Chemistry Ch 8 Test REVIEW SHEET 1 In







- Slides: 14

Chemistry Ch. 8 Test REVIEW SHEET

1. In the reaction described by the word equation sodium + water → sodium hydroxide + hydrogen gas What are the reactants? SODIUM AND WATER What are the products? SODIUM HYDROXIDE AND HYDROGEN GAS

2. What does it mean to say that a chemical equation is balanced? THE NUMBERS OF ATOMS OF EACH ELEMENT ARE THE SAME ON BOTH SIDES OF THE EQUATION. 3. What are three things that you can obtain from a complete and correctly written chemical equation? Physical states of the reactants and products. Relative amounts of the reactants and products. Chemical formulas of the reactants and products.

4. To balance a chemical equation, you must adjust the _______s in front of each compound. COEFFICIENTS 5. The formation of a solid product in a chemical reaction is represented by what symbol? (S)

6. How does the law of conservation of mass relate to chemical equations? THE NUMBERS OF ATOMS OF EACH ELEMENT ARE THE SAME ON BOTH SIDES OF THE EQUATION. 7. Write the balanced equation for the reaction between iron and oxygen. 2 Fe + O 2 2 Fe. O

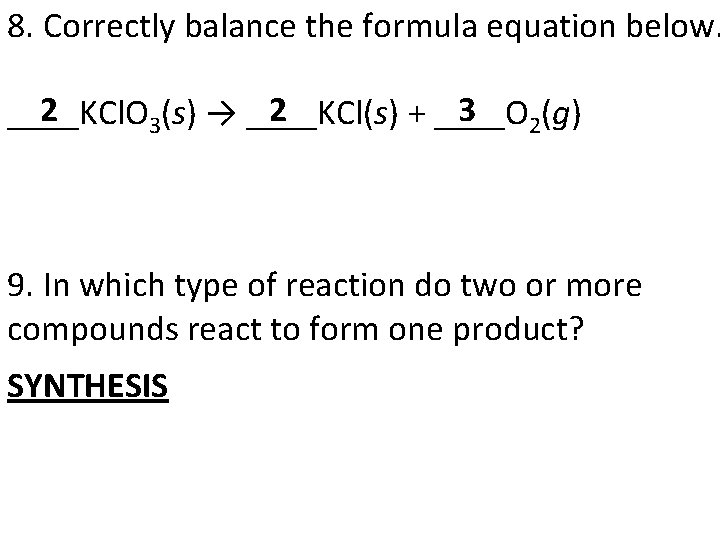
8. Correctly balance the formula equation below. 2 3 ____KCl. O 3(s) → ____KCl(s) + ____O 2(g) 9. In which type of reaction do two or more compounds react to form one product? SYNTHESIS

10. Explain how you would recognize a single-displacement reaction? ONE ELEMENT REPLACES ANOTHER IN A COMPOUND. 11. A mixture of propane and oxygen react to form carbon dioxide and water. What type of chemical reaction is this? COMBUSTION

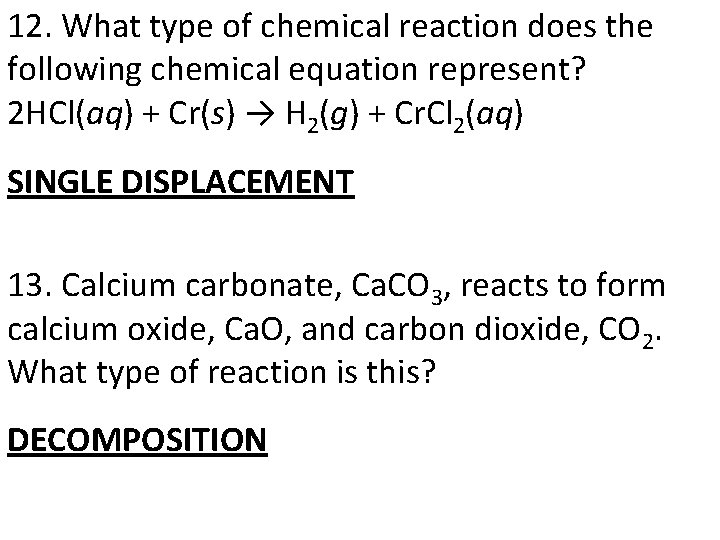
12. What type of chemical reaction does the following chemical equation represent? 2 HCl(aq) + Cr(s) → H 2(g) + Cr. Cl 2(aq) SINGLE DISPLACEMENT 13. Calcium carbonate, Ca. CO 3, reacts to form calcium oxide, Ca. O, and carbon dioxide, CO 2. What type of reaction is this? DECOMPOSITION

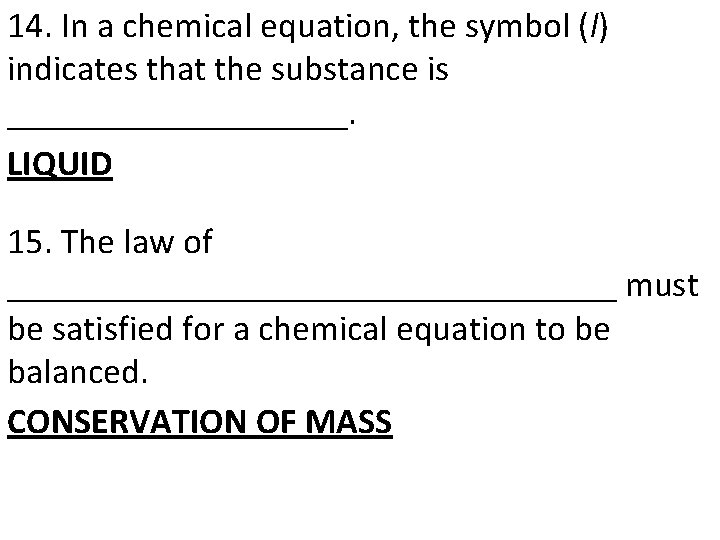
14. In a chemical equation, the symbol (l) indicates that the substance is __________. LIQUID 15. The law of _________________ must be satisfied for a chemical equation to be balanced. CONSERVATION OF MASS

Write a word equation for each chemical reaction in the space provided. 16. 2 Zn. S(s) + 3 O 2(g) → 2 Zn. O(s) + 2 SO 2(g) ZINC SULFIDE REACTS WITH OXYGEN TO CREATE ZINC OXIDE AND SULFUR DIOXIDE

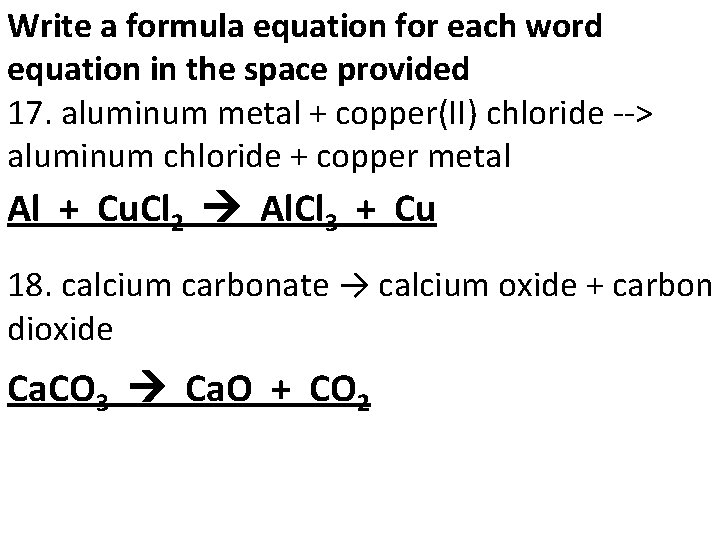
Write a formula equation for each word equation in the space provided 17. aluminum metal + copper(II) chloride --> aluminum chloride + copper metal Al + Cu. Cl Al. Cl + Cu 2 3 18. calcium carbonate → calcium oxide + carbon dioxide Ca. CO 3 Ca. O + CO 2

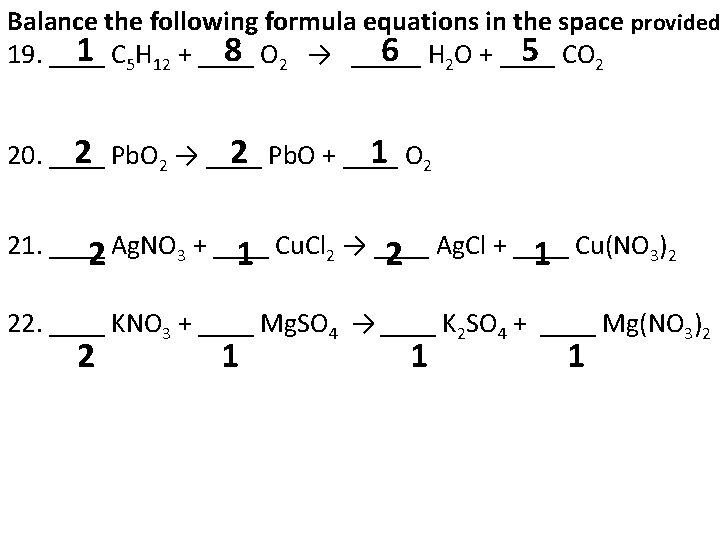
Balance the following formula equations in the space provided 1 8 6 5 19. ____ C 5 H 12 + ____ O 2 → _____ H 2 O + ____ CO 2 2 1 20. ____ Pb. O 2 → ____ Pb. O + ____ O 2 21. ____ Ag. NO 3 + ____ Cu. Cl 2 → ____ Ag. Cl + ____ Cu(NO 3)2 2 1 2 1 22. ____ KNO 3 + ____ Mg. SO 4 → ____ K 2 SO 4 + ____ Mg(NO 3)2 2 1 1

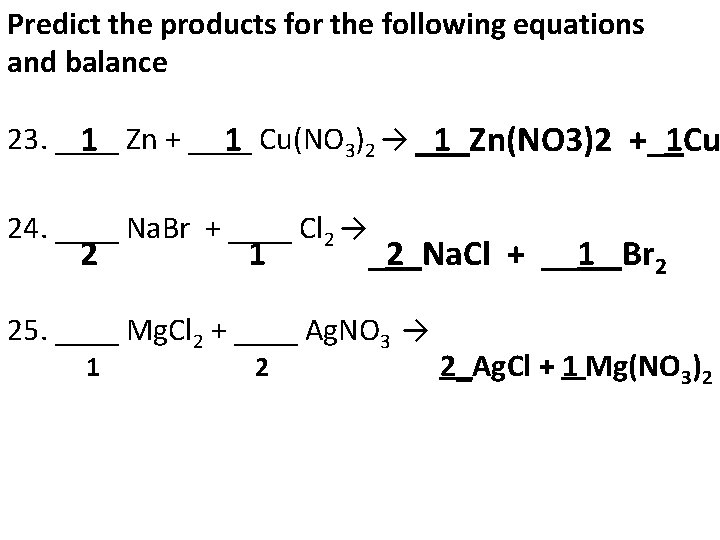
Predict the products for the following equations and balance 23. ____ Zn + ____ Cu(NO 1 1 3)2 → _1_Zn(NO 3)2 +_1 Cu 24. ____ Na. Br + ____ Cl 2 → 2 1 _2_Na. Cl + __1_ Br 2 25. ____ Mg. Cl 2 + ____ Ag. NO 3 → 1 2 2_Ag. Cl + 1 Mg(NO 3)2

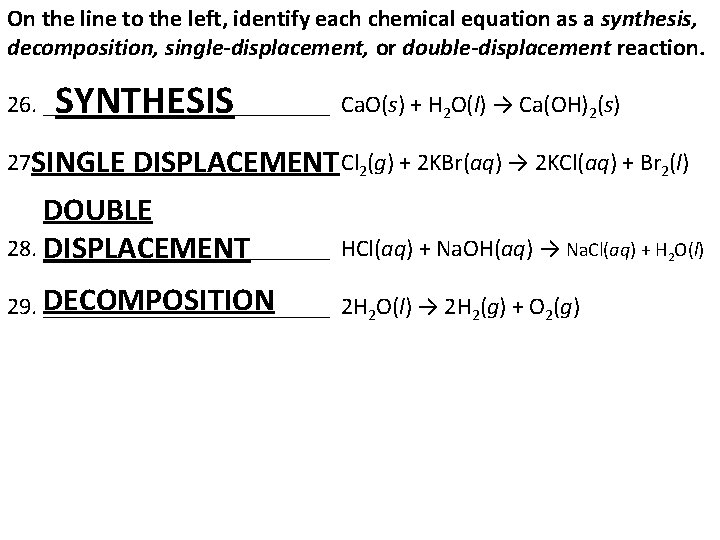
On the line to the left, identify each chemical equation as a synthesis, decomposition, single-displacement, or double-displacement reaction. 26. ____________ Ca. O(s) + H 2 O(l) → Ca(OH)2(s) 27. ____________ Cl SINGLE DISPLACEMENT 2(g) + 2 KBr(aq) → 2 KCl(aq) + Br 2(l) SYNTHESIS DOUBLE 28. ____________ HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l) DISPLACEMENT DECOMPOSITION 29. ____________ 2 H 2 O(l) → 2 H 2(g) + O 2(g)