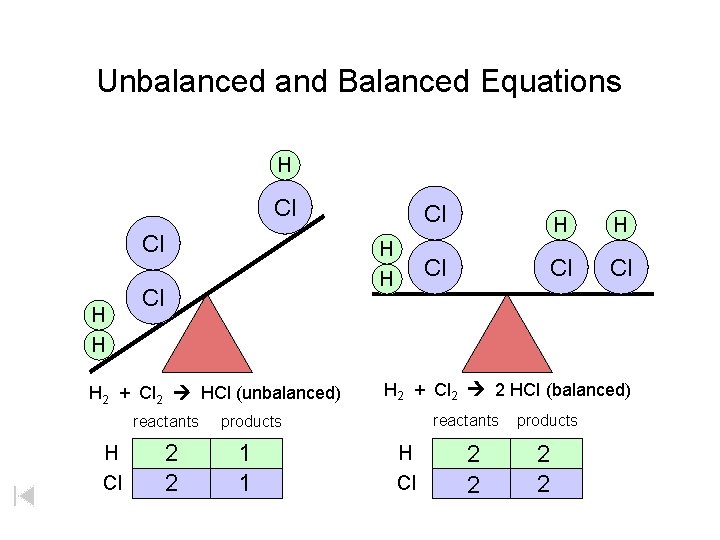
Unbalanced and Balanced Equations H Cl Cl H H Cl H 2 + Cl 2 HCl (unbalanced) reactants H Cl 2 2 H H Cl Cl Cl H 2 + Cl 2 2 HCl (balanced) reactants products 1 1 Cl H Cl 2 2 products 2 2

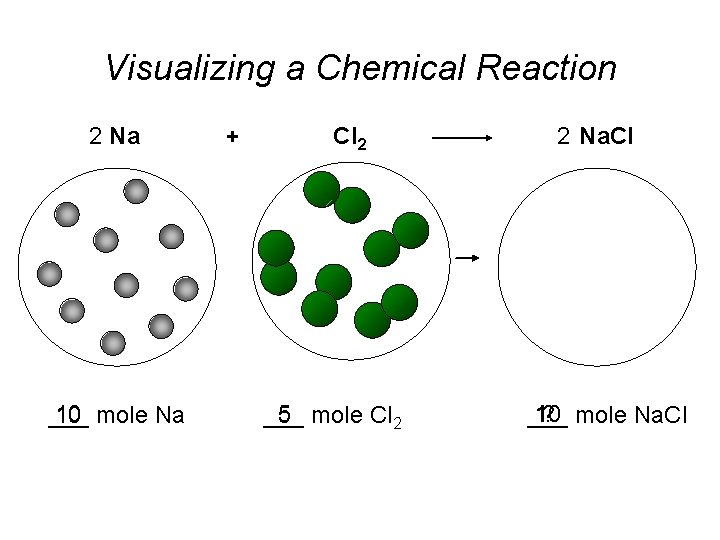
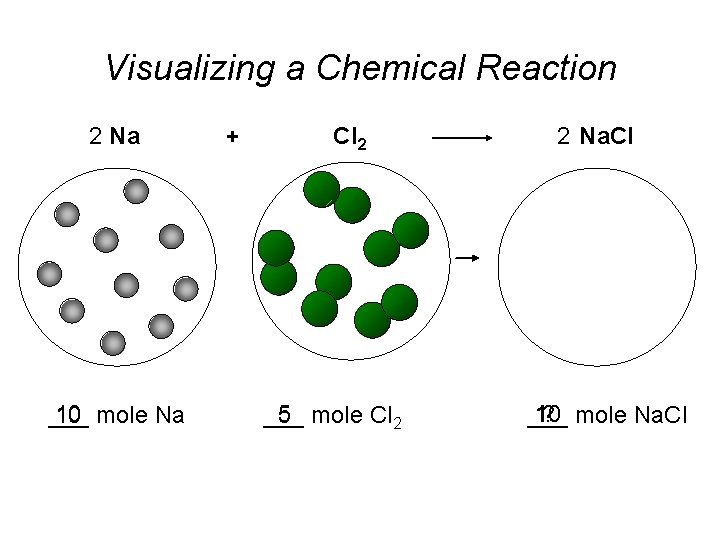
Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

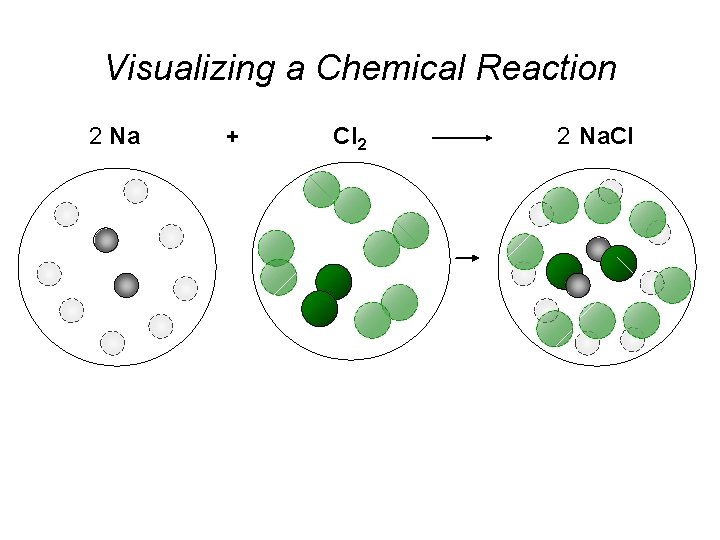
Visualizing a Chemical Reaction 2 Na + Cl 2 2 Na. Cl

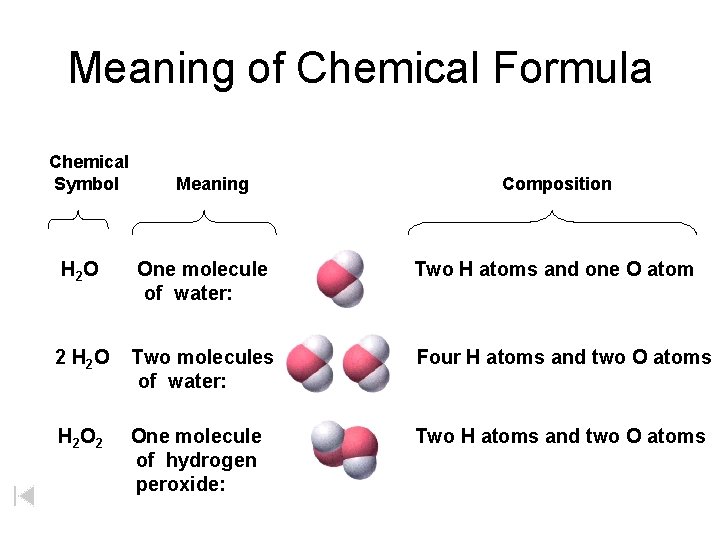
Meaning of Chemical Formula Chemical Symbol Meaning Composition H 2 O One molecule of water: Two H atoms and one O atom 2 H 2 O Two molecules of water: Four H atoms and two O atoms H 2 One molecule of hydrogen peroxide: Two H atoms and two O atoms

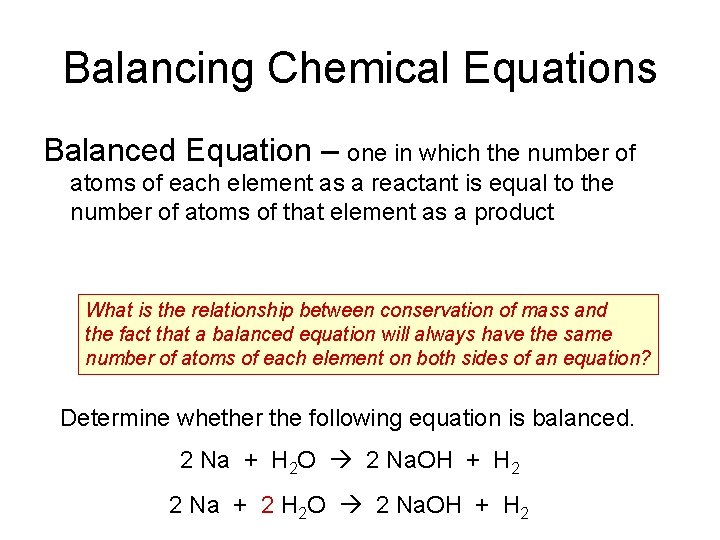
Balancing Chemical Equations Balanced Equation – one in which the number of atoms of each element as a reactant is equal to the number of atoms of that element as a product What is the relationship between conservation of mass and the fact that a balanced equation will always have the same number of atoms of each element on both sides of an equation? Determine whether the following equation is balanced. 2 Na + H 2 O 2 Na. OH + H 2 2 Na + 2 H 2 O 2 Na. OH + H 2

Balancing Chemical Equations • Write a word equation for the reaction. • Write the correct formulas for all reactants and products. • Determine the coefficients that make the equation balance.

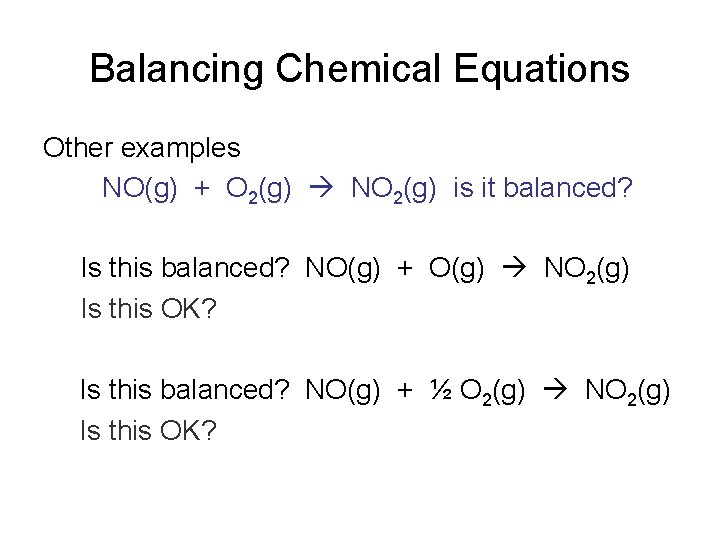
Balancing Chemical Equations Other examples NO(g) + O 2(g) NO 2(g) is it balanced? Is this balanced? NO(g) + O(g) NO 2(g) Is this OK? Is this balanced? NO(g) + ½ O 2(g) NO 2(g) Is this OK?

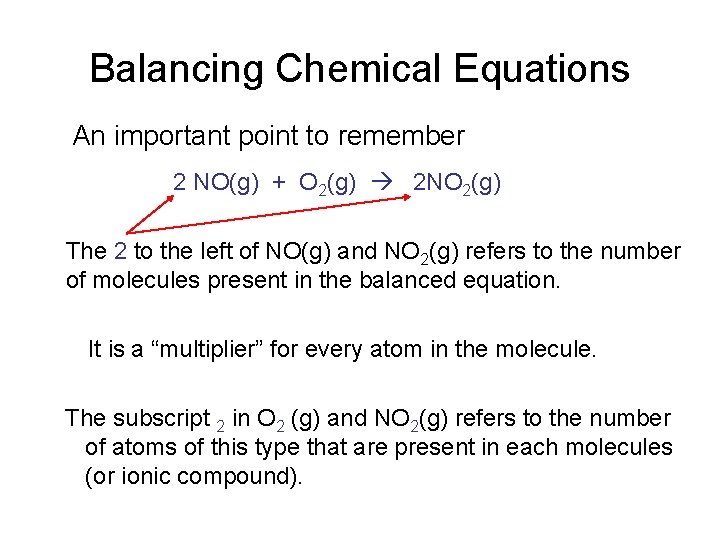
Balancing Chemical Equations An important point to remember 2 NO(g) + O 2(g) 2 NO 2(g) The 2 to the left of NO(g) and NO 2(g) refers to the number of molecules present in the balanced equation. It is a “multiplier” for every atom in the molecule. The subscript 2 in O 2 (g) and NO 2(g) refers to the number of atoms of this type that are present in each molecules (or ionic compound).

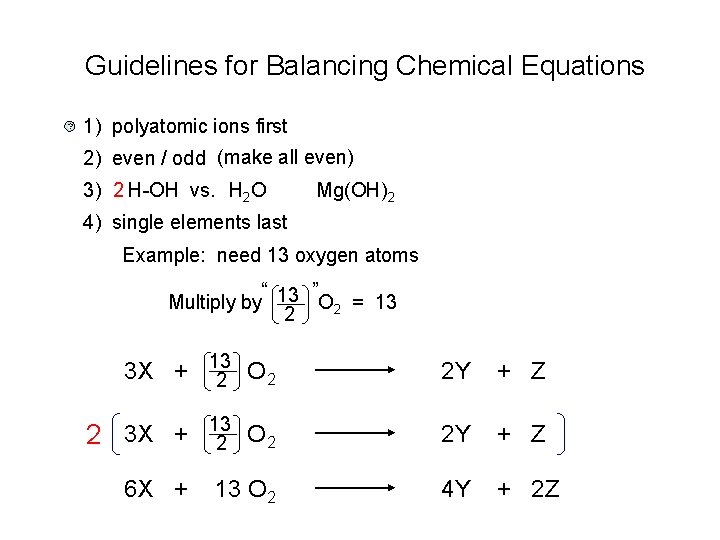
Guidelines for Balancing Chemical Equations ? 1) polyatomic ions first 2) even / odd (make all even) 3) 2 H-OH vs. H 2 O Mg(OH)2 4) single elements last Example: need 13 oxygen atoms “ ” Multiply by 13 O 2 = 13 2 3 X + 13 2 O 2 2 3 X + 6 X + 2 Y + Z O 2 2 Y + Z 13 O 2 4 Y + 2 Z 13 2
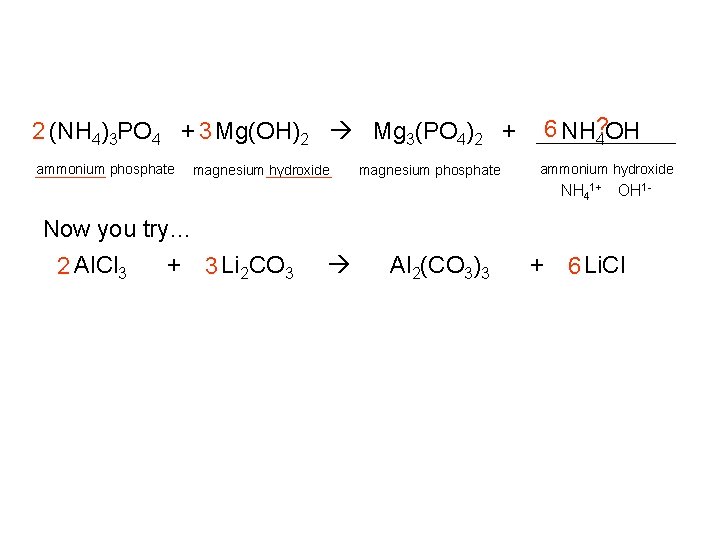
6 NH? 4 OH 2 (NH 4)3 PO 4 + 3 Mg(OH)2 Mg 3(PO 4)2 + ammonium phosphate magnesium hydroxide magnesium phosphate ammonium hydroxide NH 41+ OH 1 - Now you try… 2 Al. Cl 3 + 3 Li 2 CO 3 Al 2(CO 3)3 + 6 Li. Cl

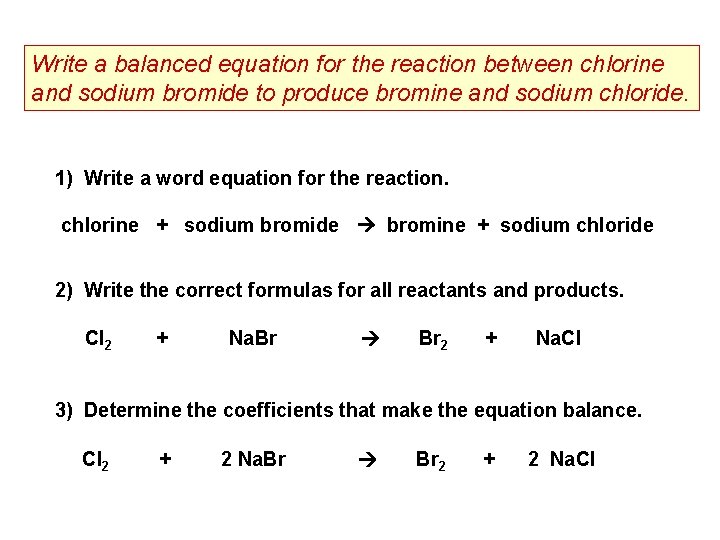
Write a balanced equation for the reaction between chlorine and sodium bromide to produce bromine and sodium chloride. 1) Write a word equation for the reaction. chlorine + sodium bromide bromine + sodium chloride 2) Write the correct formulas for all reactants and products. Cl 2 + Na. Br Br 2 + Na. Cl 3) Determine the coefficients that make the equation balance. Cl 2 + 2 Na. Br Br 2 + 2 Na. Cl

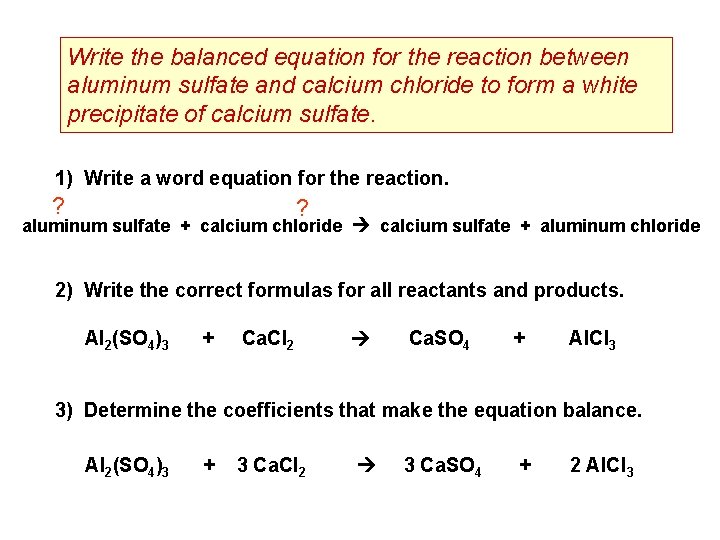
Write the balanced equation for the reaction between aluminum sulfate and calcium chloride to form a white precipitate of calcium sulfate. 1) Write a word equation for the reaction. ? ? aluminum sulfate + calcium chloride calcium sulfate + aluminum chloride 2) Write the correct formulas for all reactants and products. Al 2(SO 4)3 + Ca. Cl 2 Ca. SO 4 + Al. Cl 3 3) Determine the coefficients that make the equation balance. Al 2(SO 4)3 + 3 Ca. Cl 2 3 Ca. SO 4 + 2 Al. Cl 3

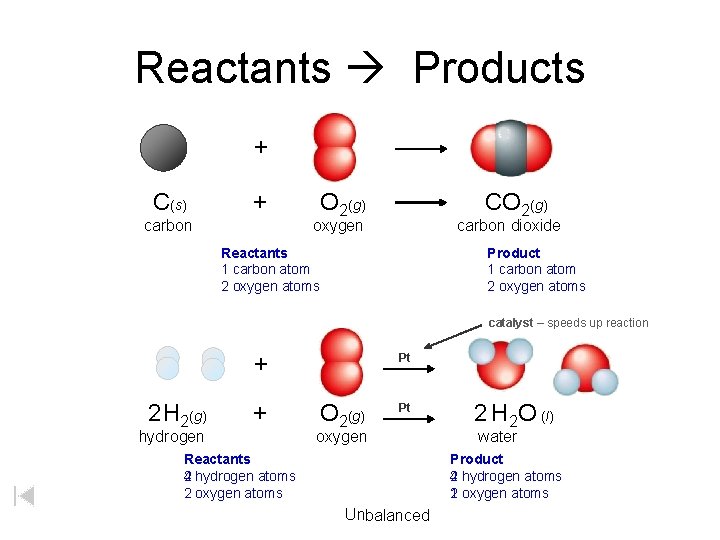
CH 4 + 2 O 2 CO 2 + 2 H 2 O Reactants 1 C atom 4 H atoms 4 O atoms Products 1 C atom 4 H atoms 4 O atoms

Reactants Products + C(s) + carbon O 2(g) CO 2(g) oxygen carbon dioxide Reactants 1 carbon atom 2 oxygen atoms Product 1 carbon atom 2 oxygen atoms catalyst – speeds up reaction + 2 H 2(g) + hydrogen Pt O 2(g) Pt oxygen Reactants 2 hydrogen atoms 4 2 oxygen atoms 2 H 2 O (l) water Product 2 hydrogen atoms 4 1 oxygen atoms 2 Unbalanced

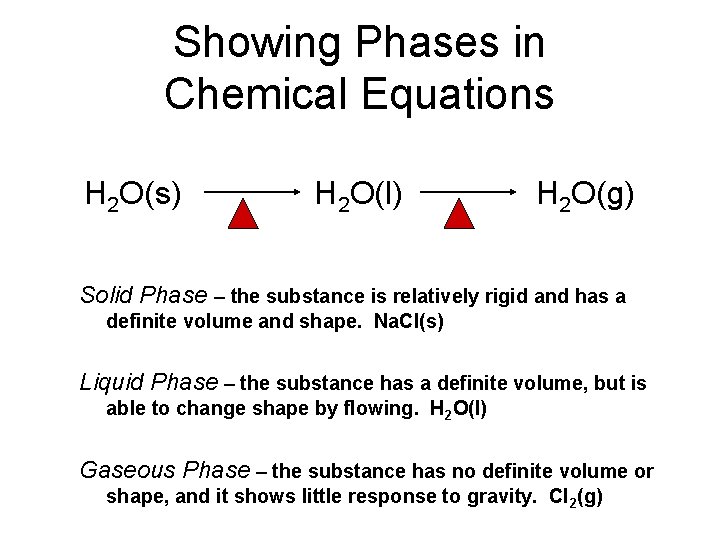
Showing Phases in Chemical Equations H 2 O(s) H 2 O(l) H 2 O(g) Solid Phase – the substance is relatively rigid and has a definite volume and shape. Na. Cl(s) Liquid Phase – the substance has a definite volume, but is able to change shape by flowing. H 2 O(l) Gaseous Phase – the substance has no definite volume or shape, and it shows little response to gravity. Cl 2(g)

Additional Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction (s) A reactant or product in the solid state; also used to indicate a precipitate Alternative to (s), but used only to indicate a precipitate (l) A reactant or product in the liquid state (aq) A reactant or product in an aqueous solution (dissolved in water) (g) A reactant or product in the gaseous state

Additional Symbols Used in Chemical Equations Alternative to (g), but used only to indicate a gaseous product D 2 atm pressure Reactants are heated Pressure at which reaction is carried out, in this case 2 atm Pressure at which reaction is carried out exceeds normal atmospheric pressure 0 o. C Temperature at which reaction is carried out, in this case 0 o. C Mn. O 2 Formula of catalyst, in this case manganese (IV) oxide, used to alter the rate of the reaction

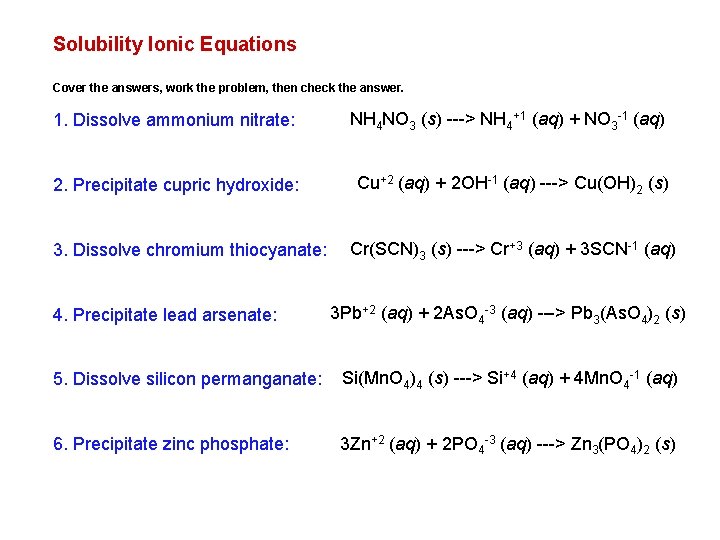
Solubility Ionic Equations Cover the answers, work the problem, then check the answer. 1. Dissolve ammonium nitrate: NH 4 NO 3 (s) ---> NH 4+1 (aq) + NO 3 -1 (aq) 2. Precipitate cupric hydroxide: Cu+2 (aq) + 2 OH-1 (aq) ---> Cu(OH)2 (s) 3. Dissolve chromium thiocyanate: 4. Precipitate lead arsenate: Cr(SCN)3 (s) ---> Cr+3 (aq) + 3 SCN-1 (aq) 3 Pb+2 (aq) + 2 As. O 4 -3 (aq) ---> Pb 3(As. O 4)2 (s) 5. Dissolve silicon permanganate: Si(Mn. O 4)4 (s) ---> Si+4 (aq) + 4 Mn. O 4 -1 (aq) 6. Precipitate zinc phosphate: 3 Zn+2 (aq) + 2 PO 4 -3 (aq) ---> Zn 3(PO 4)2 (s)

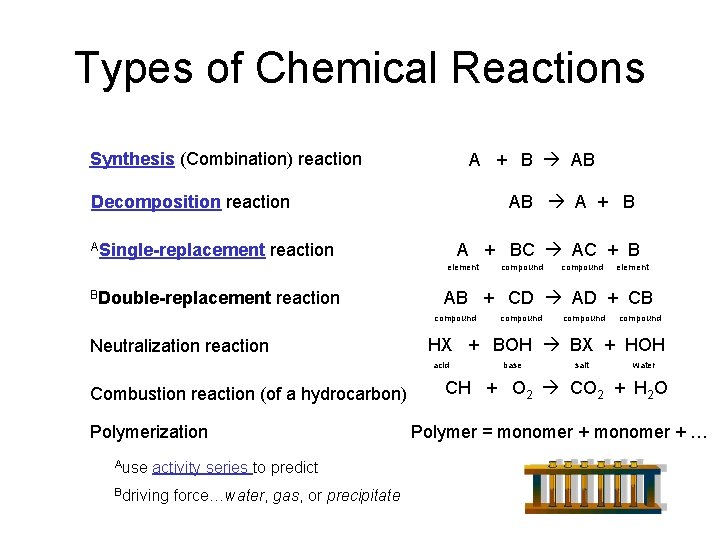
Types of Chemical Reactions Synthesis (Combination) reaction A + B AB AB A + B Decomposition reaction ASingle-replacement A + BC AC + B reaction element BDouble-replacement reaction Polymerization Ause activity series to predict Bdriving force…water, gas, or precipitate element compound HX + BOH BX + HOH acid Combustion reaction (of a hydrocarbon) compound AB + CD AD + CB compound Neutralization reaction compound base salt water CH + O 2 CO 2 + H 2 O Polymer = monomer + …

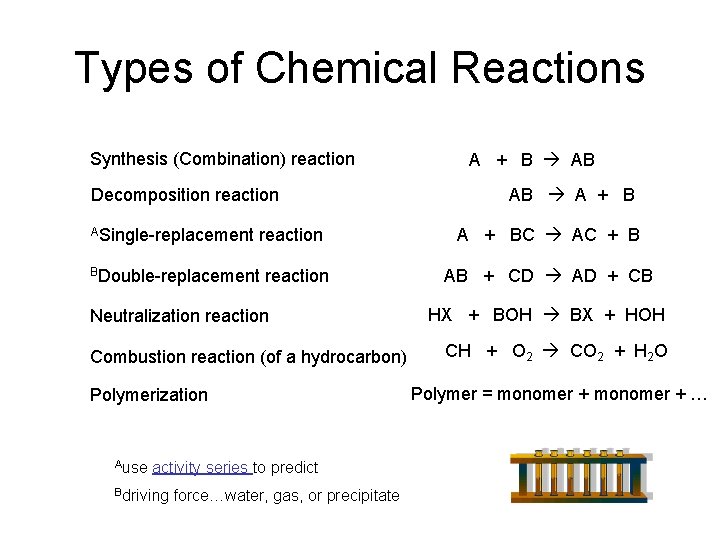
Types of Chemical Reactions Synthesis (Combination) reaction Decomposition reaction ASingle-replacement reaction BDouble-replacement reaction Neutralization reaction Combustion reaction (of a hydrocarbon) Polymerization Ause activity series to predict Bdriving force…water, gas, or precipitate A + B AB AB A + BC AC + B AB + CD AD + CB HX + BOH BX + HOH CH + O 2 CO 2 + H 2 O Polymer = monomer + …

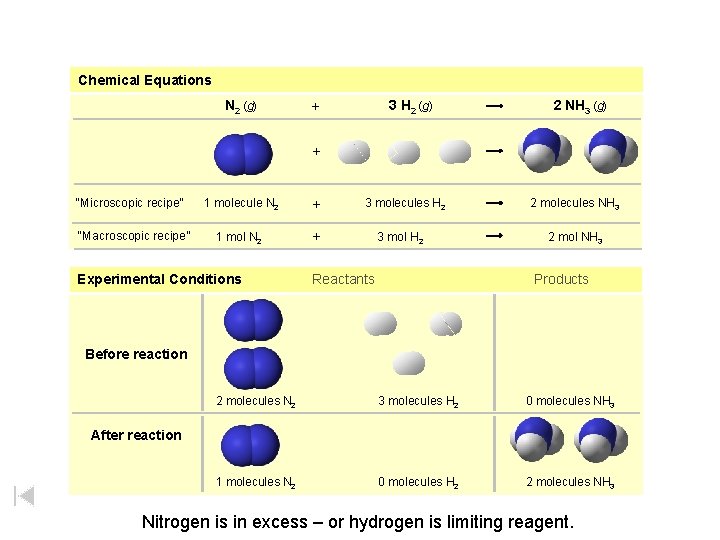
Chemical Equations N 2 (g) 3 H 2 (g) + 2 NH 3 (g) + “Microscopic recipe” “Macroscopic recipe” 1 molecule N 2 + 1 mol N 2 + Experimental Conditions 3 molecules H 2 3 mol H 2 Reactants 2 molecules NH 3 2 mol NH 3 Products Before reaction 1 mol N 2 + 3 mol H 2 2 mol NH 3 2 molecules N 2 3 molecules H 2 0 molecules NH 3 1 molecules N 2 0 molecules H 2 2 molecules NH 3 After reaction Nitrogen is in excess – or hydrogen is limiting reagent.

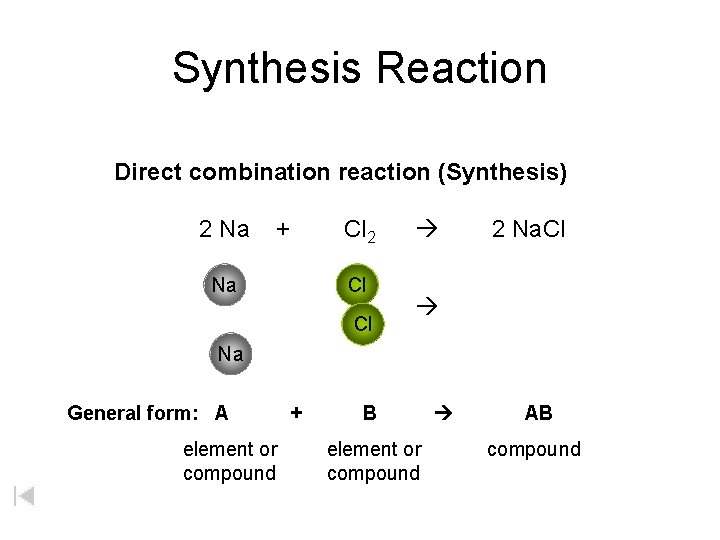
Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Na Cl 2 Cl Cl 2 Na. Cl Na General form: A element or compound + B element or compound AB compound

Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Cl 2 Na. Cl Cl Na+ Cl - Cl Cl - Na+ Na General form: A element or compound + B element or compound AB compound

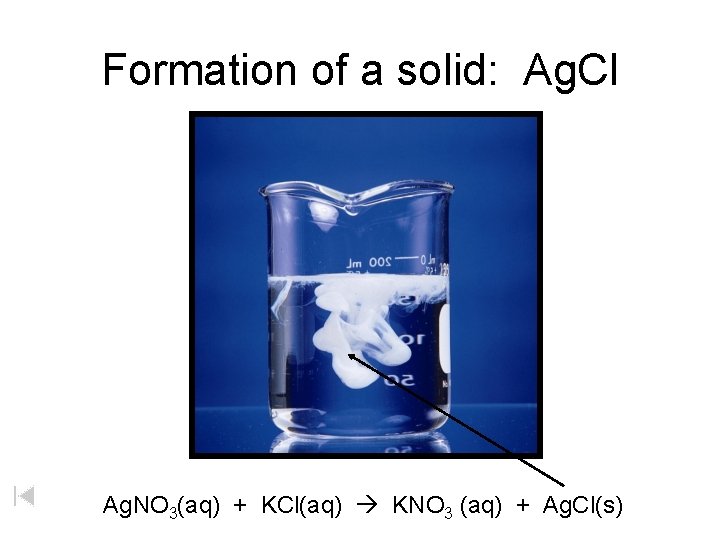
Formation of a solid: Ag. Cl Ag. NO 3(aq) + KCl(aq) KNO 3 (aq) + Ag. Cl(s)

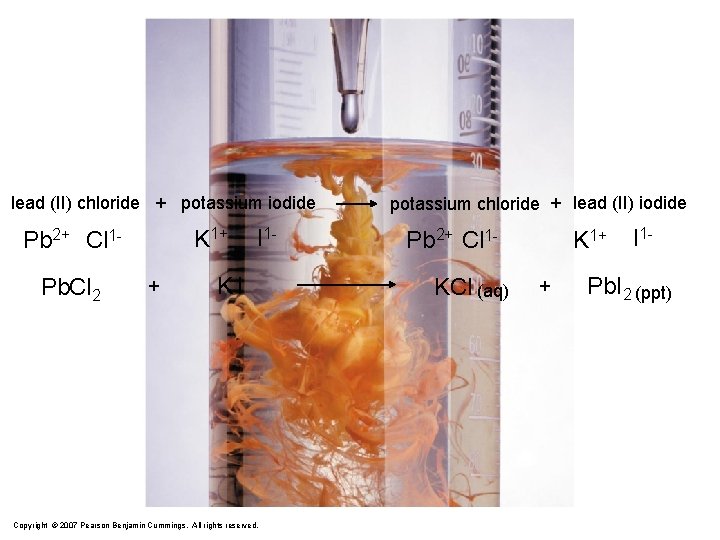
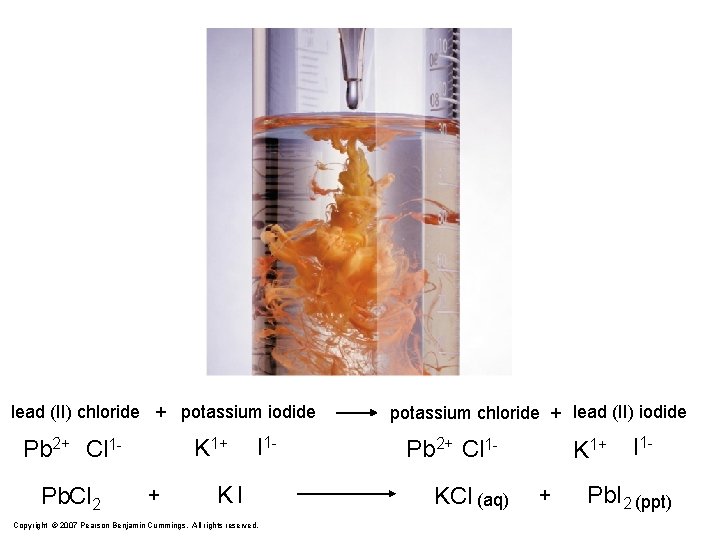
lead (II) chloride + potassium iodide K 1+ Pb 2+ Cl 1 Pb. Cl 2 + I 1 - KI Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. potassium chloride + lead (II) iodide Pb 2+ Cl 1 KCl (aq) K 1+ + I 1 - Pb. I 2 (ppt)

lead (II) chloride + potassium iodide K 1+ Pb 2+ Cl 1 Pb. Cl 2 + I 1 - KI Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. potassium chloride + lead (II) iodide Pb 2+ Cl 1 KCl (aq) K 1+ + I 1 - Pb. I 2 (ppt)

lead(II) chloride + potassium iodide Pb 2+ I 1 - K 1+ Cl 1 - Pb Cl 2 (aq) Pb 2+ + 2 K 1+(aq) + 2 I 1 -(aq) Pb 2+ Cl 1 - KCl (aq) 2 K I (aq) + Pb 2+(aq) + 2 Cl 1 -(aq) potassium chloride + lead(II) iodide 2 K 1+(aq) + 2 Cl 1 -(aq) + Pb 2+(aq) + 2 I 1 -(aq) Cl 1 - I 1 K 1+ REACTANTS I 1 Cl 1 I 1 - Pb 2+ K 1+ I 1 - PRODUCTS Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. I 1 - Pb. I 2 (ppt) K 1+ I 1 - Cl 1 - + Pb 2+ K 1+ I 1 - K 1+
![Double Replacement Reaction aqueous barium nitrate reacts with sulfuric acid H 2 SO 4aq Double Replacement Reaction aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)]](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-41.jpg)
Double Replacement Reaction aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a barium sulfate precipitate and nitric acid [HNO 3(aq)] barium nitrate Ba 2+ NO 31 - + Ba(NO 3)2(aq) + sulfuric acid H 1+ SO 42 - H 2 SO 4(aq) Ba 2+(aq) + 2 NO 31 -(aq) + 2 H 1+(aq) + SO 42 -(aq) Ba 2+ NO 31 - barium sulfate Ba 2+ SO 42 - nitric acid + 2 HNO 3 Ba. SO 4 (ppt) Ba 2+(aq) + SO 42 -(aq) + H 1+ + 2 H 1+(aq) + 2 NO 31 -(aq) Ba 2+ NO 31 SO 42 - H 1+ NO 31 - Ba 2+ SO 42 REACTANTS NO 31 - 2 HNO 3(aq) H 1+ NO 31 - + PRODUCTS H 1+
![aqueous barium nitrate reacts with sulfuric acid H 2 SO 4aq to yield a aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-42.jpg)
aqueous barium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a barium sulfate precipitate and nitric acid [HNO 3(aq)] barium nitrate Ba(NO 3)2(aq) + sulfuric acid barium sulfate + H 2 SO 4(aq) Ba. SO 4 (ppt) Ba 2+(aq) + 2 NO 31 -(aq) + 2 H 1+(aq) + SO 42 -(aq) 2+ 1 - 1+ Ba 2+(aq) + SO 42 -(aq) nitric acid + + 2 HNO 3(aq) + 2 H 1+(aq) + 2 NO 31 -(aq) 1+ 1 - 21 - 1+ REACTANTS 11+ 2+ 2 PRODUCTS
![Double Replacement Reaction aqueous calcium nitrate reacts with sulfuric acid H 2 SO 4aq Double Replacement Reaction aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)]](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-43.jpg)
Double Replacement Reaction aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a calcium sulfate precipitate and nitric acid [HNO 3(aq)] calcium nitrate Ca 2+ NO 31 - + Ca(NO 3)2(aq) + sulfuric acid H 1+ SO 42 - H 2 SO 4(aq) Ca 2+(aq) + 2 NO 31 -(aq) + 2 H 1+(aq) + SO 42 -(aq) Ca 2+ NO 31 - calcium sulfate Ca 2+ SO 42 - nitric acid + 2 HNO 3 Ca. SO 4 (ppt) Ca 2+(aq) + SO 42 -(aq) + H 1+ + 2 H 1+(aq) + 2 NO 31 -(aq) Ca 2+ NO 31 SO 42 - H 1+ NO 31 - Ca 2+ SO 42 REACTANTS NO 31 - 2 HNO 3(aq) H 1+ NO 31 - + PRODUCTS H 1+
![aqueous calcium nitrate reacts with sulfuric acid H 2 SO 4aq to yield a aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a](https://slidetodoc.com/presentation_image_h/1812c3754690e67ad740a1e1b718f5d9/image-44.jpg)
aqueous calcium nitrate reacts with sulfuric acid [H 2 SO 4(aq)] to yield a calcium sulfate precipitate and nitric acid [HNO 3(aq)] calcium nitrate + sulfuric acid barium sulfate Ca(NO 3)2(aq) + H 2 SO 4(aq) Ca. SO 4 (ppt) Ca 2+(aq) + 2 NO 31 -(aq) + 2 H 1+(aq) + SO 42 -(aq) 2+ 1 - 1+ Ca 2+(aq) + SO 42 -(aq) nitric acid + + 2 HNO 3(aq) + 2 H 1+(aq) + 2 NO 31 -(aq) 1+ 1 - 21 - 1+ REACTANTS 11+ 2+ 2 PRODUCTS

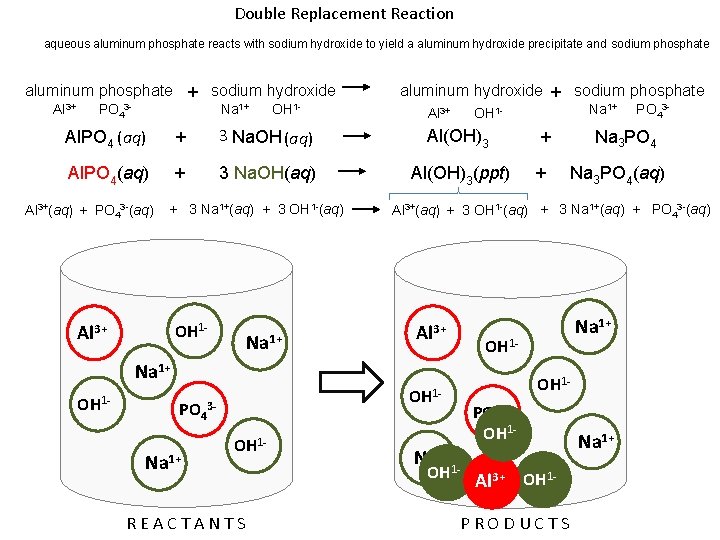
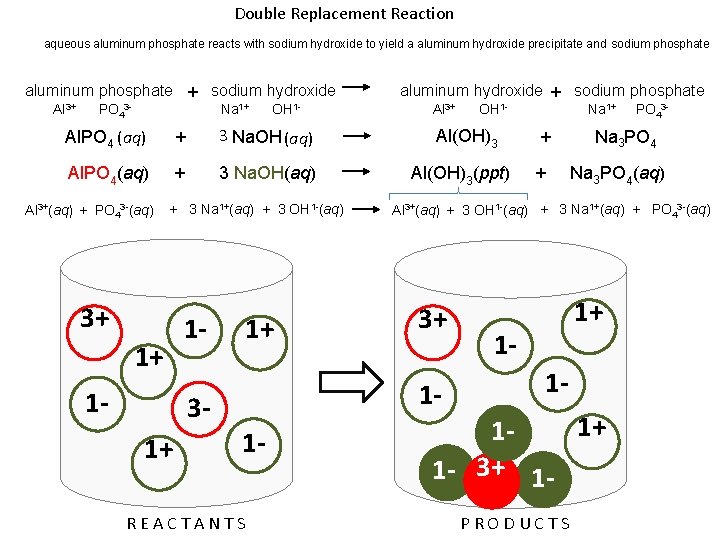
Double Replacement Reaction aqueous aluminum phosphate reacts with sodium hydroxide to yield a aluminum hydroxide precipitate and sodium phosphate aluminum phosphate + sodium hydroxide Al 3+ Na 1+ PO 43 - OH 1 - aluminum hydroxide + sodium phosphate Al 3+ Na 1+ OH 1 - Al. PO 4 (aq) + 3 Na. OH (aq) Al(OH)3 + Al. PO 4(aq) + 3 Na. OH(aq) Al(OH)3(ppt) + Al 3+(aq) + PO 43 -(aq) + 3 Na 1+(aq) + 3 OH 1 -(aq) OH 1 - Al 3+ Na 1+ Al 3+ OH 1 - PO 43 - Na 1+ OH 1 - REACTANTS Na 3 PO 4(aq) Al 3+(aq) + 3 OH 1 -(aq) + 3 Na 1+(aq) + PO 43 -(aq) Na 1+1 OH Na 1+ OH 1 - PO 43 - OH 1 PO 43 OH 1 - Al 3+ OH 1 PRODUCTS Na 1+

Double Replacement Reaction aqueous aluminum phosphate reacts with sodium hydroxide to yield a aluminum hydroxide precipitate and sodium phosphate aluminum phosphate + sodium hydroxide Al 3+ Na 1+ PO 43 - OH 1 - Al. PO 4 (aq) + 3 Na. OH (aq) Al. PO 4(aq) + 3 Na. OH(aq) Al 3+(aq) + PO 43 -(aq) + 3 Na 1+(aq) + 3 OH 1 -(aq) 3+ 1+ 1 - 13 - 1+ 1+ aluminum hydroxide + sodium phosphate Al 3+ Al(OH)3(ppt) REACTANTS Na 1+ + + PO 43 - Na 3 PO 4(aq) Al 3+(aq) + 3 OH 1 -(aq) + 3 Na 1+(aq) + PO 43 -(aq) 3+ 1 - 1 - OH 1 - 1+ 11 - 3+ 1 PRODUCTS

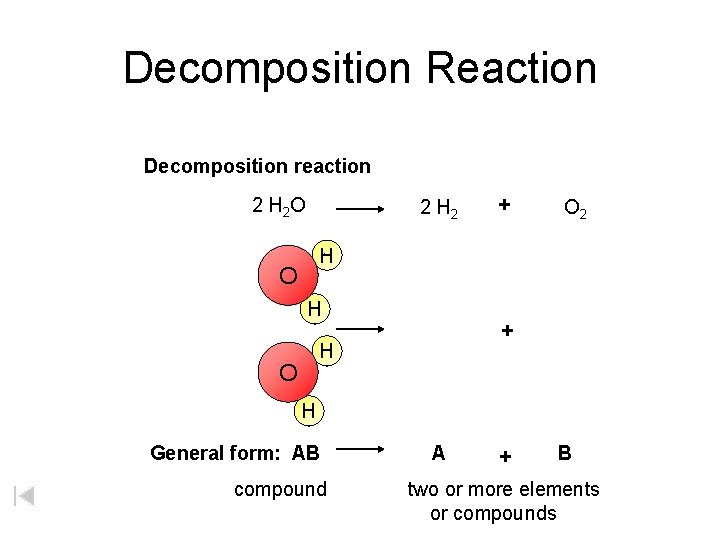
Decomposition Reaction Decomposition reaction 2 H 2 O 2 H 2 + O 2 H O H + H O H General form: AB compound A + B two or more elements or compounds

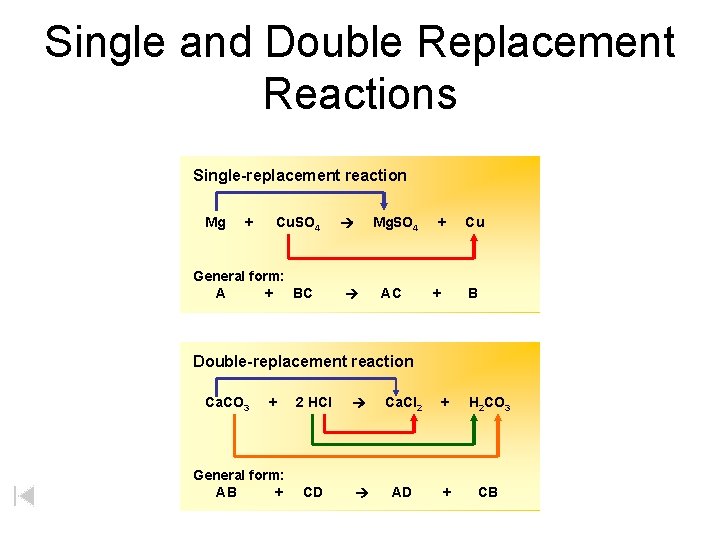
Single and Double Replacement Reactions Single-replacement reaction Mg + Cu. SO 4 General form: A + BC Mg. SO 4 AC + + Cu B Double-replacement reaction Ca. CO 3 + General form: AB + 2 HCl Ca. Cl 2 + H 2 CO 3 CD AD + CB

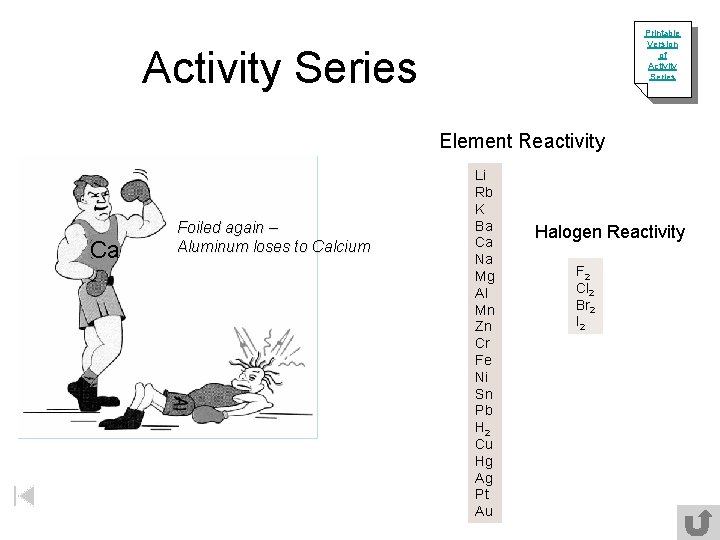
Printable Version of Activity Series Element Reactivity Ca Foiled again – Aluminum loses to Calcium Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au Halogen Reactivity F 2 Cl 2 Br 2 I 2

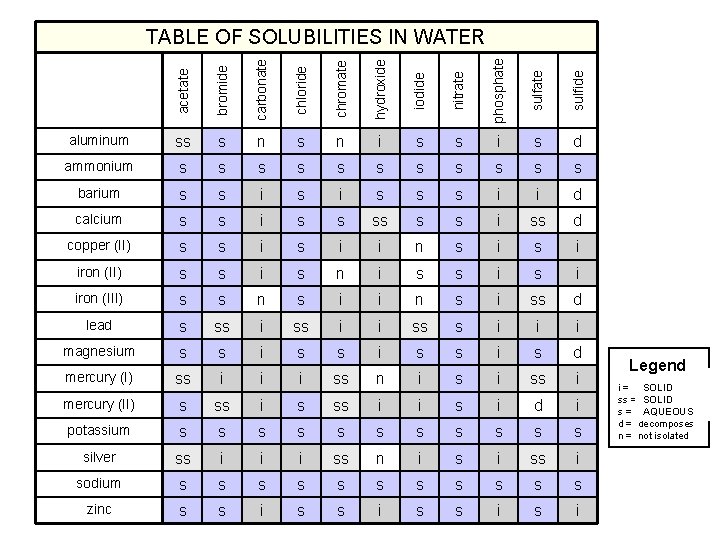
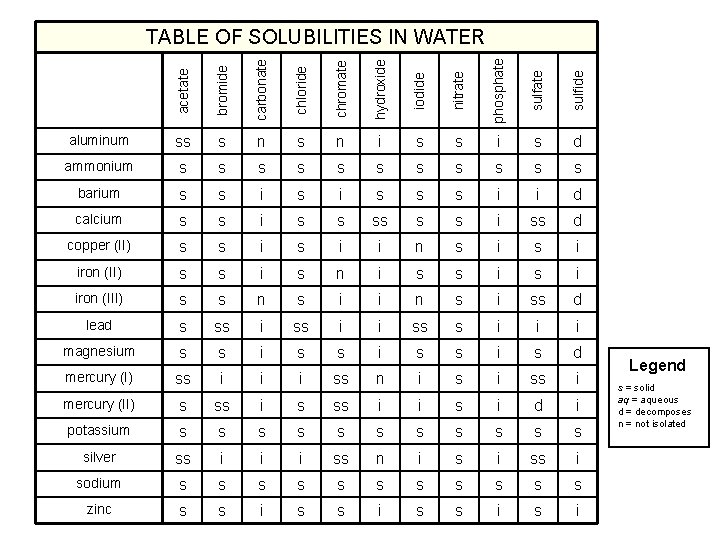
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum ss s n i s s i s d ammonium s s s barium s s i s s s i i d calcium s s i ss d copper (II) s s i i n s i iron (II) s s i s n i s s i iron (III) s s n s i i n s i ss d lead s ss i i ss s i i i magnesium s s i s d mercury (I) ss i i i ss n i ss i mercury (II) s ss i i s i d i potassium s s silver ss i i i ss n i ss i sodium s s s zinc s s i Legend SOLID i = insoluble SOLIDsoluble ss = slightly AQUEOUS s = soluble d = decomposes n = not isolated

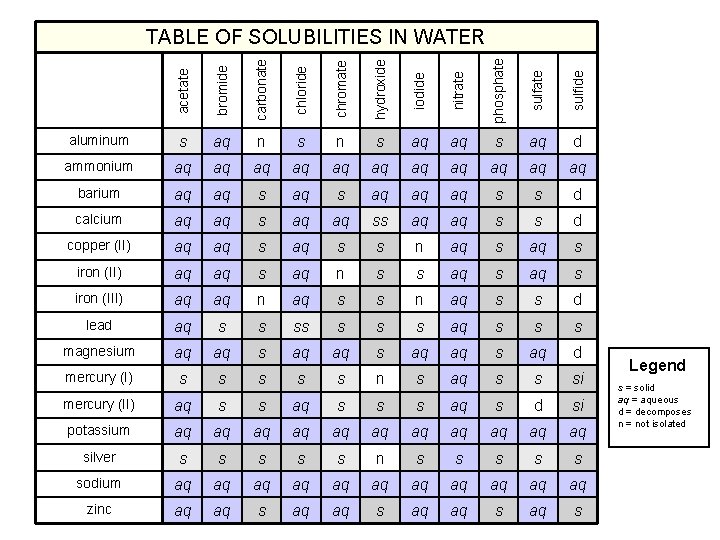
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum s aq n s aq aq s aq d ammonium aq aq aq barium aq aq s aq aq aq s s d calcium aq aq ss aq aq s s d copper (II) aq aq s s n aq s iron (II) aq aq s aq n s s aq s iron (III) aq aq n aq s s d lead aq s s s s aq s s s magnesium aq aq s aq d mercury (I) s s s n s aq s s si mercury (II) aq s s s aq s d si potassium aq aq aq silver s s s n s s sodium aq aq aq zinc aq aq s Legend s = solid aq = aqueous d = decomposes n = not isolated

acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum ss s n i s s i s d ammonium s s s barium s s i s s s i i d calcium s s i ss d copper (II) s s i i n s i iron (II) s s i s n i s s i iron (III) s s n s i i n s i ss d lead s ss i i ss s i i i magnesium s s i s d mercury (I) ss i i i ss n i ss i mercury (II) s ss i i s i d i potassium s s silver ss i i i ss n i ss i sodium s s s zinc s s i Legend s = solid aq = aqueous d = decomposes n = not isolated

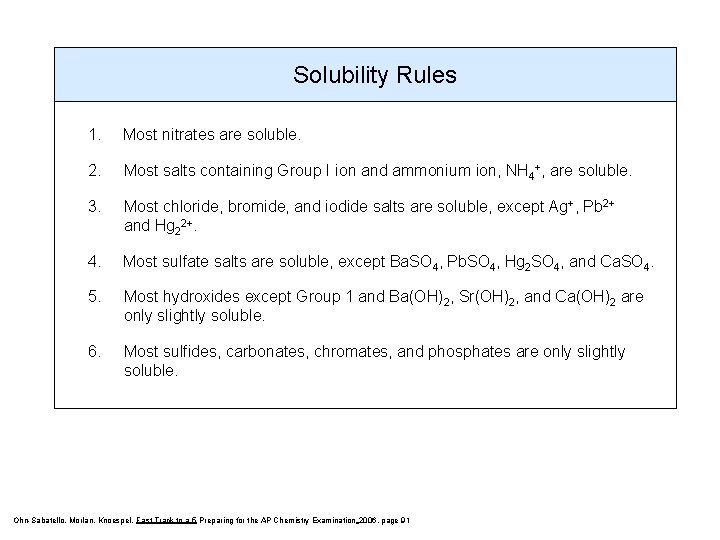
Solubility Rules 1. Most nitrates are soluble. 2. Most salts containing Group I ion and ammonium ion, NH 4+, are soluble. 3. Most chloride, bromide, and iodide salts are soluble, except Ag+, Pb 2+ and Hg 22+. 4. Most sulfate salts are soluble, except Ba. SO 4, Pb. SO 4, Hg 2 SO 4, and Ca. SO 4. 5. Most hydroxides except Group 1 and Ba(OH)2, Sr(OH)2, and Ca(OH)2 are only slightly soluble. 6. Most sulfides, carbonates, chromates, and phosphates are only slightly soluble. Ohn-Sabatello, Morlan, Knoespel, Fast Track to a 5 Preparing for the AP Chemistry Examination 2006, page 91

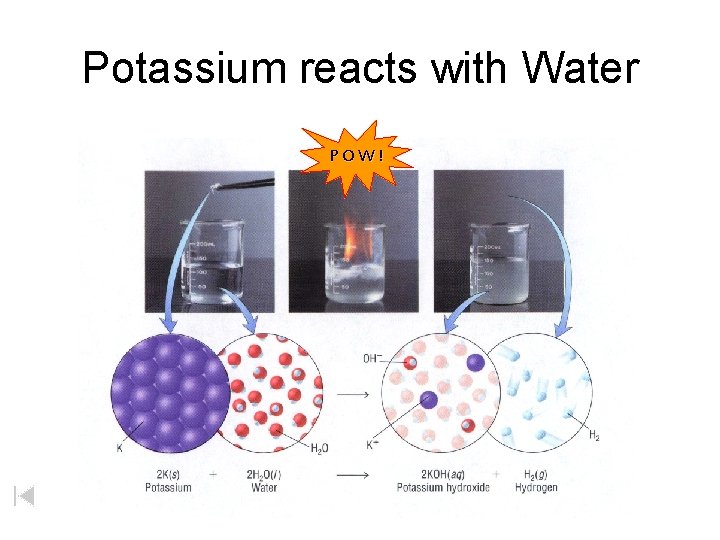
Potassium reacts with Water POW!

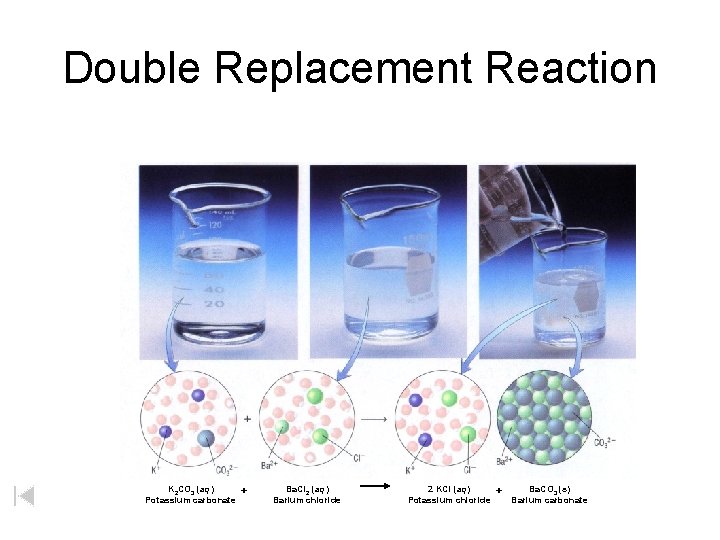
Double Replacement Reaction K 2 CO 3 (aq) Potassium carbonate + Ba. Cl 2 (aq) Barium chloride 2 KCl (aq) Potassium chloride + Ba. CO 3 (s) Barium carbonate

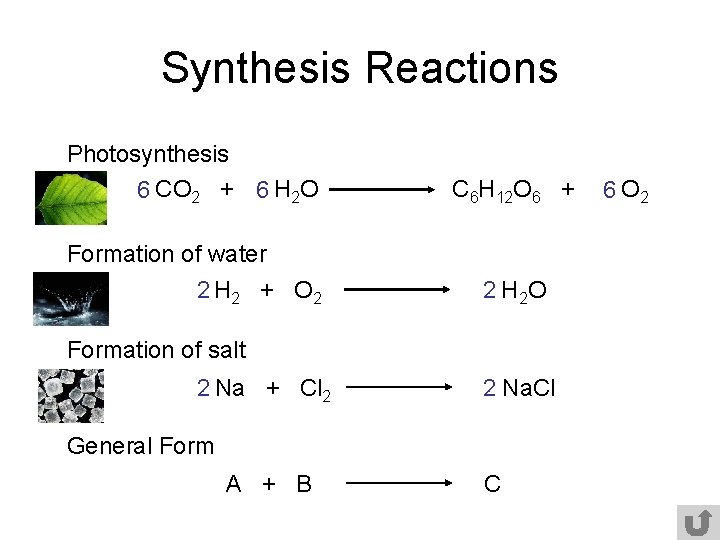
Synthesis Reactions Photosynthesis 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + Formation of water 2 H 2 + O 2 2 H 2 O Formation of salt 2 Na + Cl 2 2 Na. Cl General Form A + B C 6 O 2

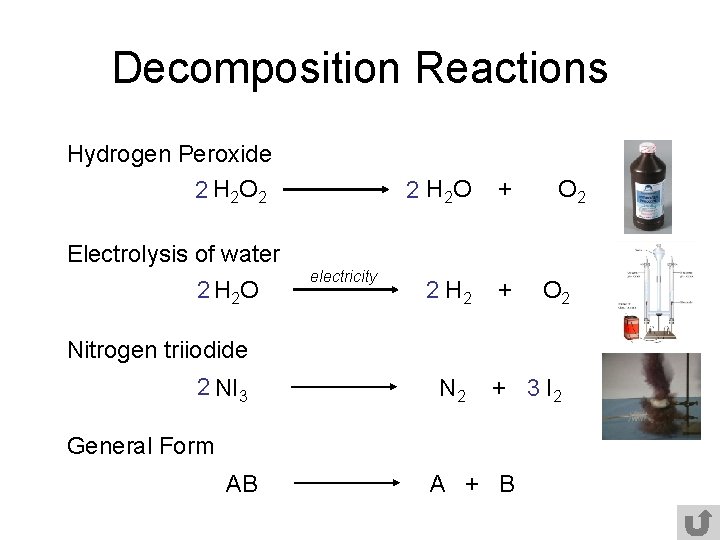
Decomposition Reactions Hydrogen Peroxide 2 H 2 O 2 2 H 2 O + 2 H 2 + O 2 Electrolysis of water 2 H 2 O electricity O 2 Nitrogen triiodide 2 NI 3 N 2 + 3 I 2 General Form AB A + B

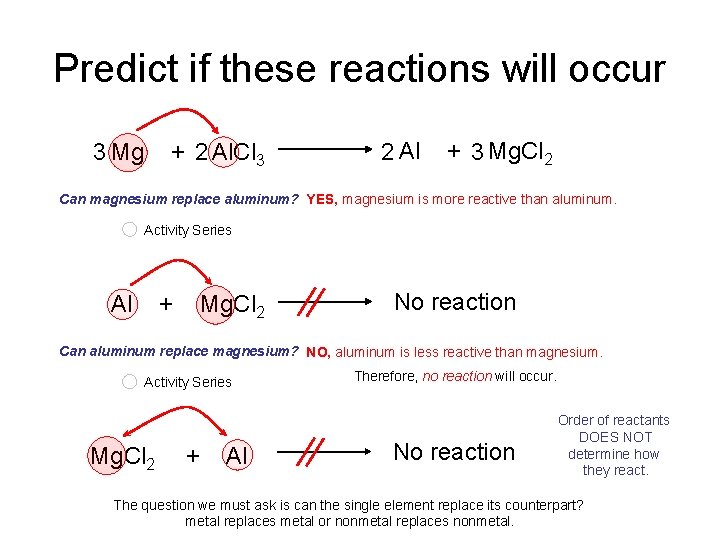
Predict if these reactions will occur 3 Mg + 2 Al. Cl 3 2 Al + 3 Mg. Cl 2 Can magnesium replace aluminum? YES, magnesium is more reactive than aluminum. Activity Series Al + Mg. Cl 2 No reaction Can aluminum replace magnesium? NO, aluminum is less reactive than magnesium. Activity Series Mg. Cl 2 + Al Therefore, no reaction will occur. No reaction Order of reactants DOES NOT determine how they react. The question we must ask is can the single element replace its counterpart? metal replaces metal or nonmetal replaces nonmetal.

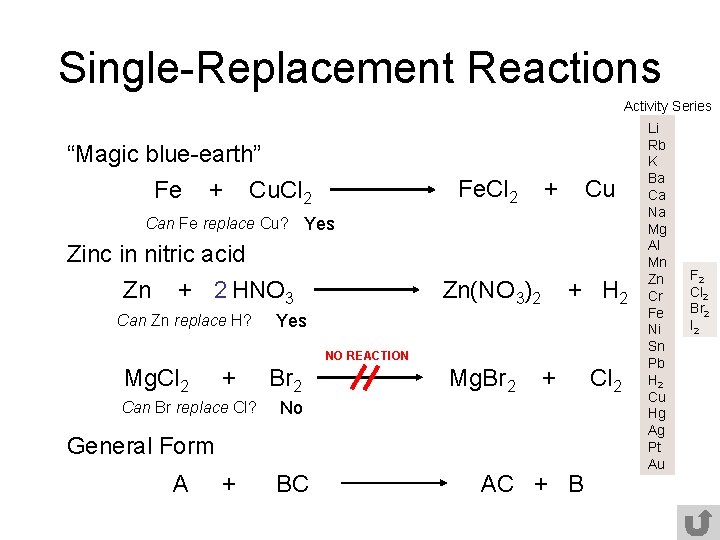
Single-Replacement Reactions Activity Series “Magic blue-earth” Fe + Cu. Cl 2 Fe. Cl 2 + Cu Can Fe replace Cu? Yes Zinc in nitric acid Zn + 2 HNO 3 Can Zn replace H? Zn(NO 3)2 + H 2 Yes NO REACTION Mg. Cl 2 + Can Br replace Cl? Br 2 Mg. Br 2 + No General Form A + BC AC + B Cl 2 Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au F 2 Cl 2 Br 2 I 2

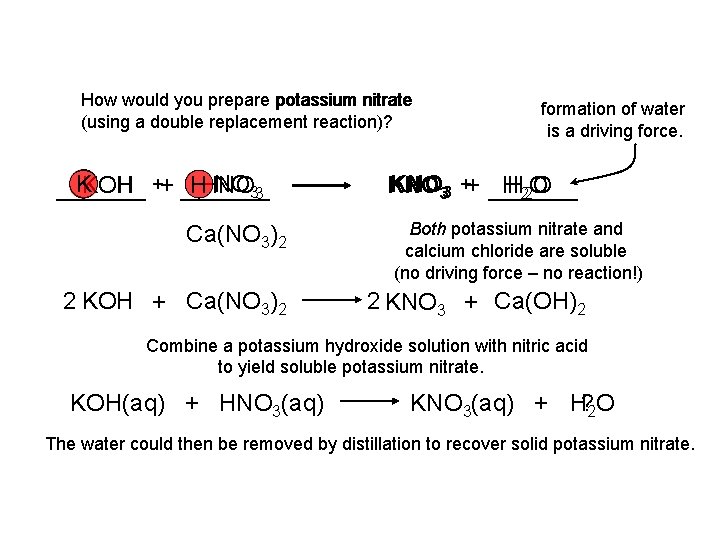
How would you prepare potassium nitrate (using a double replacement reaction)? KKOH NO 33 HHNO OH ++ _________ Ca(NO 3)2 2 KOH + Ca(NO 3)2 formation of water is a driving force. KNO 33 ++ _____ H 22 O O KNO H Both potassium nitrate and calcium chloride are soluble (no driving force – no reaction!) 2 KNO 3 + Ca(OH)2 Combine a potassium hydroxide solution with nitric acid to yield soluble potassium nitrate. KOH(aq) + HNO 3(aq) KNO 3(aq) + H? 2 O The water could then be removed by distillation to recover solid potassium nitrate.

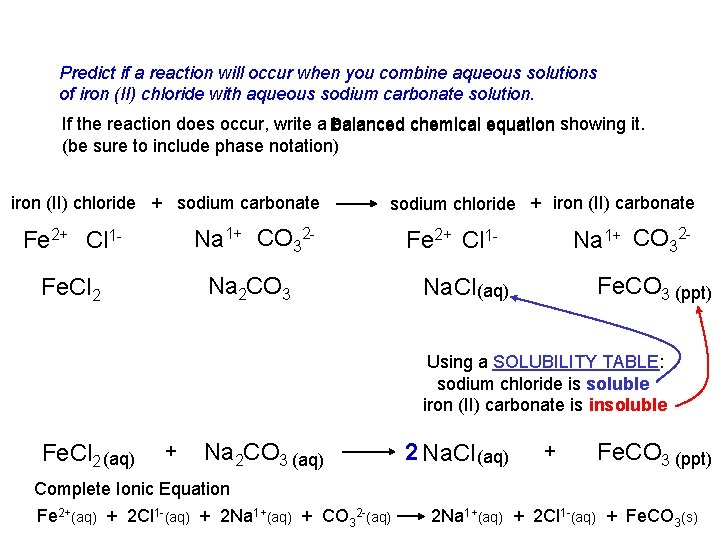
Predict if a reaction will occur when you combine aqueous solutions of iron (II) chloride with aqueous sodium carbonate solution. If the reaction does occur, write a Balanced balanced chemical equation showing it. (be sure to include phase notation) iron (II) chloride + sodium carbonate Fe 2+ Cl 1 - Na 1+ CO 32 - Fe. Cl 2 Na 2 CO 3 sodium chloride + iron (II) carbonate Na 1+ CO 32 - Fe 2+ Cl 1 - Fe. CO 3 (ppt) Na. Cl (aq) Using a SOLUBILITY TABLE: sodium chloride is soluble iron (II) carbonate is insoluble Fe. Cl 2 (aq) + Na 2 CO 3 (aq) Complete Ionic Equation Fe 2+(aq) + 2 Cl 1 -(aq) + 2 Na 1+(aq) + CO 32 -(aq) 2 Na. Cl (aq) + Fe. CO 3 (ppt) 2 Na 1+(aq) + 2 Cl 1 -(aq) + Fe. CO 3(s)

Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

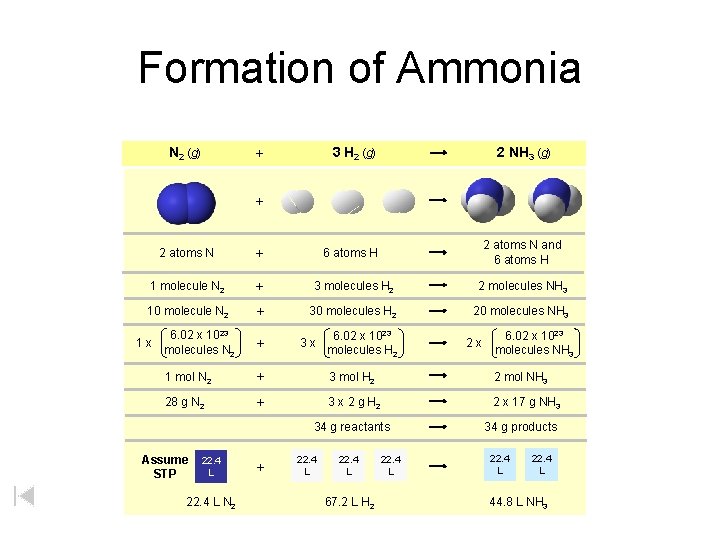
Formation of Ammonia N 2 (g) 3 H 2 (g) + 2 NH 3 (g) + 2 atoms N + 6 atoms H 2 atoms N and 6 atoms H 1 molecule N 2 + 3 molecules H 2 2 molecules NH 3 10 molecule N 2 + 30 molecules H 2 20 molecules NH 3 1 x 6. 02 x 1023 molecules N 2 + 1 mol N 2 + 3 mol H 2 2 mol NH 3 28 g N 2 + 3 x 2 g H 2 2 x 17 g NH 3 3 x 6. 02 x 1023 molecules H 2 34 g reactants Assume STP 22. 4 L N 2 + 22. 4 L 67. 2 L H 2 22. 4 L 2 x 6. 02 x 1023 molecules NH 3 34 g products 22. 4 L 44. 8 L NH 3

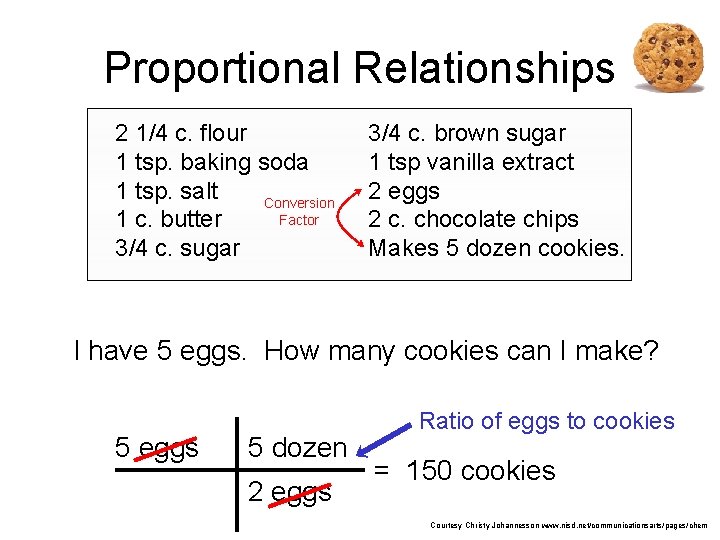
Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt Conversion Factor 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. I have 5 eggs. How many cookies can I make? 5 eggs 5 dozen 2 eggs Ratio of eggs to cookies 150 dozen cookies = 12. 5 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Proportional Relationships • Stoichiometry – mass relationships between substances in a chemical reaction – based on the mole ratio • Mole Ratio – indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

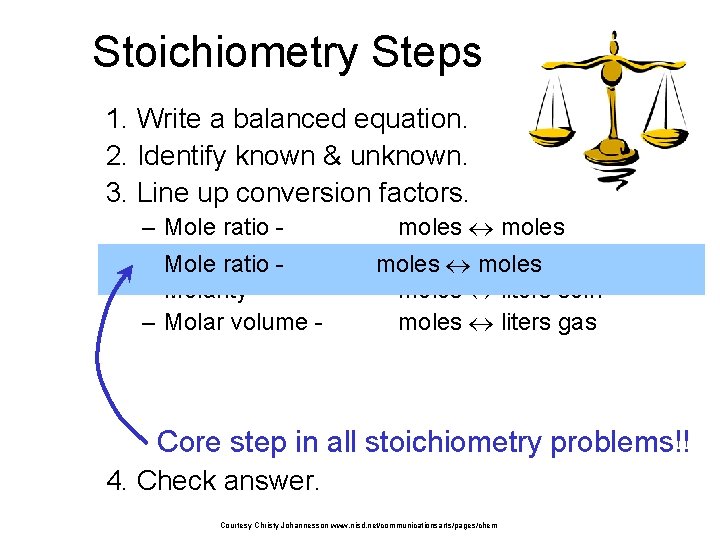
Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. – – – Mole ratio Molarratio mass Mole - Molarity Molar volume - moles grams moles liters soln moles liters gas Core step in all stoichiometry problems!! 4. Check answer. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

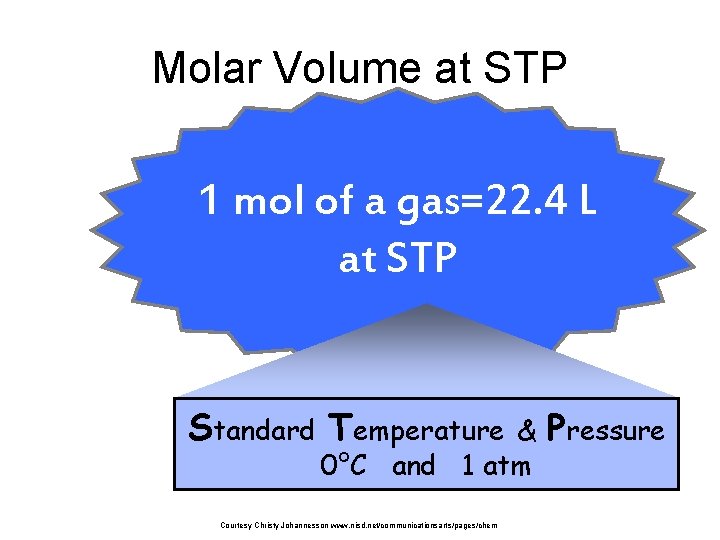
Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Pressure

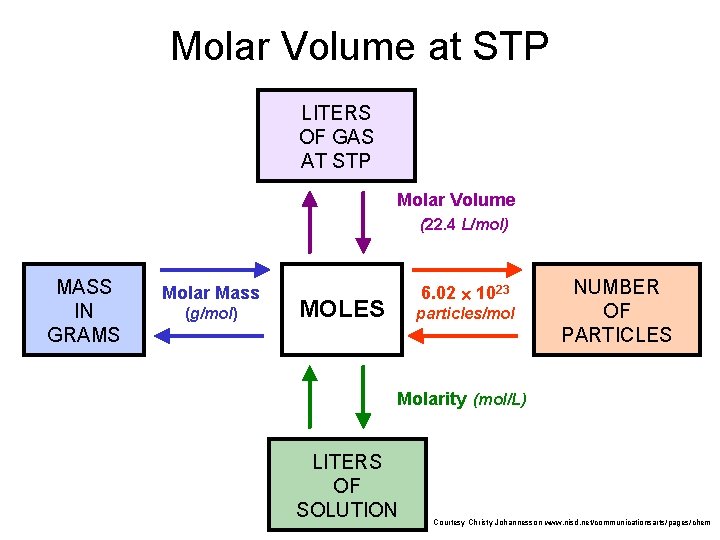
Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) 6. 02 1023 MOLES particles/mol NUMBER OF PARTICLES Molarity (mol/L) LITERS OF SOLUTION Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

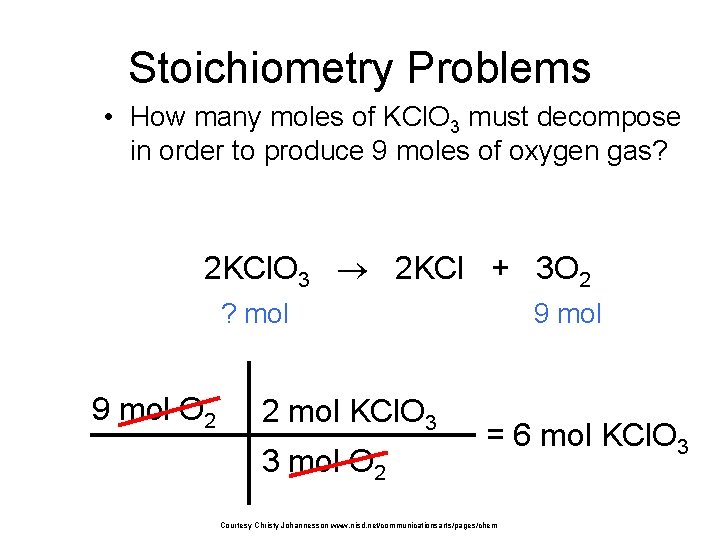
Stoichiometry Problems • How many moles of KCl. O 3 must decompose in order to produce 9 moles of oxygen gas? 2 KCl. O 3 2 KCl + 3 O 2 ? mol 9 mol O 2 2 mol KCl. O 3 3 mol O 2 9 mol = 6 mol KCl. O 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

1. 2 Sb + 3 Cl 2 2 Sb. Cl 3 2. 2 Mg + O 2 2 Mg. O 3. Ca. Cl 2 Ca + Cl 2 4. 2 Na. Cl. O 3 2 Na. Cl + 3 O 2 5. Fe + 2 HCl Fe. Cl 2 + H 2 6. Cu. O + H 2 Cu + H 2 O 7. 2 Al + 3 H 2 SO 4 Al 2(SO 4)3 + 3 H 2

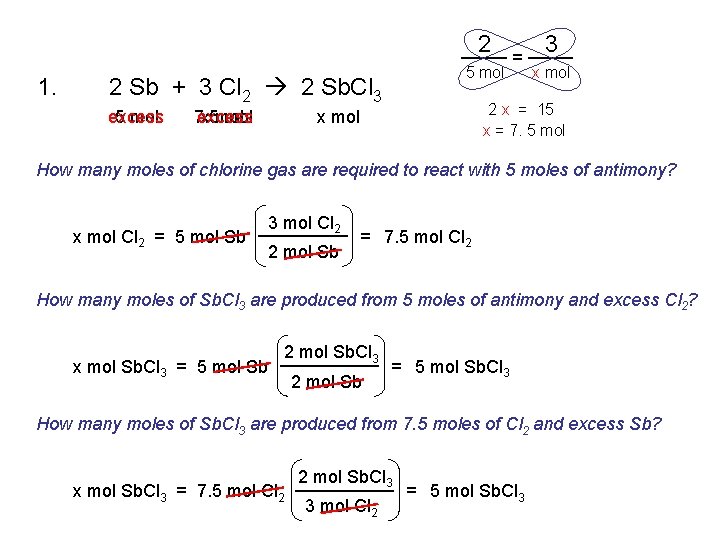
2 1. 5 mol 2 Sb + 3 Cl 2 2 Sb. Cl 3 excess 5 mol 7. 5 excess x mol = 3 x mol 2 x = 15 x = 7. 5 mol x mol How many moles of chlorine gas are required to react with 5 moles of antimony? x mol Cl 2 = 5 mol Sb 3 mol Cl 2 2 mol Sb = 7. 5 mol Cl 2 How many moles of Sb. Cl 3 are produced from 5 moles of antimony and excess Cl 2? x mol Sb. Cl 3 = 5 mol Sb 2 mol Sb. Cl 3 2 mol Sb = 5 mol Sb. Cl 3 How many moles of Sb. Cl 3 are produced from 7. 5 moles of Cl 2 and excess Sb? x mol Sb. Cl 3 = 7. 5 mol Cl 2 2 mol Sb. Cl 3 3 mol Cl 2 = 5 mol Sb. Cl 3
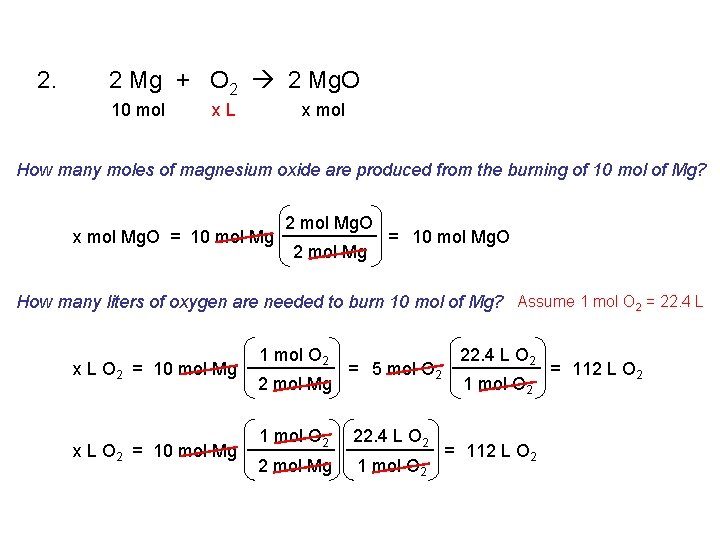
2. 2 Mg + O 2 2 Mg. O 10 mol x. L x mol How many moles of magnesium oxide are produced from the burning of 10 mol of Mg? x mol Mg. O = 10 mol Mg 2 mol Mg. O 2 mol Mg = 10 mol Mg. O How many liters of oxygen are needed to burn 10 mol of Mg? Assume 1 mol O 2 = 22. 4 L x L O 2 = 10 mol Mg 1 mol O 2 2 mol Mg = 5 mol O 2 1 mol O 2 22. 4 L O 2 2 mol Mg 1 mol O 2 22. 4 L O 2 1 mol O 2 = 112 L O 2

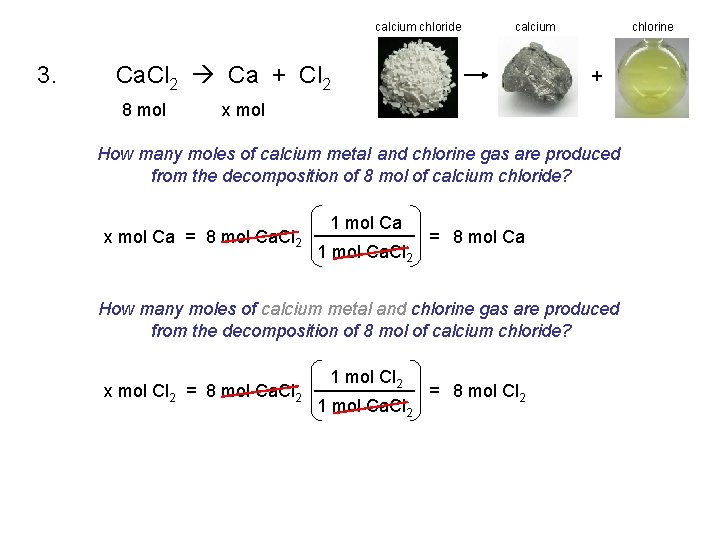
calcium chloride 3. calcium Ca. Cl 2 Ca + Cl 2 8 mol chlorine + x mol How many moles of calcium metal and chlorine gas are produced from the decomposition of 8 mol of calcium chloride? x mol Ca = 8 mol Ca. Cl 2 1 mol Ca. Cl 2 = 8 mol Ca How many moles of calcium metal and chlorine gas are produced from the decomposition of 8 mol of calcium chloride? x mol Cl 2 = 8 mol Ca. Cl 2 1 mol Ca. Cl 2 = 8 mol Cl 2

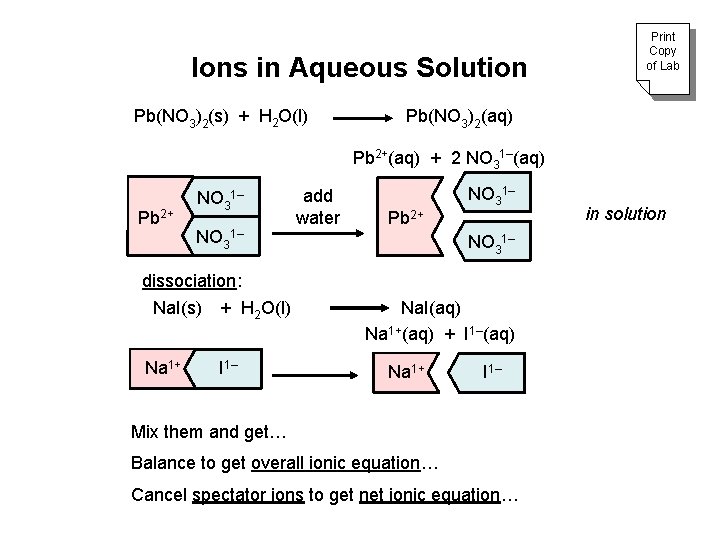
Ions in Aqueous Solution Pb(NO 3)2(s) + H 2 O(l) Print Copy of Lab Pb(NO 3)2(aq) Pb 2+(aq) + 2 NO 31–(aq) Pb 2+ NO 31– dissociation: Na. I(s) + H 2 O(l) Na 1+ I 1– add water Pb 2+ NO 31– Na. I(aq) Na 1+(aq) + I 1–(aq) Na 1+ I 1– Mix them and get… Balance to get overall ionic equation… Cancel spectator ions to get net ionic equation… in solution

Solubility Chart Mix them and get… Pb(NO 3)2(aq) + 2 Na. I(aq) Pb 2+ Na 1+ Pb. I 2(s) ++ 2 Na. NO (aq) + 2 Na 1+(aq) NO 31– 3 (aq) I 1– NO 31– Pb 2+ NO 31– I 1– Na 1+ solid I 1– NO 31– in solution Na 1+ I 1– Na 1+ NO 31– Balance to get overall ionic equation… Pb 2+(aq) + 2 NO 31–(aq) + 2 Na 1+(aq) + 2 I 1–(aq) Pb. I 2(s) + 2 NO 31–(aq) + 2 Na 1+(aq) Cancel spectator ions to get net ionic equation… Pb 2+(aq) + 2 I 1–(aq) Pb. I 2(s)

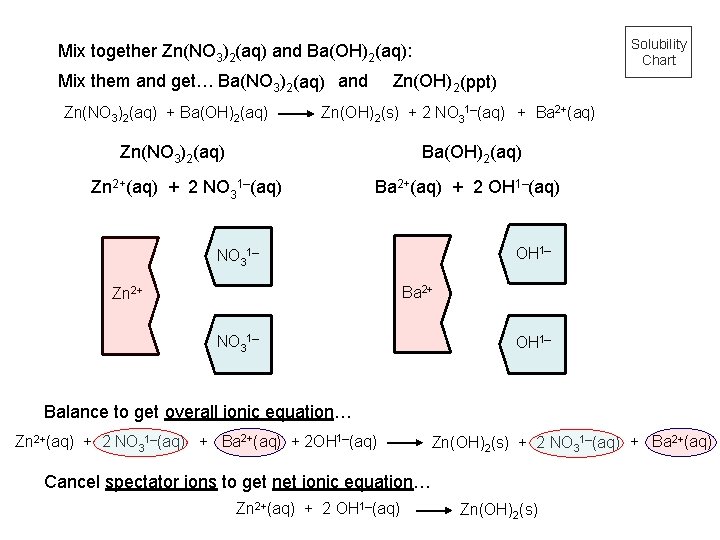
Solubility Chart Mix together Zn(NO 3)2(aq) and Ba(OH)2(aq): Mix them and get… Ba(NO 3)2(aq) and Zn(NO 3)2(aq) + Ba(OH)2(aq) Zn(OH)2 (ppt) Zn(OH)2(s) + 2 NO 31–(aq) + Ba 2+(aq) Zn(NO 3)2(aq) Ba(OH)2(aq) Zn 2+(aq) + 2 NO 31–(aq) Ba 2+(aq) + 2 OH 1–(aq) OH 1– NO 31– Ba 2+ Zn 2+ NO 31– OH 1– Balance to get overall ionic equation… Zn 2+(aq) + 2 NO 31–(aq) + Ba 2+(aq) + 2 OH 1–(aq) Zn(OH)2(s) + 2 NO 31–(aq) + Ba 2+(aq) Cancel spectator ions to get net ionic equation… Zn 2+(aq) + 2 OH 1–(aq) Zn(OH)2(s)
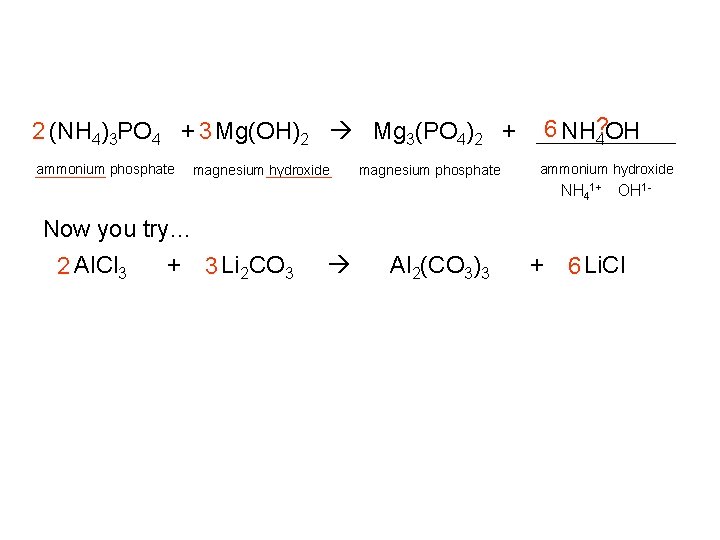
6 NH? 4 OH 2 (NH 4)3 PO 4 + 3 Mg(OH)2 Mg 3(PO 4)2 + ammonium phosphate magnesium hydroxide magnesium phosphate ammonium hydroxide NH 41+ OH 1 - Now you try… 2 Al. Cl 3 + 3 Li 2 CO 3 Al 2(CO 3)3 + 6 Li. Cl

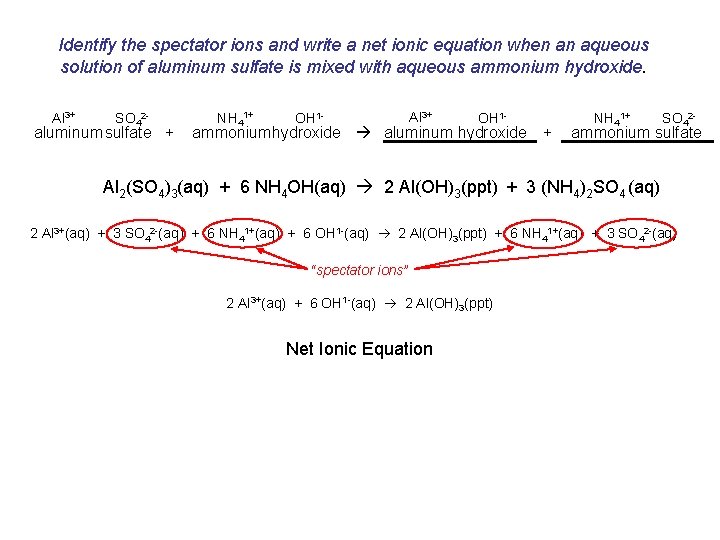
Identify the spectator ions and write a net ionic equation when an aqueous solution of aluminum sulfate is mixed with aqueous ammonium hydroxide. Al 3+ SO 42 - aluminum sulfate + NH 41+ OH 1 - Al 3+ OH 1 - ammoniumhydroxide aluminum hydroxide + NH 41+ SO 42 - ammonium sulfate Al 2(SO 4)3(aq) + 6 NH 4 OH(aq) 2 Al(OH)3(ppt) + 3 (NH 4)2 SO 4 (aq) 2 Al 3+(aq) + 3 SO 42 -(aq) + 6 NH 41+(aq) + 6 OH 1 -(aq) 2 Al(OH)3(ppt) + 6 NH 41+(aq) + 3 SO 42 -(aq) “spectator ions” 2 Al 3+(aq) + 6 OH 1 -(aq) 2 Al(OH)3(ppt) Net Ionic Equation

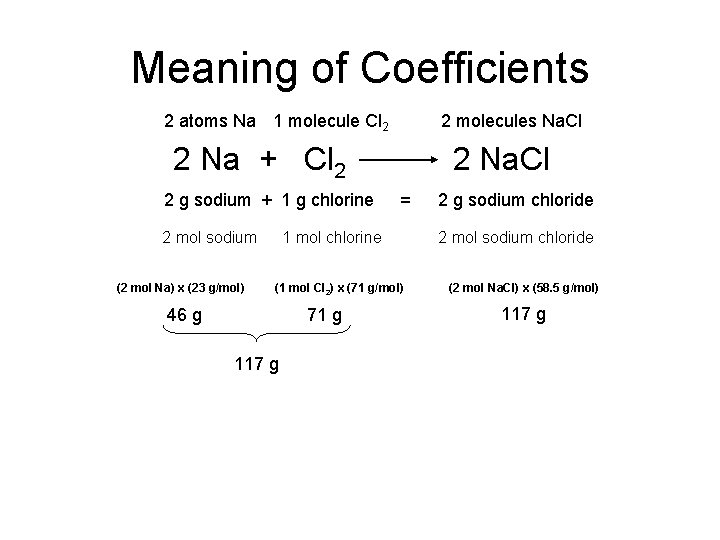
Meaning of Coefficients 2 atoms Na 1 molecule Cl 2 2 molecules Na. Cl 2 Na + Cl 2 2 g sodium + 1 g chlorine 2 mol sodium (2 mol Na) x (23 g/mol) 2 Na. Cl = 1 mol chlorine (1 mol Cl 2) x (71 g/mol) 46 g 71 g 117 g 2 g sodium chloride 2 mol sodium chloride (2 mol Na. Cl) x (58. 5 g/mol) 117 g

Classes of Reactions Chemical reactions Precipitation reactions Oxidation-Reduction Reactions Combustion Reactions Acid-Base Reactions

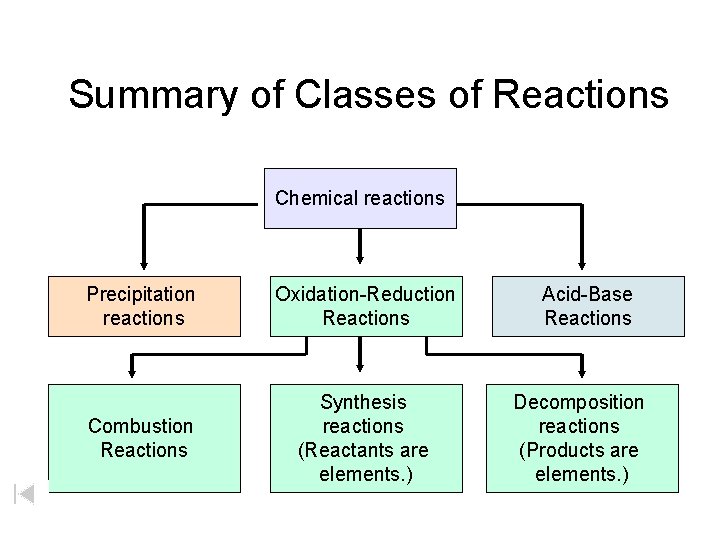
Summary of Classes of Reactions Chemical reactions Precipitation reactions Oxidation-Reduction Reactions Combustion Reactions Synthesis reactions (Reactants are elements. ) Acid-Base Reactions Decomposition reactions (Products are elements. )

Summary of Classes of Reactions Chemical reactions Precipitation reactions Oxidation-Reduction Reactions Combustion Reactions Synthesis reactions Acid-Base Reactions Decomposition reactions

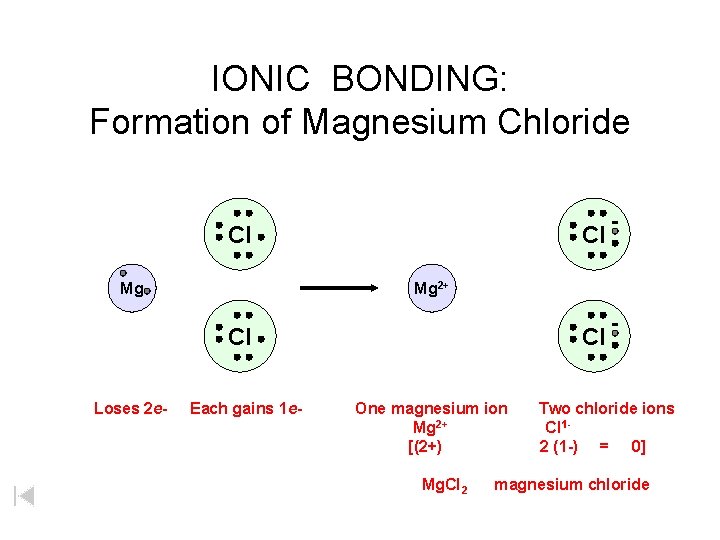
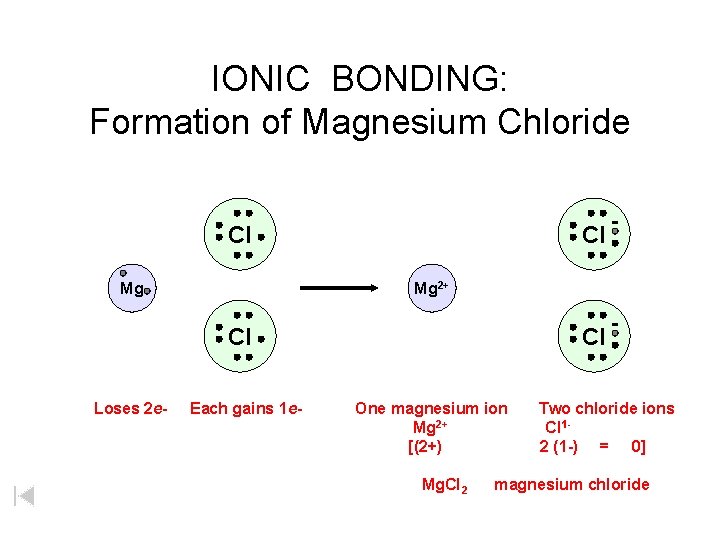
IONIC BONDING: Formation of Magnesium Chloride Cl Mg 2+ Cl Loses 2 e- Each gains 1 e- Cl One magnesium ion Mg 2+ [(2+) Mg. Cl 2 Two chloride ions Cl 12 (1 -) = 0] magnesium chloride

IONIC BONDING: Formation of Magnesium Chloride Cl Mg Mg 2+ Cl Loses 2 e- Each gains 1 e- Cl One magnesium ion Mg 2+ [(2+) Mg. Cl 2 Two chloride ions Cl 12 (1 -) = 0] magnesium chloride
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Unit 5 chemical reactions
Unit 5 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 1 chemical changes
Section 1 chemical changes Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations Toxic reactions chemical equations
Toxic reactions chemical equations Unit 11 chemical reactions
Unit 11 chemical reactions Translating chemical equations
Translating chemical equations Reduction half reaction
Reduction half reaction Stoichiometry island diagram
Stoichiometry island diagram Different types of redox reactions
Different types of redox reactions Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions 5 types of chemical reactions
5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions Predicting synthesis reactions
Predicting synthesis reactions Activity series of metals
Activity series of metals Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Five chemical changes
Five chemical changes Reactants and products
Reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Equilibrium occurs when
Equilibrium occurs when Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Types of reactions
Types of reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Functional isomers of carboxylic acids
Functional isomers of carboxylic acids Summary of chemical reactions
Summary of chemical reactions Chemical reactions in bread
Chemical reactions in bread Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Solvent in chemical reactions
Solvent in chemical reactions Three types of chemical reactions
Three types of chemical reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of chemical reaction
Indications of chemical reaction Describing chemical reactions
Describing chemical reactions Chemical reaction rules
Chemical reaction rules Chemical reaction rearrangement of atoms
Chemical reaction rearrangement of atoms Classification of chemical reactions worksheet
Classification of chemical reactions worksheet What are the five general types of chemical reactions
What are the five general types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Limiting reactant lab report answers
Limiting reactant lab report answers Chemical reactions in welding
Chemical reactions in welding Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Describing chemical reactions
Describing chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions What is released or absorbed whenever chemical
What is released or absorbed whenever chemical What is this?
What is this? Non aqueous solvents examples
Non aqueous solvents examples Equilibrium
Equilibrium Chemical reactions in water
Chemical reactions in water Solvent in chemical reactions
Solvent in chemical reactions Chemical reactions in soil
Chemical reactions in soil Homogenous solution
Homogenous solution Solvent in chemical reactions
Solvent in chemical reactions Alkene and alkyne reactions
Alkene and alkyne reactions Jared investigated chemical reactions
Jared investigated chemical reactions