Chapter 12 Solutions Chapter 12 Test Review Section























































- Slides: 106

Chapter 12 Solutions

Chapter 12 Test – Review Section • Two AP Problems – PV = n. RT – Calculating an Empirical/Molecular Formula – Graham’s Law – Real vs. Ideal Gas – Some other simple concepts

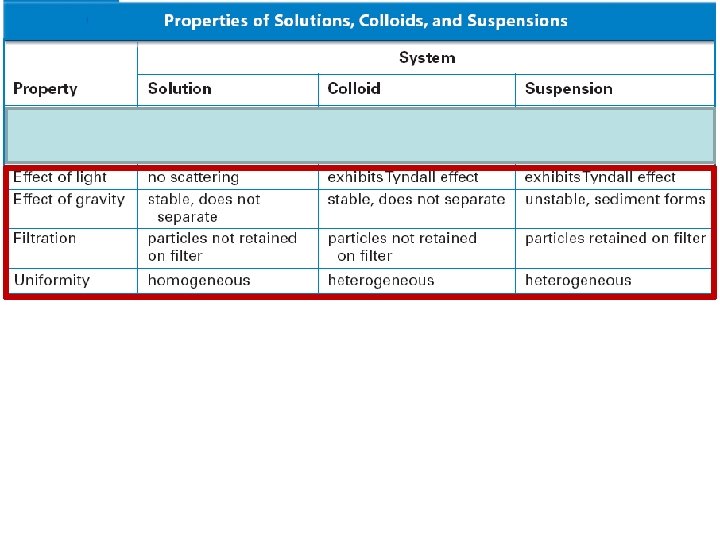
Mixtures • When materials are mixed together the distinction between heterogeneous and homogeneous depends on the size of the dispersed/dissolved particles.

15. 3 Suspensions • Suspensions are heterogeneous because at least two substances can be clearly identified. Suspensions can be separated by filtering.

15. 3 Suspensions • Suspensions –What is the difference between a suspension and a solution?

15. 3 Suspensions • A suspension is a heterogeneous mixture with dispersed particles much larger than the solute particles in a solution. These dispersed particles settle out upon standing.

15. 3 Colloids • Gelatin is a heterogeneous mixture called a colloid. • What distinguishes a colloid from a suspension and a solution?

15. 3 Colloids • Colloids are heterogeneous mixtures that have dispersed particles smaller than those in suspensions and larger than those in solutions. • Examples include: whipped cream, marshmallows, fog, jelly.

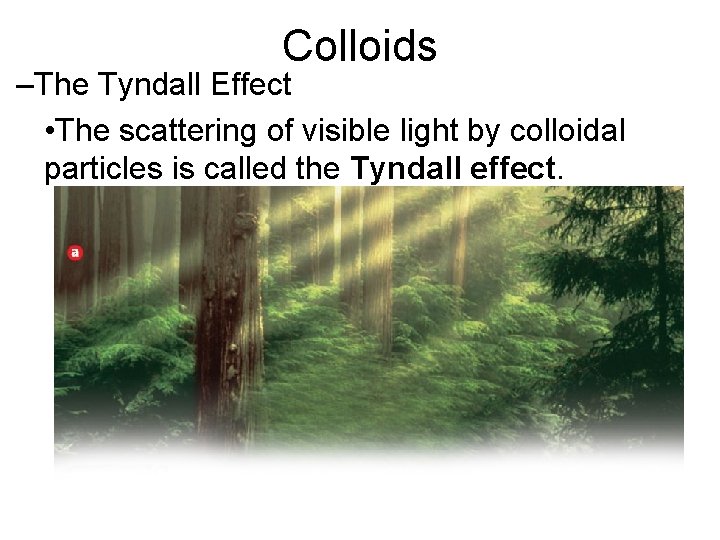
15. 3 Colloids –The Tyndall Effect • The scattering of visible light by colloidal particles is called the Tyndall effect.

15. 3 Colloids • Particles in colloids and suspensions reflect or scatter light in all directions. Solutions do not scatter light.

1

3. 8: Solutions • Solute: The substance that dissolves (the minor component of a solution). KMn. O 4

3. 8: Solutions • Solvent: The substance in which the solute dissolves (the major component of a solution).

Solution: A homogeneous mixture of the solute and solvent. KMn. O 4 solution

Solutions • Solutions come in the form of solids, liquids and gases. • Solutes/solvents can be a gas, liquid, or solid.

Solute-solvent combinations: • • oxygen in nitrogen (gas-gas) = air carbon dioxide in water (gas-liquid) = soda pop water vapor in air (liquid-gas) = humidity alcohol in water (liquid-liquid) = liquor mercury in silver (liquid-solid) = dental amalgam sugar in water (solid-liquid) = kool-aid tin in copper (solid-solid) = bronze alloy How many other combinations are possible?

Other solute – solvent combinations • (solid-gas) = Mothballs sublime in air • (gas-solid) = Hydrogen dissolves rather well in metals; an example is hydrogen in palladium which is used as a gas stove lighter.

Properties of Solutions • In solutions (homogeneous mixtures) the macroscopic properties do not vary throughout the sample. • This is in contrast to heterogeneous mixtures in which the macroscopic properties vary from one part of the mixture to another.

Heterogeneous Mixture of Copper and Zinc

Pennies are a Heterogeneous Mixture of Copper and Zinc • For many years pennies were 95 percent copper and 5 percent zinc until 1982, when the composition was changed to 97. 5 percent zinc and 2. 5 percent copper.

Brass is a solution of Copper and Zinc.

3. 8: Solutions Dilute Concentrated

Solubility • The solubility of a solute is the quantity that will dissolve in a given amount of solvent to produce a saturated solution. • Expressed as grams of solute in 100 grams of solvent, usually water. g of solute 100 g water

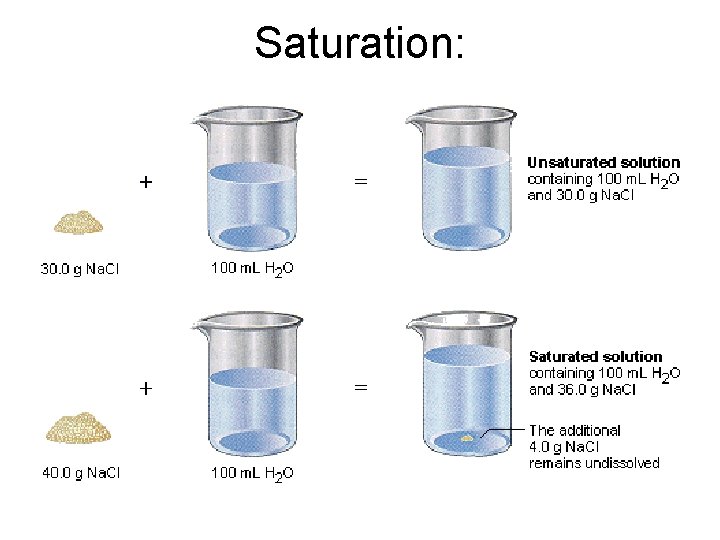
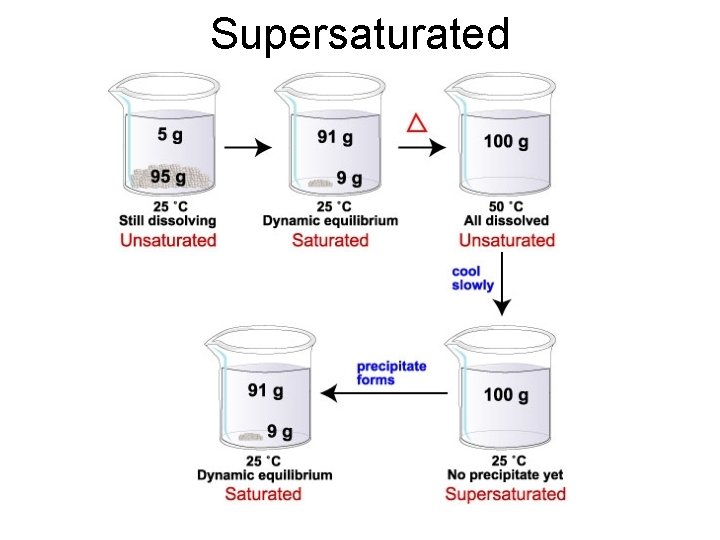
Unsaturated Solutions • Contains less than the maximum amount of solute. • No undissolved solute present. • Can dissolve more solute. Dissolved solute

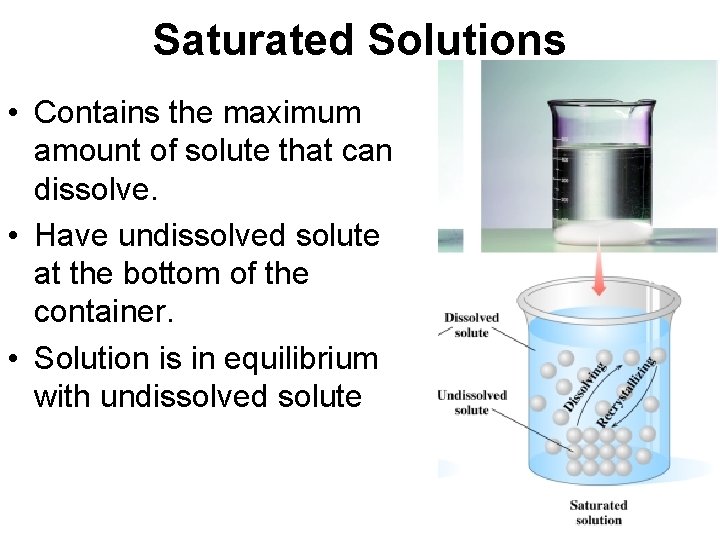
Saturated Solutions • Contains the maximum amount of solute that can dissolve. • Have undissolved solute at the bottom of the container. • Solution is in equilibrium with undissolved solute

Saturation:

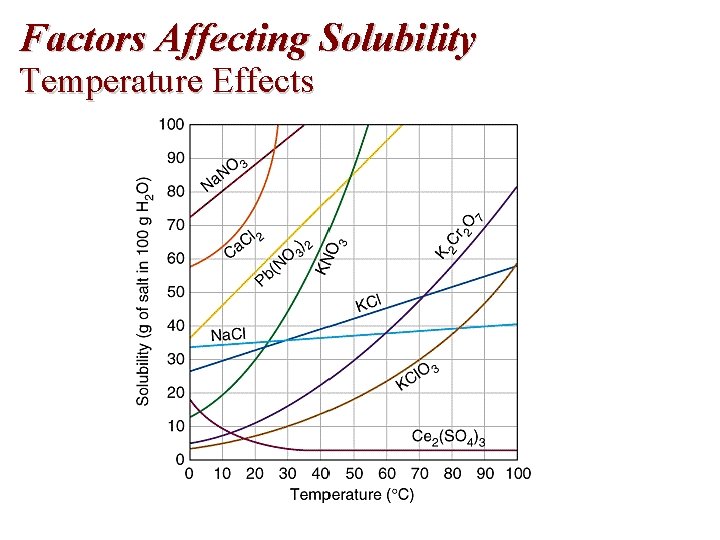
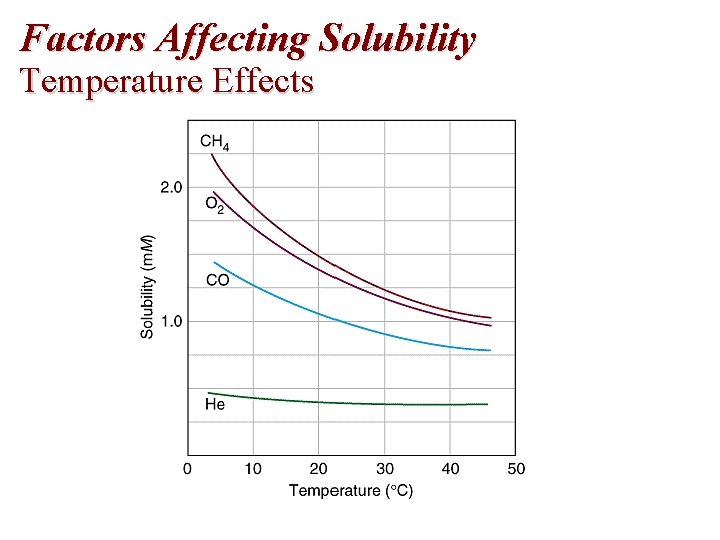
Factors Affecting Solubility Temperature Effects

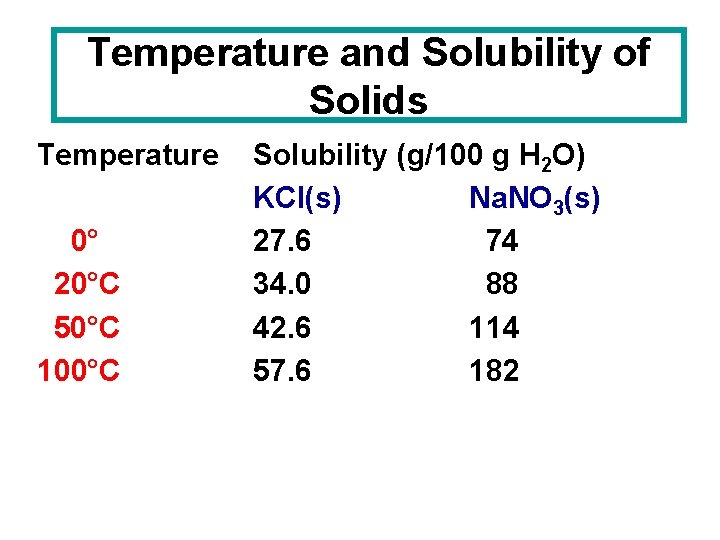
Temperature and Solubility of Solids Temperature 0° 20°C 50°C 100°C Solubility (g/100 g H 2 O) KCl(s) Na. NO 3(s) 27. 6 74 34. 0 88 42. 6 114 57. 6 182

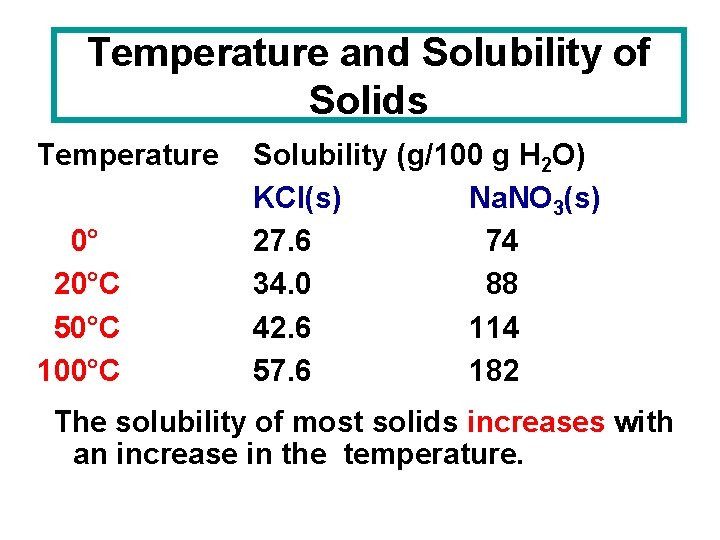
Temperature and Solubility of Solids Temperature 0° 20°C 50°C 100°C Solubility (g/100 g H 2 O) KCl(s) Na. NO 3(s) 27. 6 74 34. 0 88 42. 6 114 57. 6 182 The solubility of most solids increases with an increase in the temperature.

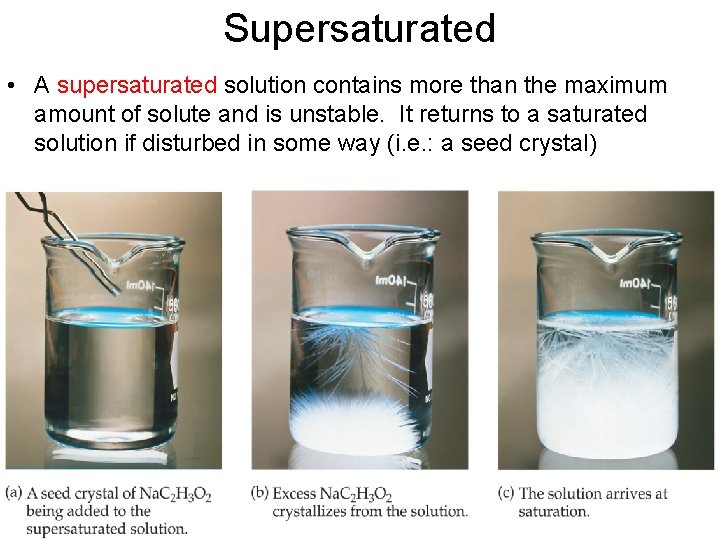
Supersaturated • A supersaturated solution contains more than the maximum amount of solute and is unstable. It returns to a saturated solution if disturbed in some way (i. e. : a seed crystal)

Supersaturated

Factors Affecting Solubility Temperature Effects

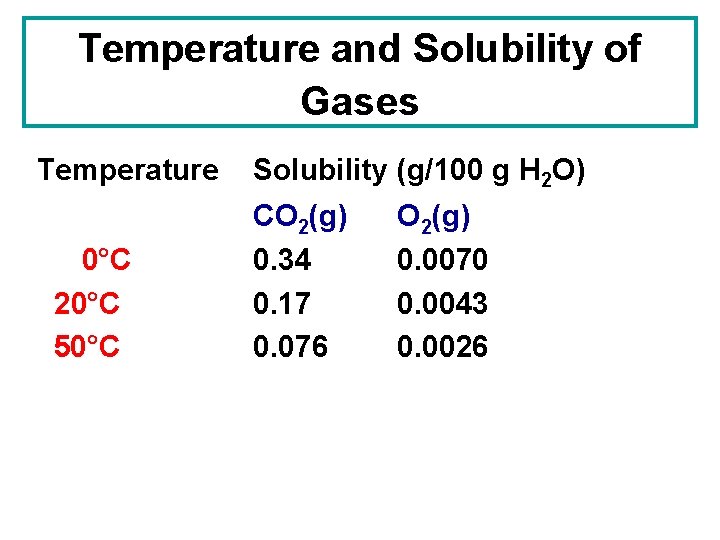
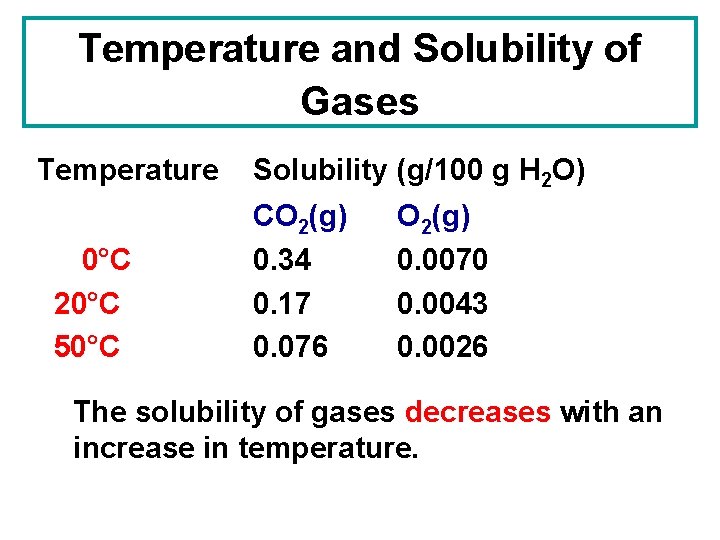
Temperature and Solubility of Gases Temperature 0°C 20°C 50°C Solubility (g/100 g H 2 O) CO 2(g) 0. 34 0. 17 0. 076 O 2(g) 0. 0070 0. 0043 0. 0026

Temperature and Solubility of Gases Temperature 0°C 20°C 50°C Solubility (g/100 g H 2 O) CO 2(g) 0. 34 0. 17 0. 076 O 2(g) 0. 0070 0. 0043 0. 0026 The solubility of gases decreases with an increase in temperature.

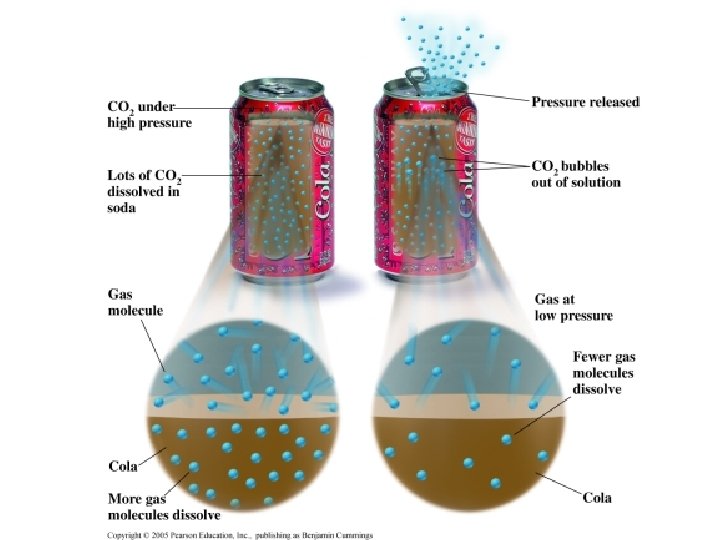
Factors That Affect Solubility • Pressure – If we increase the pressure on a liquid/gas solution, we will increase the solubility of the gas into the liquid solvent • Example: soda pop


What conditions affect solubility?

What conditions affect solubility? • For solids in liquids as the temperature goes up the solubility goes up.

What conditions affect solubility? • For solids in liquids as the temperature goes up the solubility goes up. • For gases in a liquid as the temperature goes up the solubility goes down.

What conditions affect solubility? • For solids in liquids as the temperature goes up the solubility goes up. • For gases in a liquid as the temperature goes up the solubility goes down. • For gases in a liquid- as the pressure goes up the solubility goes up.

GENERAL PROPERTIES OF SOLUTIONS

GENERAL PROPERTIES OF SOLUTIONS 1. A solution is a homogeneous.

GENERAL PROPERTIES OF SOLUTIONS 1. A solution is a homogeneous. 2. The solute remains uniformly distributed throughout the solution and will not settle out over time.

GENERAL PROPERTIES OF SOLUTIONS 1. A solution is a homogeneous. 2. The solute remains uniformly distributed throughout the solution and will not settle out over time. 3. The solute is dissolved as molecules or ions.

GENERAL PROPERTIES OF SOLUTIONS 1. A solution is a homogeneous. 2. The solute remains uniformly distributed throughout the solution and will not settle out over time. 3. The solute is dissolved as molecules or ions. 4. It has variable composition within limits of its solubility.

The solution process involves changes in energy among the attractive forces within the solute and solvent. i

Generally solution formation increases the amount of disorder (entropy) • The dissolving of many salts such as Na. Cl in water is just one example of a change that occurs spontaneously even though it is endothermic. • Na. Cl dissolves as a result of the drive towards increased entropy.

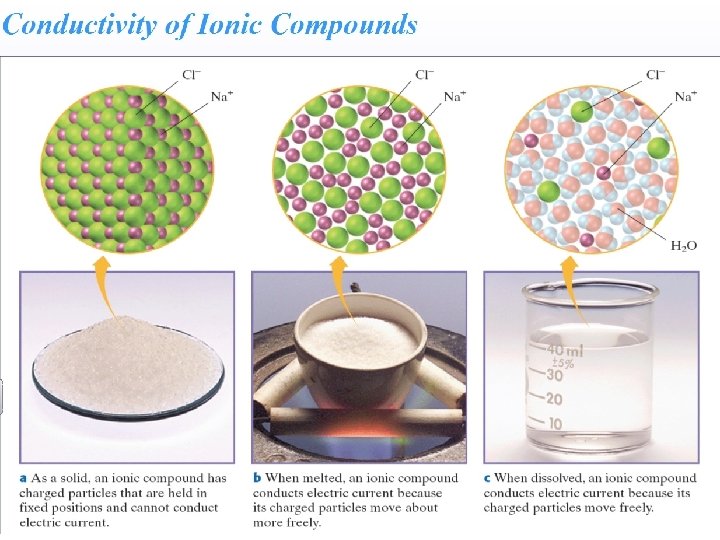
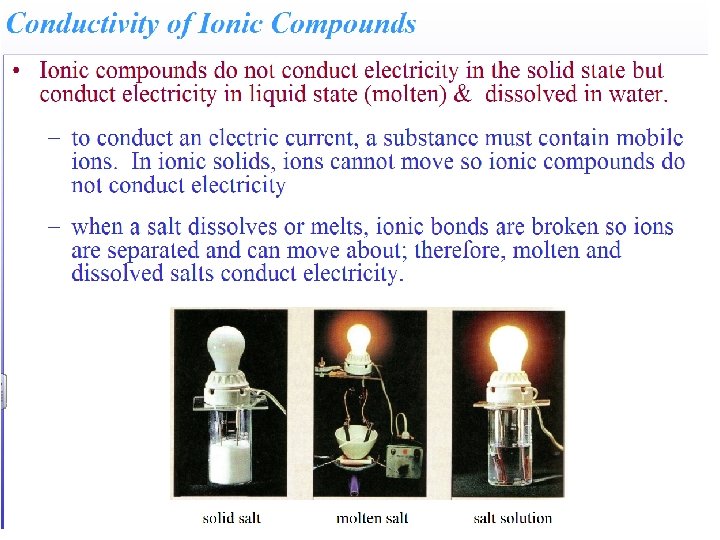
Strong Electrolytes • Strong electrolytes are either strong acids, strong bases or soluble salts. • Strong electrolytes dissociate completely into ions when they dissolve in water.

Strong Electrolytes • Generally when an ionic compound is dissolved in water the ions are separated and dispersed.

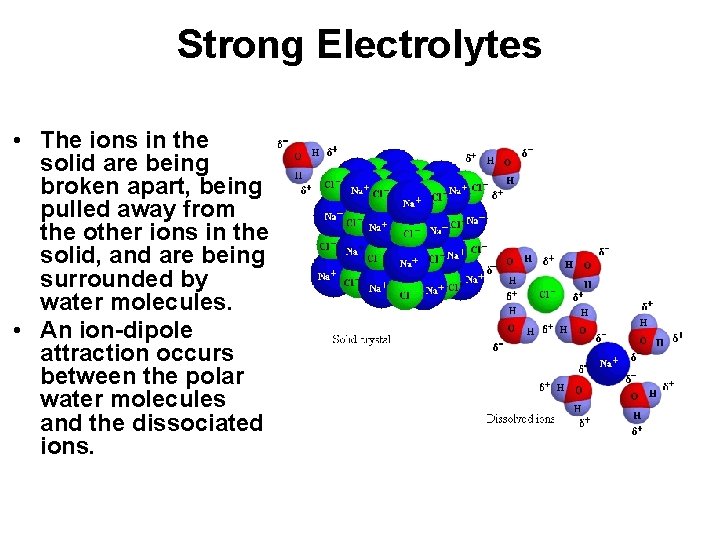
Strong Electrolytes • The ions in the solid are being broken apart, being pulled away from the other ions in the solid, and are being surrounded by water molecules. • An ion-dipole attraction occurs between the polar water molecules and the dissociated ions.

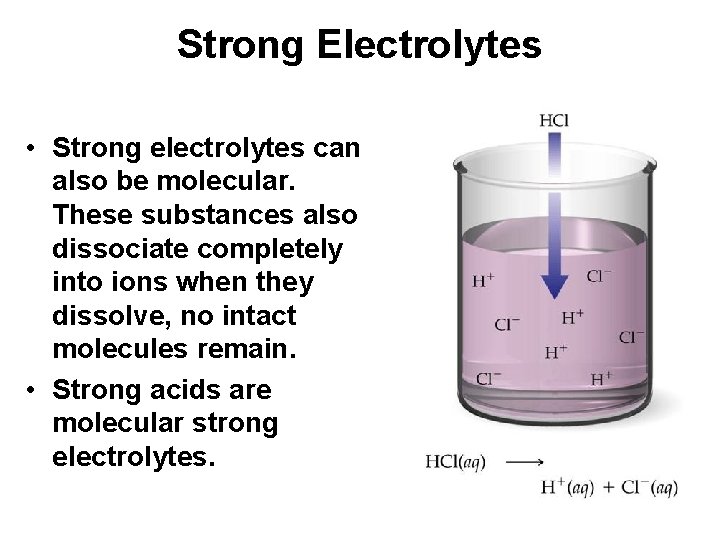
Strong Electrolytes • Strong electrolytes can also be molecular. These substances also dissociate completely into ions when they dissolve, no intact molecules remain. • Strong acids are molecular strong electrolytes.

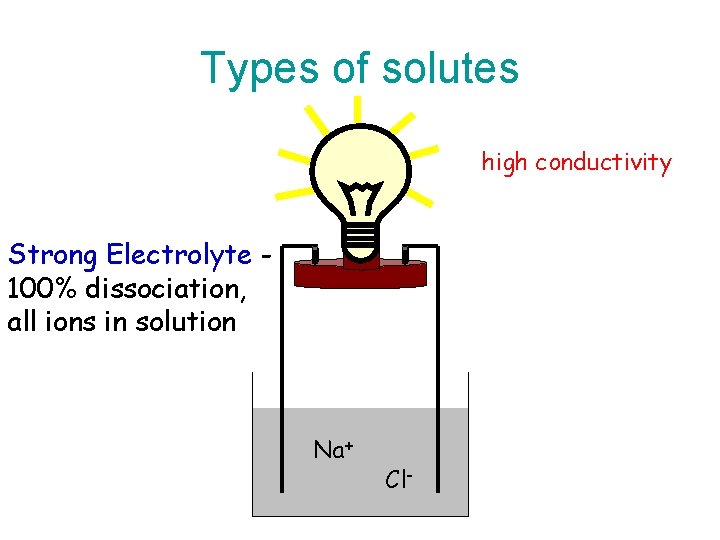
Strong Electrolytes • Solutions of strong electrolytes conduct electricity well. • This is because dissociated ions are free to move and can therefore be made to create a difference in potential.

Types of solutes high conductivity Strong Electrolyte 100% dissociation, all ions in solution Na+ Cl-

What is another way that ions can allowed freedom of movement?



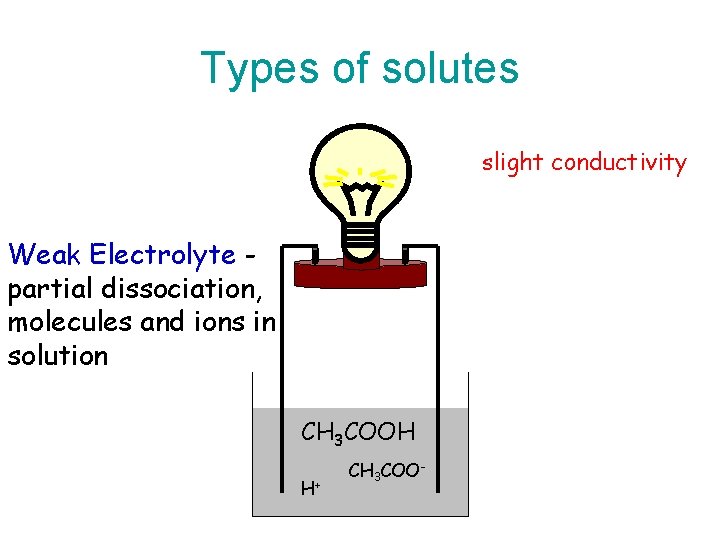
Weak Electrolytes • A weak electrolyte is a compound that when dissolved in water only partially ionizes (partially dissociates into ions). • The compound exists in water as a mixture of individual ions and intact molecules. • This solution conducts electricity weakly. • Therefore the term weak electrolyte is applied to this type of compound.

Types of solutes slight conductivity Weak Electrolyte partial dissociation, molecules and ions in solution CH 3 COOH H+ CH 3 COO-

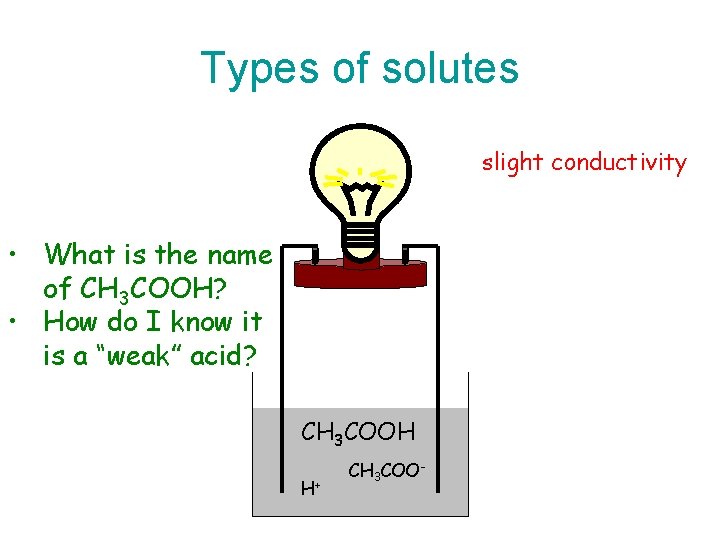
Types of solutes slight conductivity • What is the name of CH 3 COOH? • How do I know it is a “weak” acid? CH 3 COOH H+ CH 3 COO-

Weak Electrolytes • Weak electrolytes include weak acids and weak bases. • There will be dipole-dipole, or possibly even hydrogen bonding, between the solute molecules and the water. • For the few molecules which do dissociate into ions, you will have iondipole attractions between the dissociated ions and water molecules.

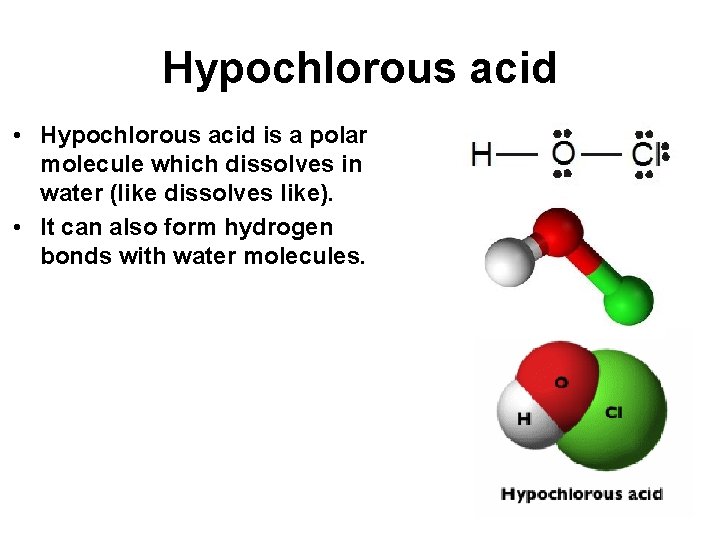
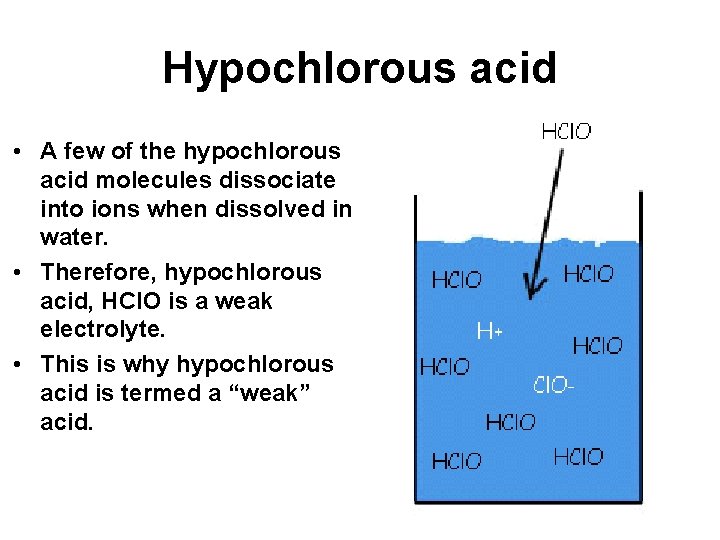
Hypochlorous acid • Hypochlorous acid is a polar molecule which dissolves in water (like dissolves like). • It can also form hydrogen bonds with water molecules.

Hypochlorous acid • A few of the hypochlorous acid molecules dissociate into ions when dissolved in water. • Therefore, hypochlorous acid, HCl. O is a weak electrolyte. • This is why hypochlorous acid is termed a “weak” acid.

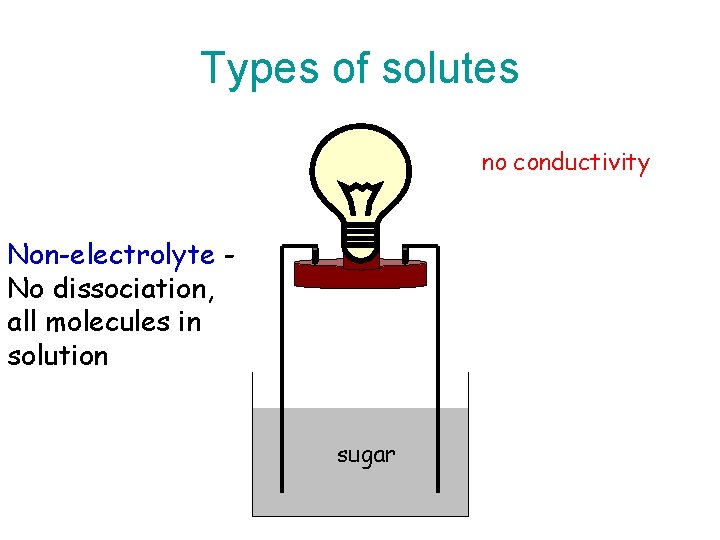
Nonelectrolytes • A nonelectrolyte is a compound that when dissolved in water does not ionize or dissociate into ions at all. • In water, this compound exists entirely as intact molecules.

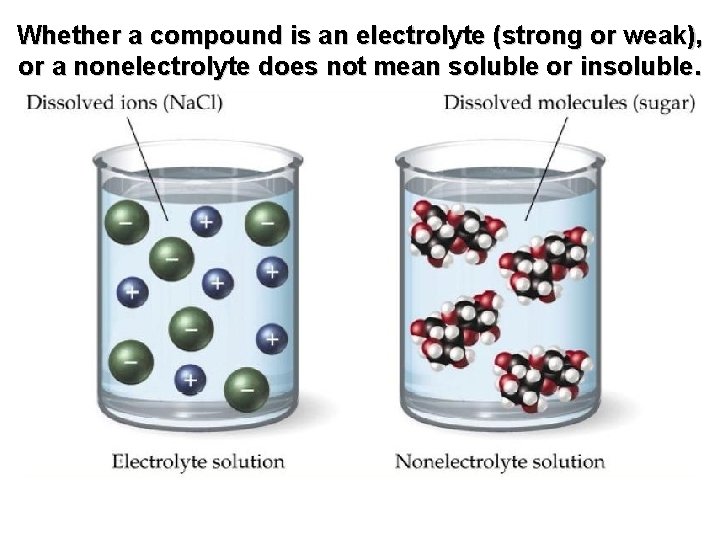
Whether a compound is an electrolyte (strong or weak), or a nonelectrolyte does not mean soluble or insoluble.

Nonelectrolytes • Solutions of nonelectrolytes do not conduct electricity at all.

Types of solutes no conductivity Non-electrolyte No dissociation, all molecules in solution sugar

Nonelectrolytes • Most nonelectrolytes are molecular compounds other than the acids. • Examples: alcohol, sugar.

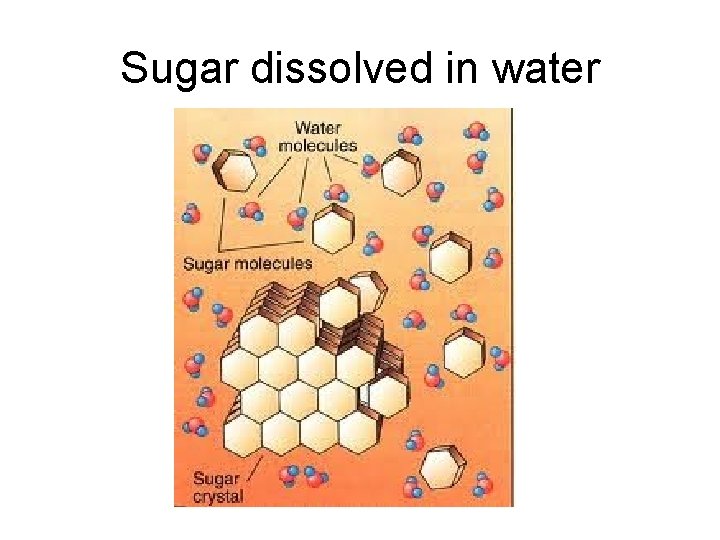
Sugar dissolved in water

Sugar dissolved in water • Hydrogen bonding between the sugar molecules and water molecules causes sugar to dissolve. • The negative end of water (oxygen end) is attracted to the hydrogen atoms in the “OH” groups within the sugar molecules. • The positive end of water (hydrogen end) is attracted to the oxygen atoms in the “OH” groups within the sugar molecules. • Sugar dissolves in water but does not dissociate into any ions. It is therefore a nonelectrolyte.

Video: Electrolytes

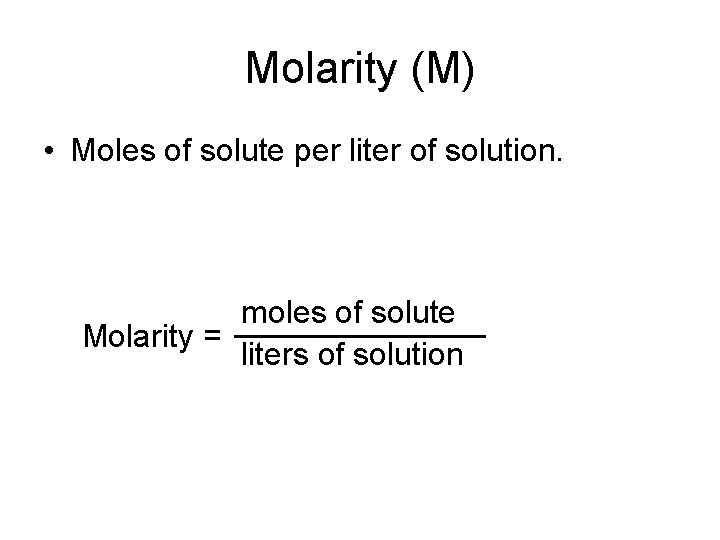
Molarity (M) • Moles of solute per liter of solution. moles of solute Molarity = liters of solution

What is the molarity of a solution prepared by dissolving 10. 0 g of Na. Cl in 250. 0 m. L of solution? 0. 684 M

How would you prepare 500. ml of a 6. 0 M Na. OH solution?

How would you prepare 500. ml of a 6. 0 M Na. OH solution? • Would it be sufficent to say that I need to use 120 g of Na. OH? • This does not answer the question. • The question was not how much Na. OH do I need prepare the solution.

How would you prepare 500. ml of a 6. 0 M Na. OH solution? • Measure out 120 g of Na. OH on a balance. 120 g

How would you prepare 500. ml of a 6. 0 M Na. OH solution? • Add the 120 g of Na. OH to a 500. ml volumetric flask.

How would you prepare 500. ml of a 6. 0 M Na. OH solution? • Add distilled water and swirl the flask to dissolve the solid.

How would you prepare 500. ml of a 6. 0 M Na. OH solution? • Add distilled to the 500. ml line on the volumetric flask.

How would you prepare 500. ml of a 6. 0 M Na. OH solution? • Cap and then invert the flask several times to thoroughly mix the solution.

I need to make 250 ml of a 0. 50 M Na. OH solution using the 6. 0 M Na. OH prepared in the previous example. How do I prepare this solution? This is a dilution problem

Dilution Problems

I need to make 250 ml of a 0. 50 M Na. OH solution using the 6. 0 M Na. OH prepared in the previous example. How do I prepare this solution? • Measure out 21 ml of the 6. 0 M Na. OH in a graduated cylinder or pipet.

I need to make 250 ml of a 0. 50 M Na. OH solution using the 6. 0 M Na. OH prepared in the previous example. How do I prepare this solution? • Transfer to a 250. ml volumetric flask and add water to the 250. ml mark.

I need to make 250 ml of a 0. 50 M Na. OH solution using the 6. 0 M Na. OH prepared in the previous example. How do I prepare this solution? • Cap and then invert the flask several times to thoroughly mix the solution.

0. 850 ml of a 5. 00 M Cu(NO 3)2 solution is diluted to a total volume of 1. 80 L. What is the molarity of the resulting diluted solution? 0. 00236 M

How many milliliters of water are needed to dilute 12 ml of a 0. 44 M acetic acid solution to 0. 12 M? 32 ml of water added

Methods of separation of the components of a solution • Distillation • Chromatography – Paper – Column

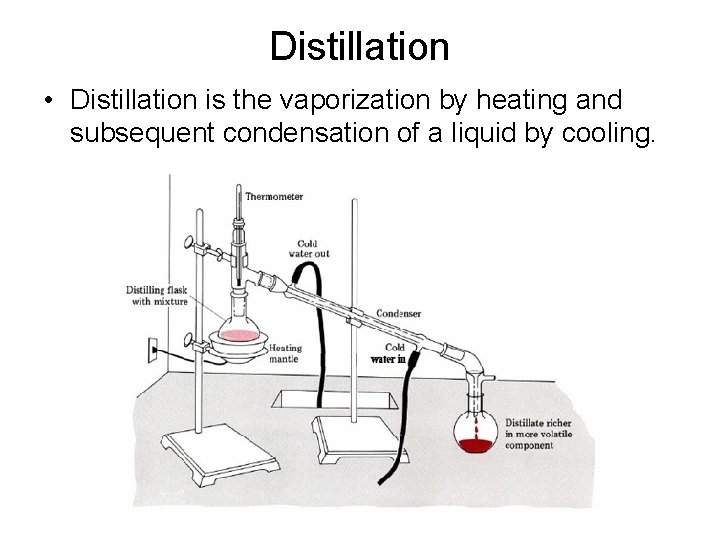
Distillation • Distillation is the vaporization by heating and subsequent condensation of a liquid by cooling.

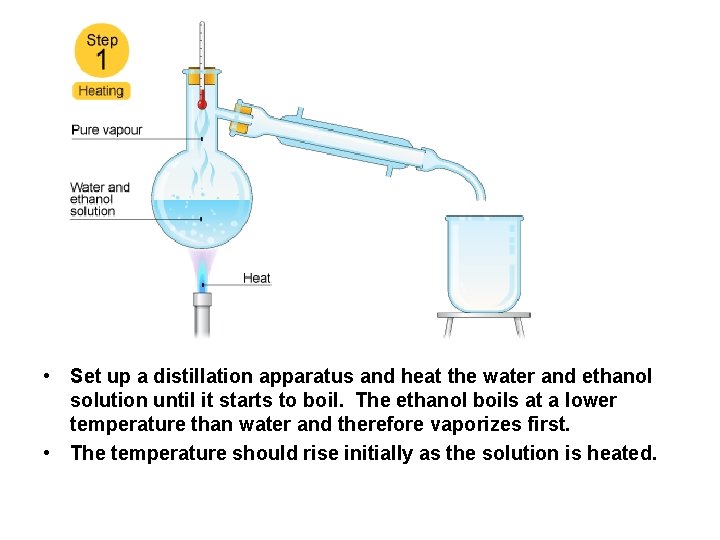
How Could a Solution of Ethanol and Water be Separated by Distillation?

• Set up a distillation apparatus and heat the water and ethanol solution until it starts to boil. The ethanol boils at a lower temperature than water and therefore vaporizes first. • The temperature should rise initially as the solution is heated.

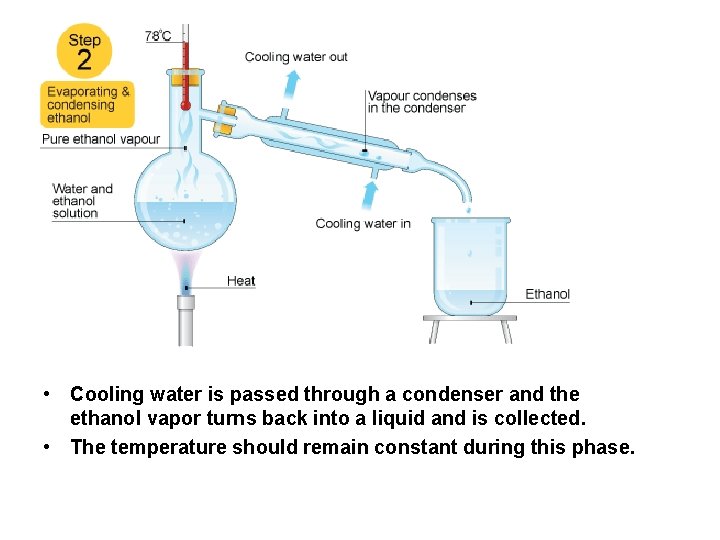
• Cooling water is passed through a condenser and the ethanol vapor turns back into a liquid and is collected. • The temperature should remain constant during this phase.

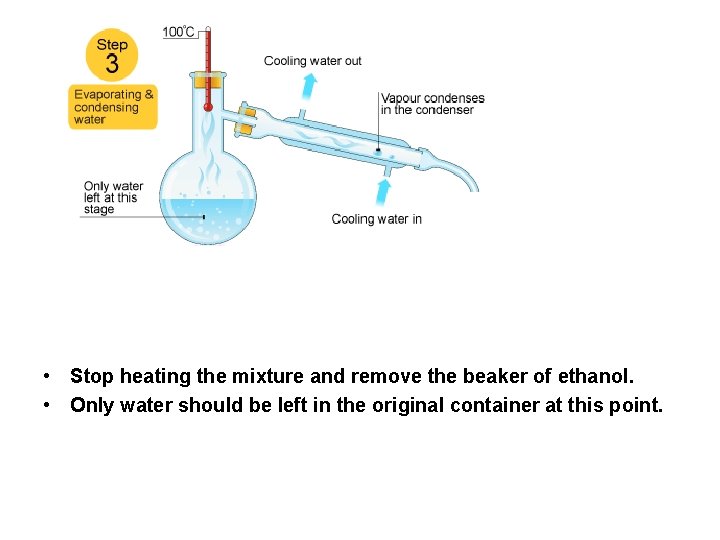
. . • Stop heating the mixture and remove the beaker of ethanol. • Only water should be left in the original container at this point.

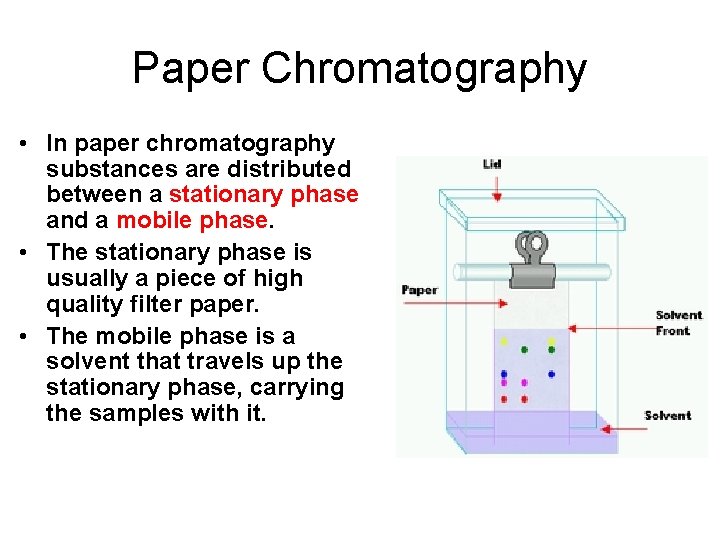
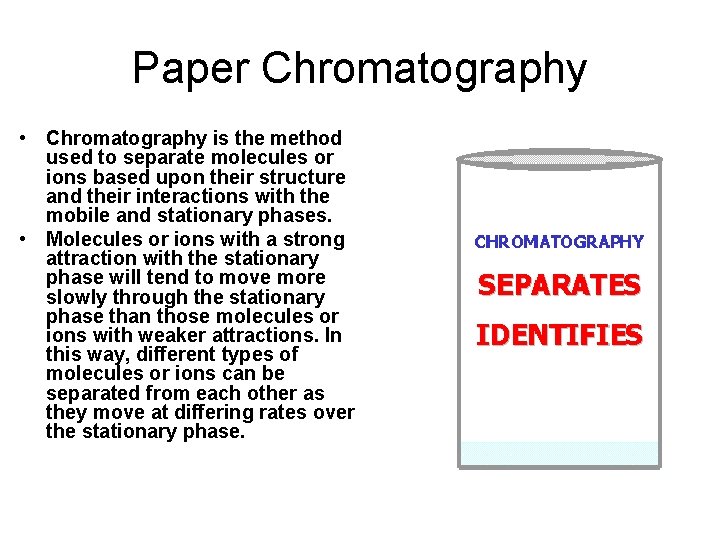
Paper Chromatography • In paper chromatography substances are distributed between a stationary phase and a mobile phase. • The stationary phase is usually a piece of high quality filter paper. • The mobile phase is a solvent that travels up the stationary phase, carrying the samples with it.

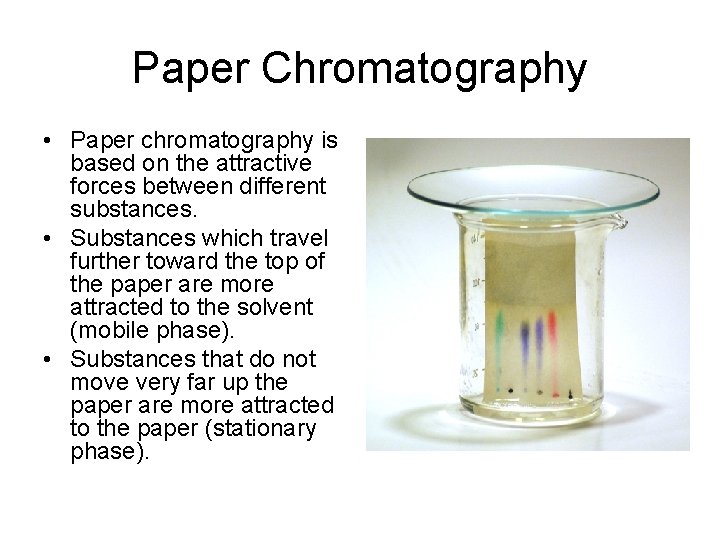
Paper Chromatography • Paper chromatography is based on the attractive forces between different substances. • Substances which travel further toward the top of the paper are more attracted to the solvent (mobile phase). • Substances that do not move very far up the paper are more attracted to the paper (stationary phase).

Paper Chromatography • Chromatography is the method used to separate molecules or ions based upon their structure and their interactions with the mobile and stationary phases. • Molecules or ions with a strong attraction with the stationary phase will tend to move more slowly through the stationary phase than those molecules or ions with weaker attractions. In this way, different types of molecules or ions can be separated from each other as they move at differing rates over the stationary phase.

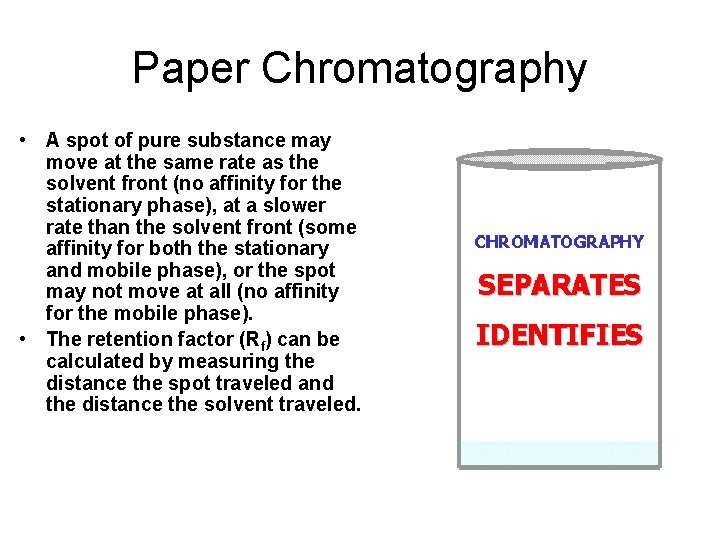
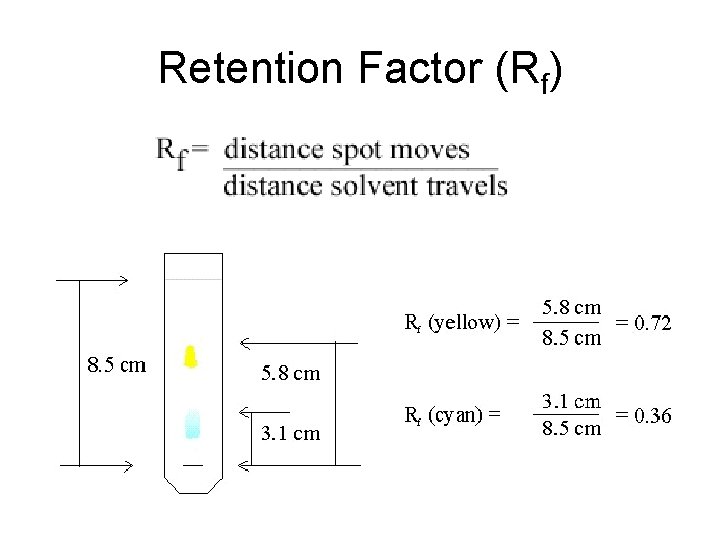
Paper Chromatography • A spot of pure substance may move at the same rate as the solvent front (no affinity for the stationary phase), at a slower rate than the solvent front (some affinity for both the stationary and mobile phase), or the spot may not move at all (no affinity for the mobile phase). • The retention factor (Rf) can be calculated by measuring the distance the spot traveled and the distance the solvent traveled.

Retention Factor (Rf)

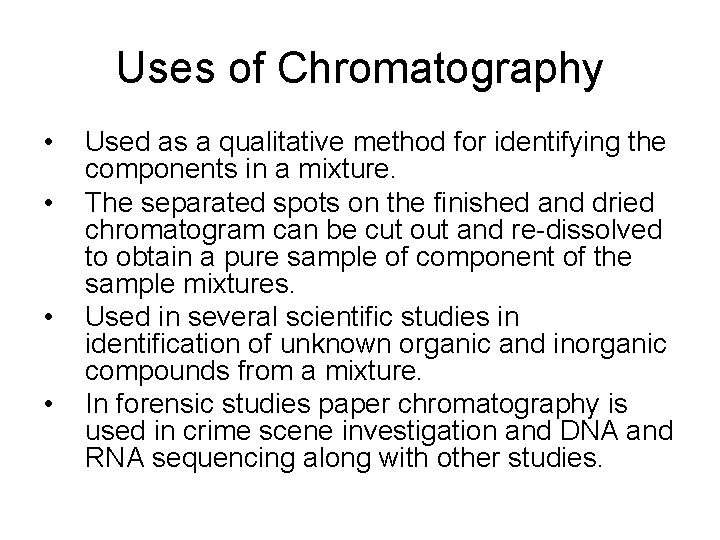
Uses of Chromatography • • Used as a qualitative method for identifying the components in a mixture. The separated spots on the finished and dried chromatogram can be cut out and re-dissolved to obtain a pure sample of component of the sample mixtures. Used in several scientific studies in identification of unknown organic and inorganic compounds from a mixture. In forensic studies paper chromatography is used in crime scene investigation and DNA and RNA sequencing along with other studies.

Some Forensic Uses of Chromatography • Drugs from narcotics to aspirin can be identified in urine and blood by using chromatography. • Detectives can analyze a sample of paint to find out the car it came from, including the model and year it was made. • Ink can be separated into its components to help identify the pen used for ransom notes and forgeries, as well as the type of ink used for counterfeiting.

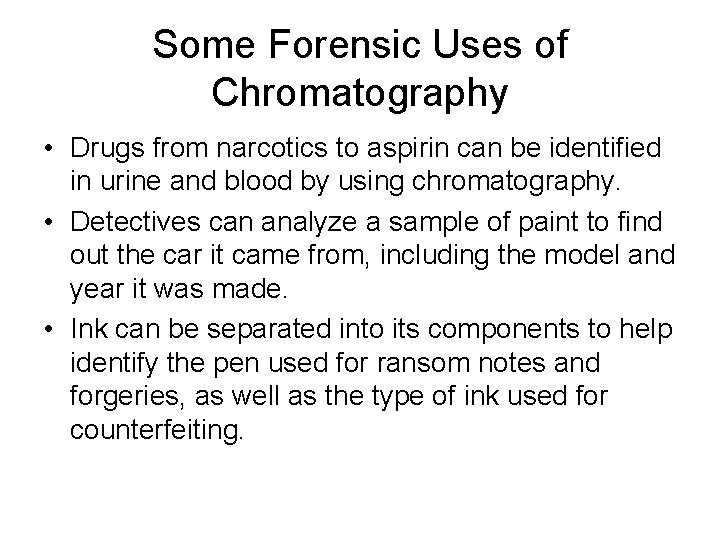
Paper Chromatography Example • Suppose you have three blue pens and you want to find out which one was used to write a message.

Paper Chromatography Example • Samples of each ink are spotted on a pencil line drawn on a sheet of chromatography paper. • Some of the ink from the message is also spotted onto the same line. • In the diagram, the pens are labeled 1, 2 and 3, and the message ink as M.

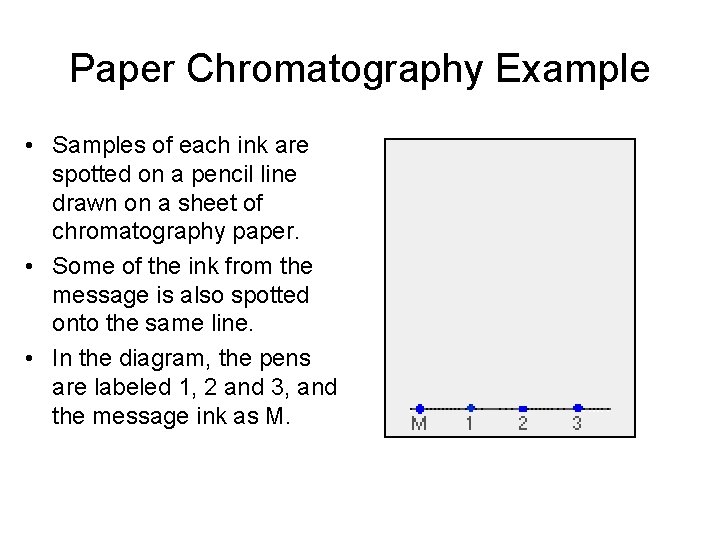
Paper Chromatography Example • The paper is suspended in a container with a shallow layer of a “suitable” solvent or a mixture of solvents in it. • It is important that the solvent level is below the line with the spots on it. • The container is covered to make sure that the solvent does not completely evaporate away.

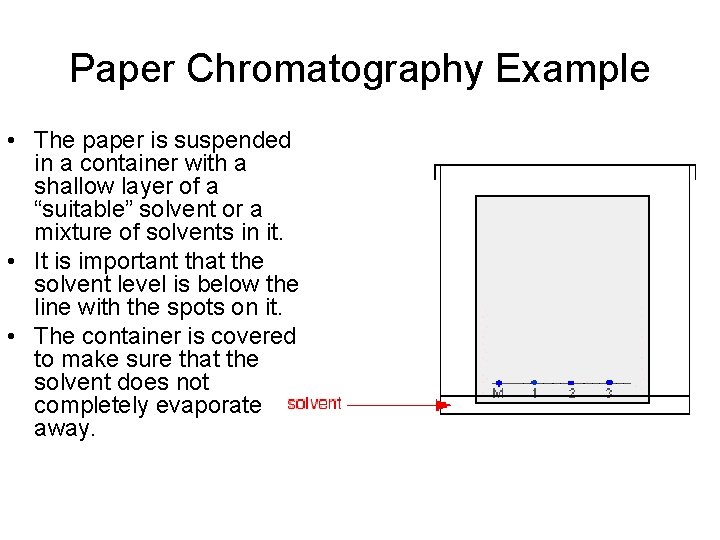
Paper Chromatography Example • As the solvent slowly travels up the paper, the different components of the ink mixtures travel at different rates and the mixtures are separated into different colored spots. • The pen that wrote the message contained the same dyes as pen 2. You can also see that pen 1 contains a mixture of two different blue dyes - one of which might be the same as the single dye in pen 3.

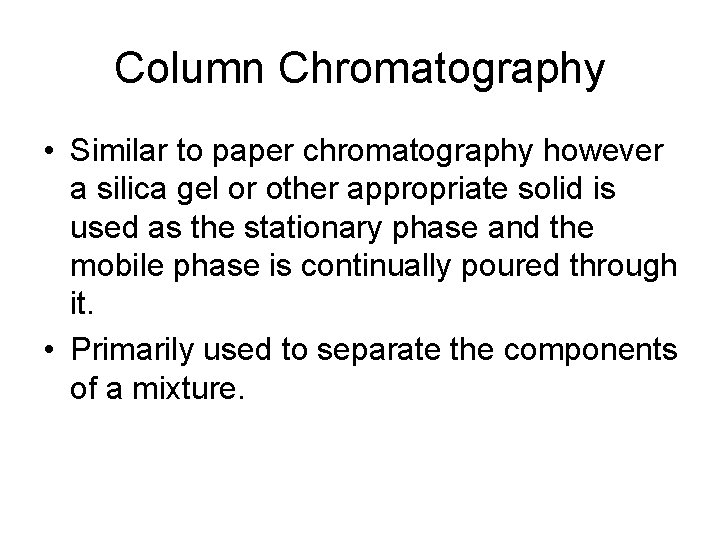
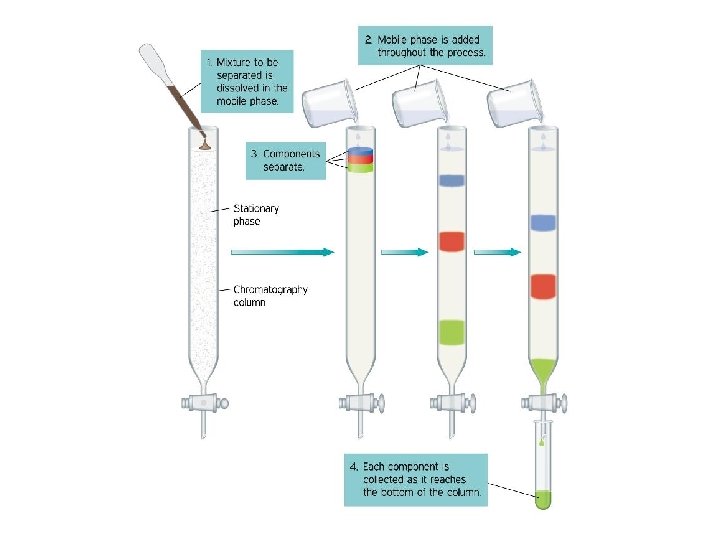
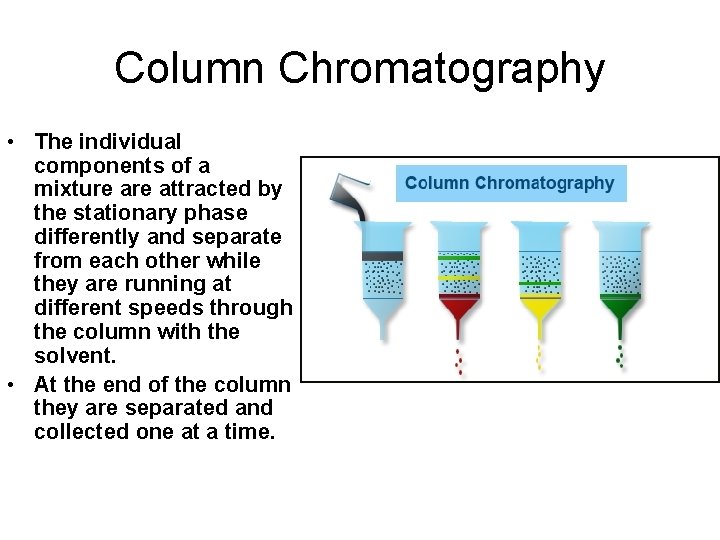
Column Chromatography • Similar to paper chromatography however a silica gel or other appropriate solid is used as the stationary phase and the mobile phase is continually poured through it. • Primarily used to separate the components of a mixture.


Column Chromatography • The individual components of a mixture attracted by the stationary phase differently and separate from each other while they are running at different speeds through the column with the solvent. • At the end of the column they are separated and collected one at a time.