Chapter 13 Properties of Solutions Solutions Solutions are













- Slides: 43

Chapter 13 Properties of Solutions

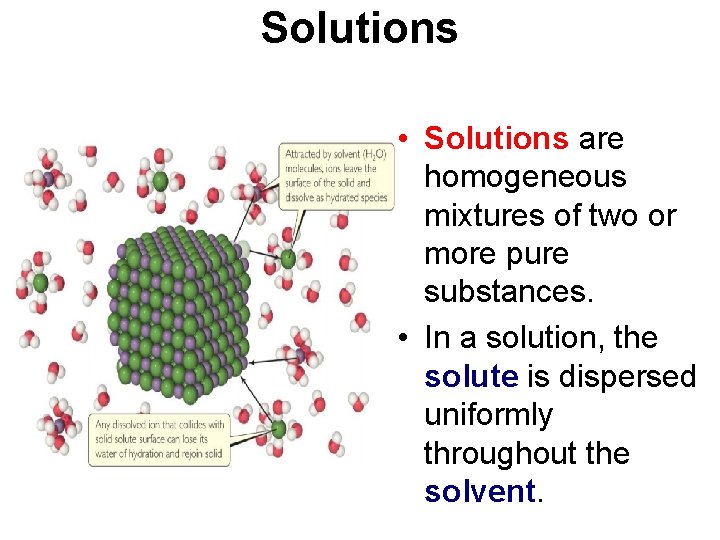
Solutions • Solutions are homogeneous mixtures of two or more pure substances. • In a solution, the solute is dispersed uniformly throughout the solvent.

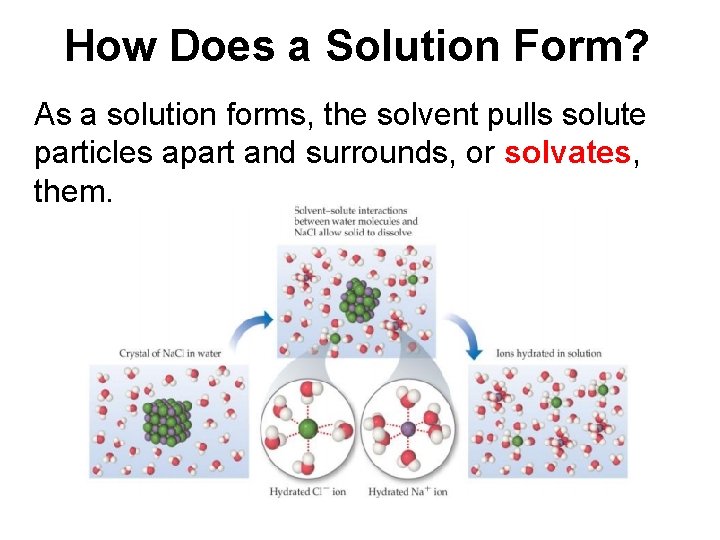
How Does a Solution Form? As a solution forms, the solvent pulls solute particles apart and surrounds, or solvates, them.

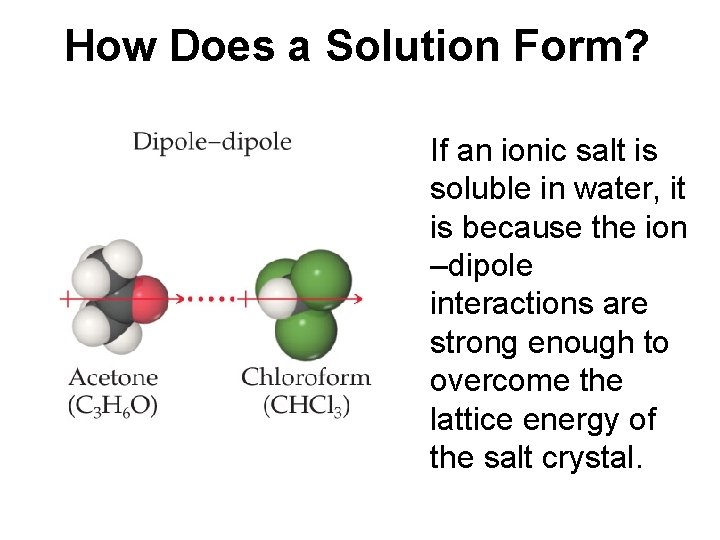
How Does a Solution Form? If an ionic salt is soluble in water, it is because the ion –dipole interactions are strong enough to overcome the lattice energy of the salt crystal.

Student, Beware! Just because a substance disappears when it comes in contact with a solvent, it doesn’t mean the substance dissolved. It may have reacted.

Student, Beware! • Dissolution is a physical change—you can get back the original solute by evaporating the solvent. • If you can’t get it back, the substance didn’t dissolve, it reacted.

Types of Solutions • Saturated – In a saturated solution, the solvent holds as much solute as is possible at that temperature. – Dissolved solute is in dynamic equilibrium with solid solute particles. • Unsaturated – If a solution is unsaturated, less solute than can dissolve in the solvent at that temperature is dissolved in the solvent. • Supersaturated – In supersaturated solutions, the solvent holds more solute than is normally possible at that temperature. – These solutions are unstable; crystallization can usually be stimulated by adding a “seed crystal” or scratching the side of the flask.

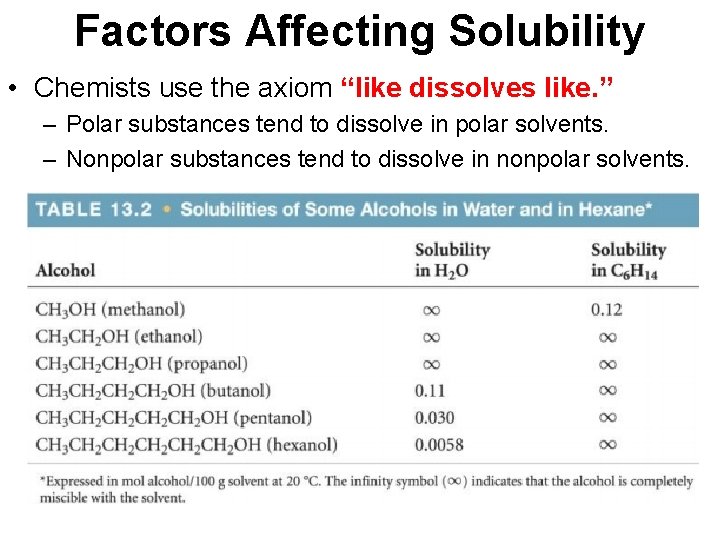
Factors Affecting Solubility • Chemists use the axiom “like dissolves like. ” – Polar substances tend to dissolve in polar solvents. – Nonpolar substances tend to dissolve in nonpolar solvents.

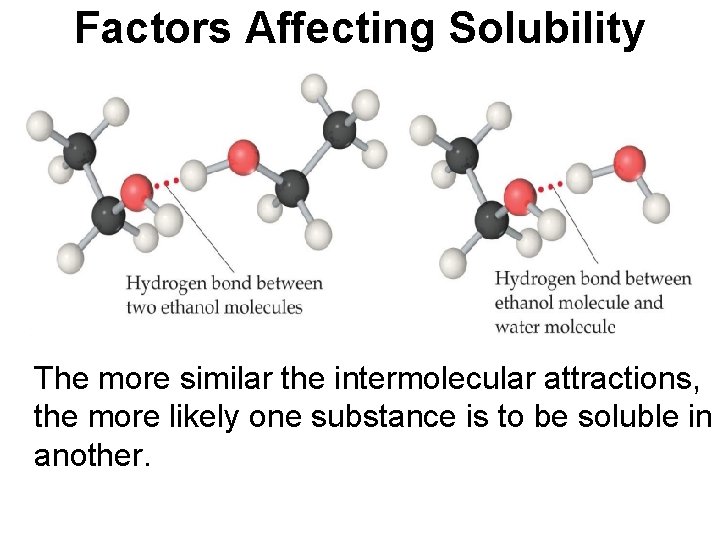
Factors Affecting Solubility The more similar the intermolecular attractions, the more likely one substance is to be soluble in another.

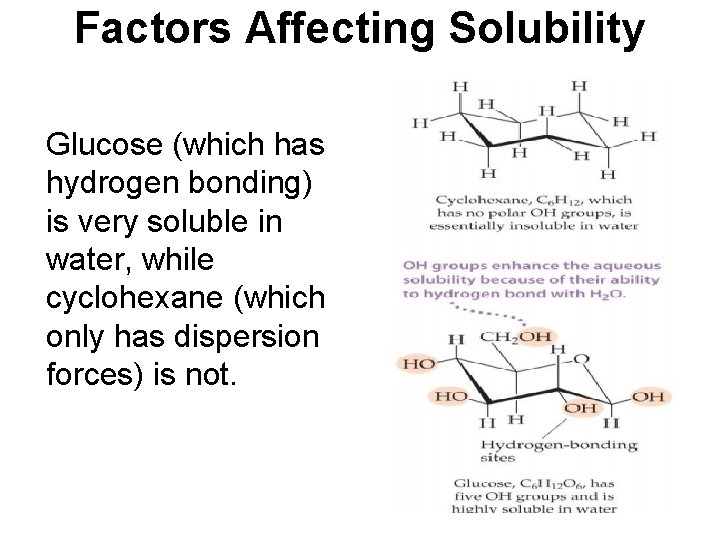
Factors Affecting Solubility Glucose (which has hydrogen bonding) is very soluble in water, while cyclohexane (which only has dispersion forces) is not.

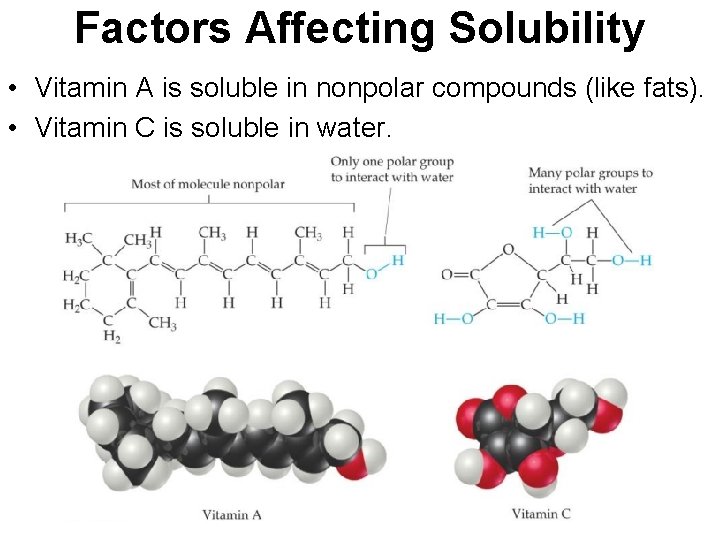
Factors Affecting Solubility • Vitamin A is soluble in nonpolar compounds (like fats). • Vitamin C is soluble in water.

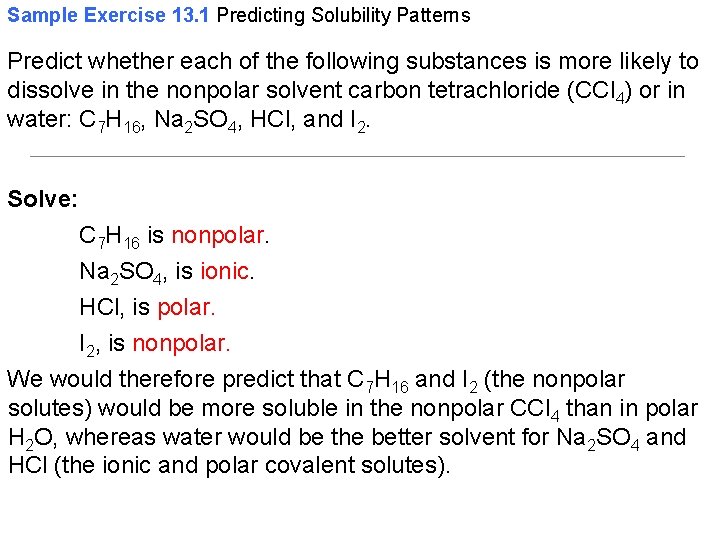
Sample Exercise 13. 1 Predicting Solubility Patterns Predict whether each of the following substances is more likely to dissolve in the nonpolar solvent carbon tetrachloride (CCl 4) or in water: C 7 H 16, Na 2 SO 4, HCl, and I 2. Solve: C 7 H 16 is nonpolar. Na 2 SO 4, is ionic. HCl, is polar. I 2, is nonpolar. We would therefore predict that C 7 H 16 and I 2 (the nonpolar solutes) would be more soluble in the nonpolar CCl 4 than in polar H 2 O, whereas water would be the better solvent for Na 2 SO 4 and HCl (the ionic and polar covalent solutes).

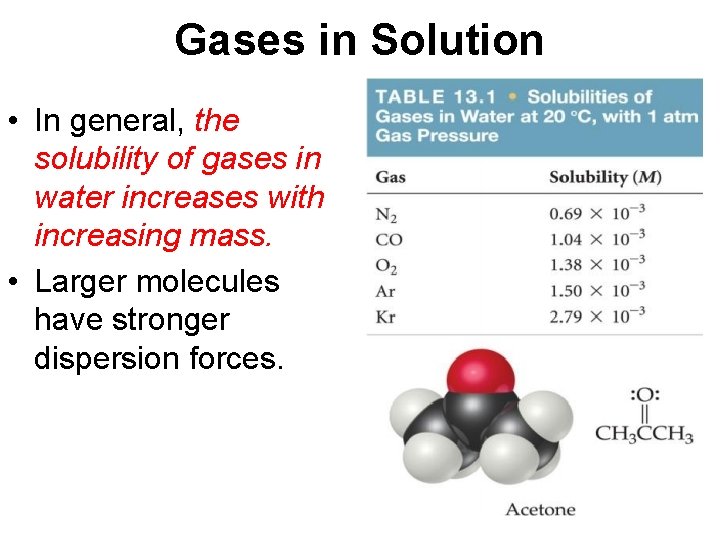
Gases in Solution • In general, the solubility of gases in water increases with increasing mass. • Larger molecules have stronger dispersion forces.

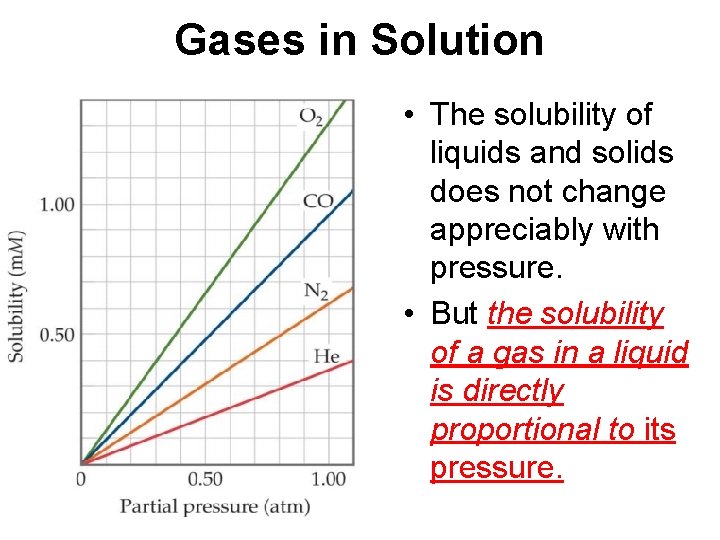
Gases in Solution • The solubility of liquids and solids does not change appreciably with pressure. • But the solubility of a gas in a liquid is directly proportional to its pressure.

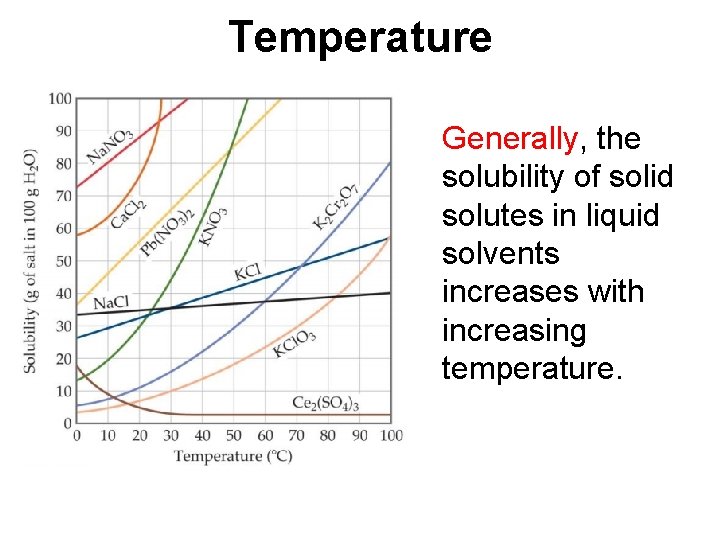
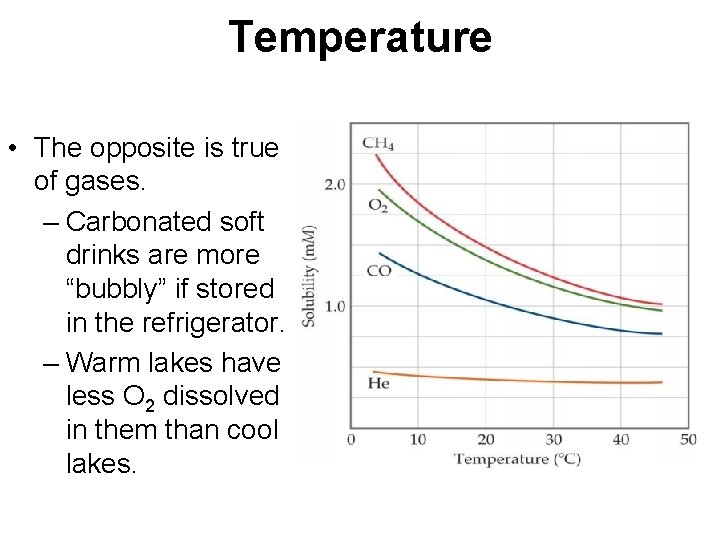
Temperature Generally, the solubility of solid solutes in liquid solvents increases with increasing temperature.

Temperature • The opposite is true of gases. – Carbonated soft drinks are more “bubbly” if stored in the refrigerator. – Warm lakes have less O 2 dissolved in them than cool lakes.

Ways of Expressing Concentrations of Solutions

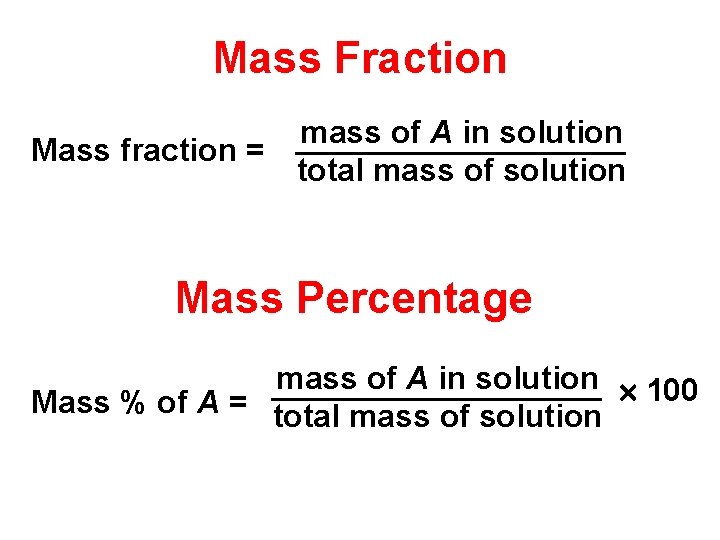
Mass Fraction mass of A in solution Mass fraction = total mass of solution Mass Percentage mass of A in solution 100 Mass % of A = total mass of solution

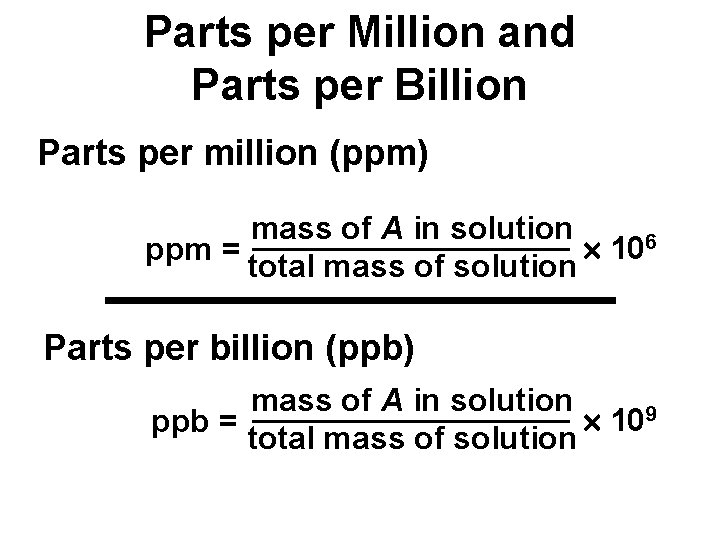
Parts per Million and Parts per Billion Parts per million (ppm) mass of A in solution 106 ppm = total mass of solution Parts per billion (ppb) mass of A in solution 109 ppb = total mass of solution

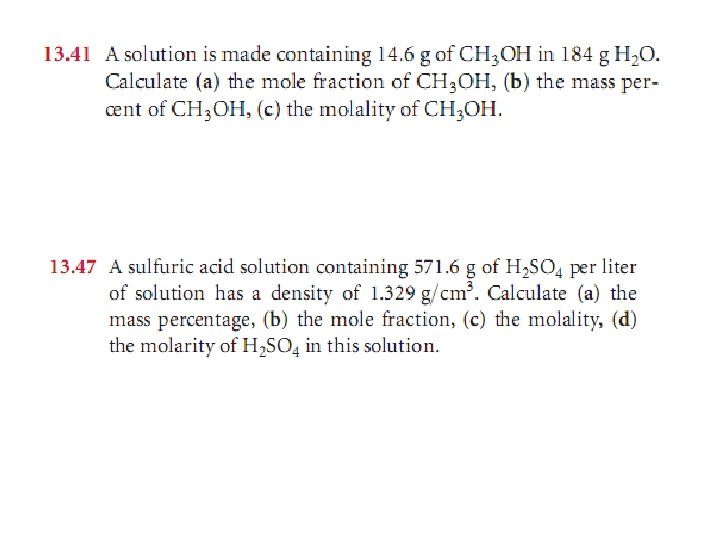
Sample Exercise 13. 3 Calculation of Mass-Related Concentrations (a) A solution is made by dissolving 13. 5 g of glucose (C 6 H 12 O 6) in 0. 100 kg of water. What is the mass percentage of solute in this solution? (b) A 2. 5 -g sample of groundwater was found to contain 5. 4 g of Zn 2+. What is the concentration of Zn 2+ in parts per million? Solution:

Mole Fraction (X ) moles of A XA = total moles of all components • In some applications, one needs the mole fraction of solvent, not solute—make sure you find the quantity you need!

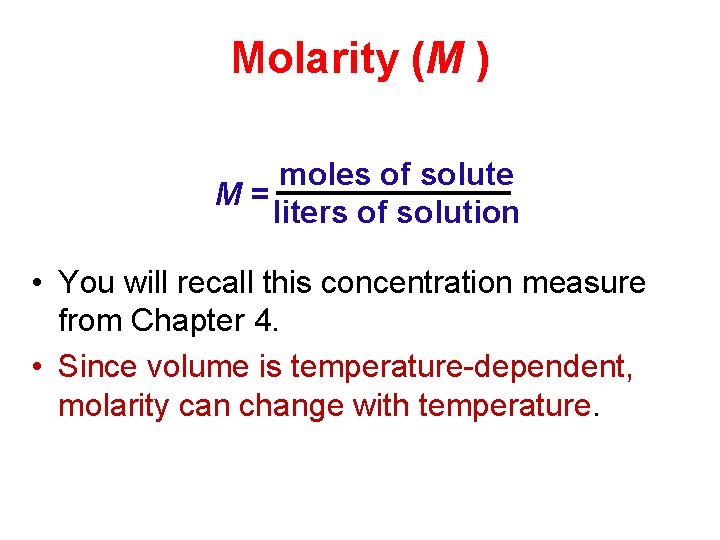
Molarity (M ) moles of solute M= liters of solution • You will recall this concentration measure from Chapter 4. • Since volume is temperature-dependent, molarity can change with temperature.

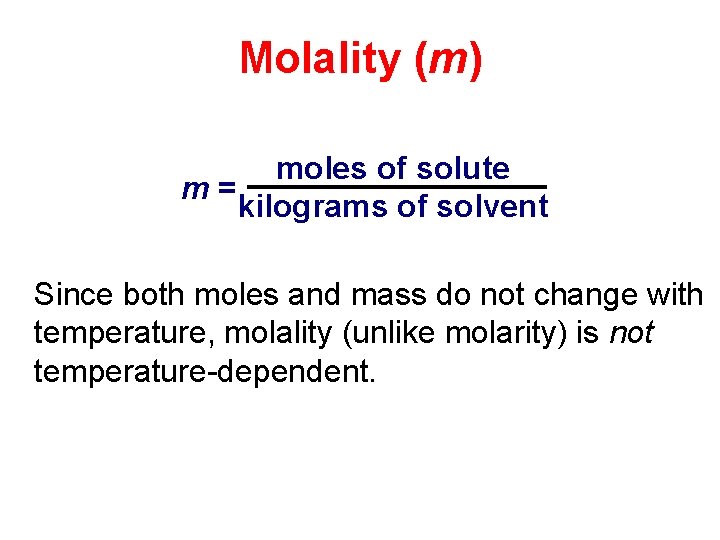
Molality (m) moles of solute m= kilograms of solvent Since both moles and mass do not change with temperature, molality (unlike molarity) is not temperature-dependent.

Sample Exercise 13. 4 Calculation of Molality A solution is made by dissolving 4. 35 g glucose (C 6 H 12 O 6) in 25. 0 m. L of water at 25 C. Calculate the molality of glucose in the solution. Water has a density of 1. 00 g/m. L. Solution: Because water has a density of 1. 00 g/m. L, the mass of the solvent is: (25. 0 m. L)(1. 00 g/m. L) = 25. 0 g = 0. 0250 kg

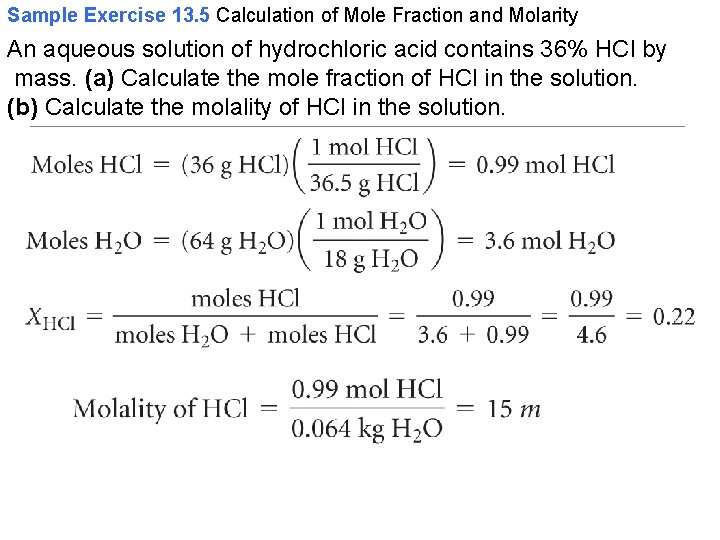
Sample Exercise 13. 5 Calculation of Mole Fraction and Molarity An aqueous solution of hydrochloric acid contains 36% HCl by mass. (a) Calculate the mole fraction of HCl in the solution. (b) Calculate the molality of HCl in the solution.

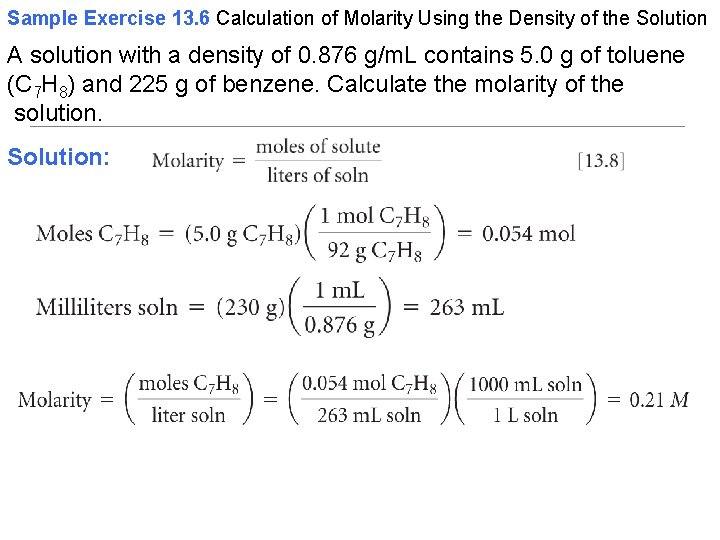
Sample Exercise 13. 6 Calculation of Molarity Using the Density of the Solution A solution with a density of 0. 876 g/m. L contains 5. 0 g of toluene (C 7 H 8) and 225 g of benzene. Calculate the molarity of the solution. Solution:

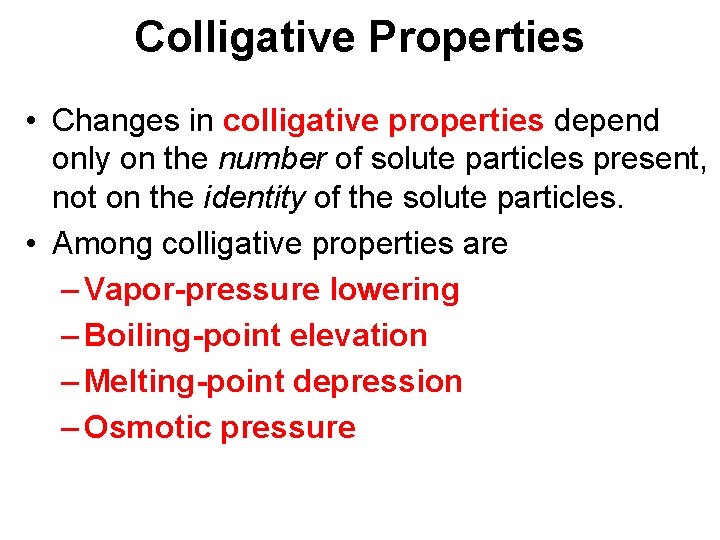
Colligative Properties • Changes in colligative properties depend only on the number of solute particles present, not on the identity of the solute particles. • Among colligative properties are – Vapor-pressure lowering – Boiling-point elevation – Melting-point depression – Osmotic pressure

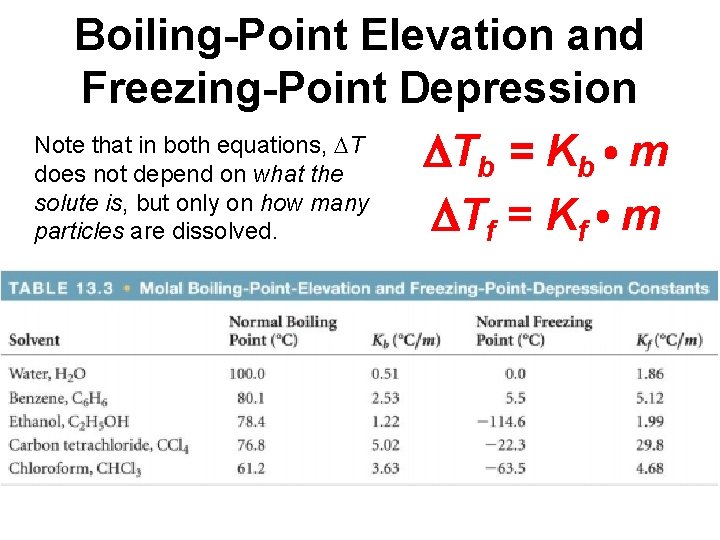
Boiling-Point Elevation and Freezing-Point Depression Note that in both equations, T T = K m b b does not depend on what the solute is, but only on how many Tf = Kf m particles are dissolved.

Sample Exercise 13. 8 Calculating Of Boiling-Point Elevation and Freezing-Point Depression Automotive antifreeze consists of ethylene glycol, CH 2(OH), a nonvolatile nonelectrolyte. Calculate the boiling point and freezing point of a 25. 0 mass % solution of ethylene glycol in water. Tb = Kbm = (0. 51 C/m)(5. 37 m) = 2. 7 C Tf = Kfm = (1. 86 C/m)(5. 37 m) = 10. 0 C Boiling point = (normal bp of solvent) + Tb = 100. 0 C + 2. 7 C = 102. 7 C Freezing point = (normal fp of solvent) Tf = 0. 0 C 10. 0 C = 10. 0 C

Colligative Properties of Electrolytes Since the colligative properties of electrolytes depend on the number of particles dissolved, solutions of electrolytes (which dissociate in solution) should show greater changes than those of nonelectrolytes.

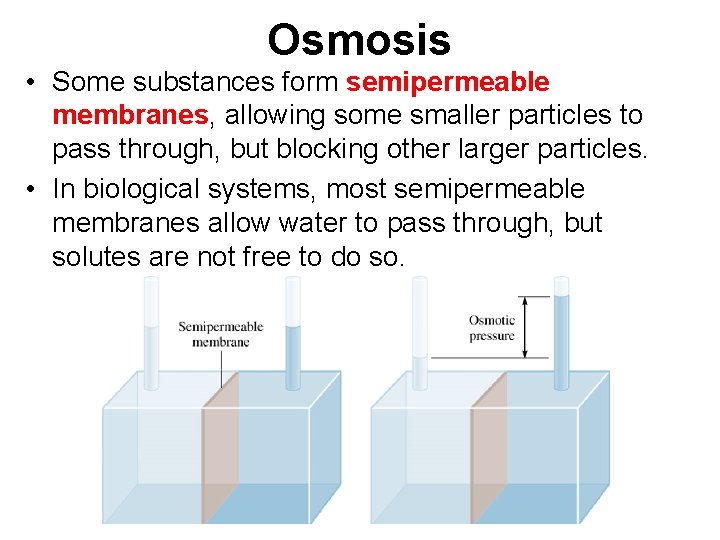
Osmosis • Some substances form semipermeable membranes, allowing some smaller particles to pass through, but blocking other larger particles. • In biological systems, most semipermeable membranes allow water to pass through, but solutes are not free to do so.

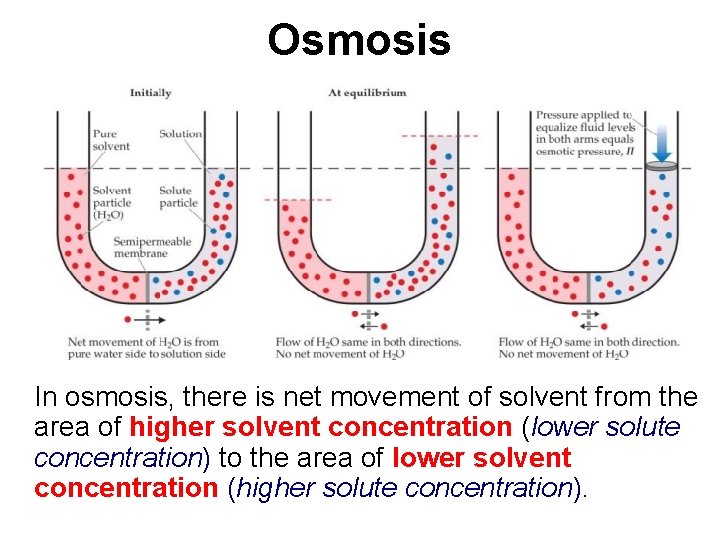
Osmosis In osmosis, there is net movement of solvent from the area of higher solvent concentration (lower solute concentration) to the area of lower solvent concentration (higher solute concentration).

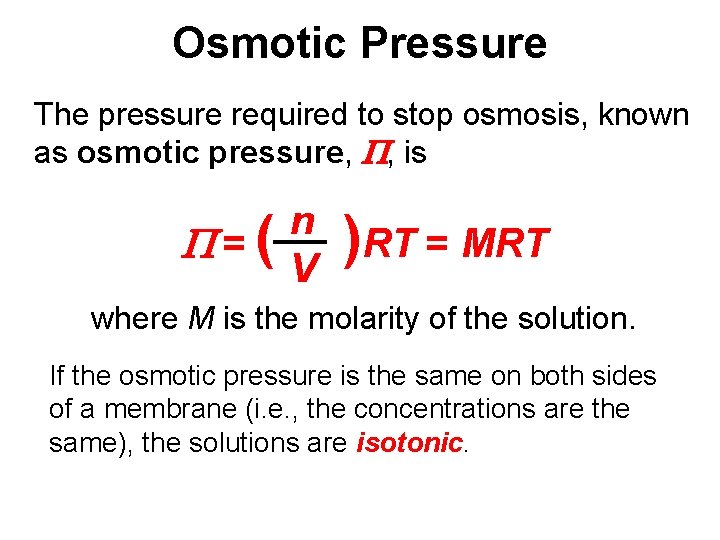
Osmotic Pressure The pressure required to stop osmosis, known as osmotic pressure, P, is P=( n V )RT = MRT where M is the molarity of the solution. If the osmotic pressure is the same on both sides of a membrane (i. e. , the concentrations are the same), the solutions are isotonic.

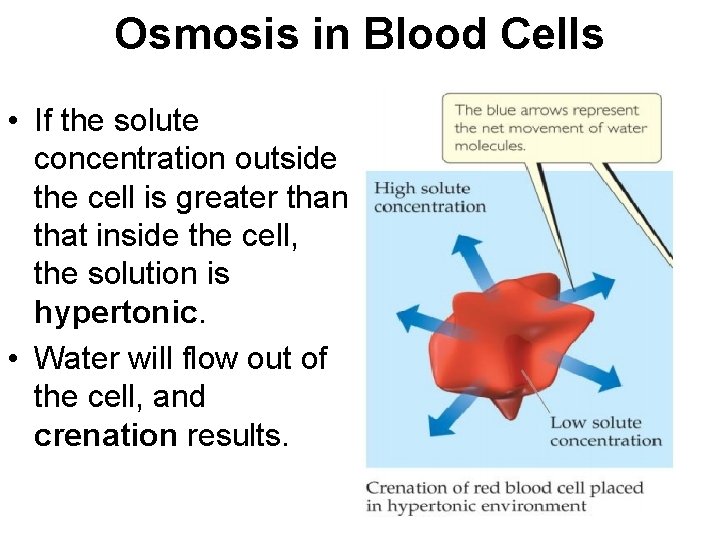
Osmosis in Blood Cells • If the solute concentration outside the cell is greater than that inside the cell, the solution is hypertonic. • Water will flow out of the cell, and crenation results.

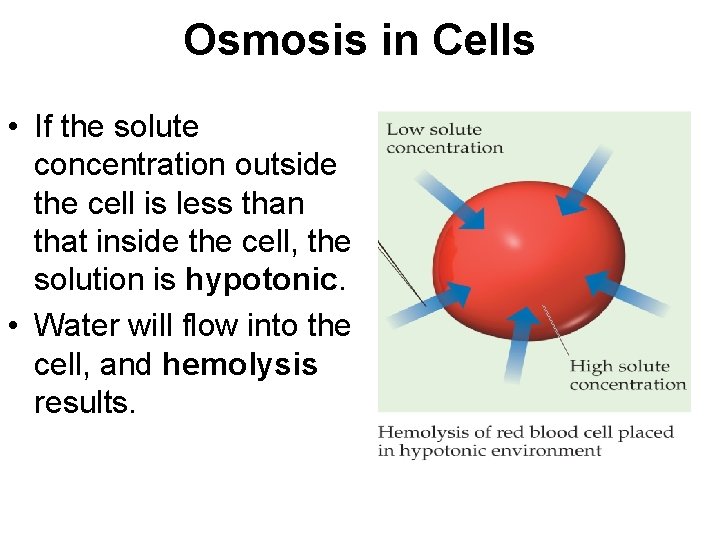
Osmosis in Cells • If the solute concentration outside the cell is less than that inside the cell, the solution is hypotonic. • Water will flow into the cell, and hemolysis results.

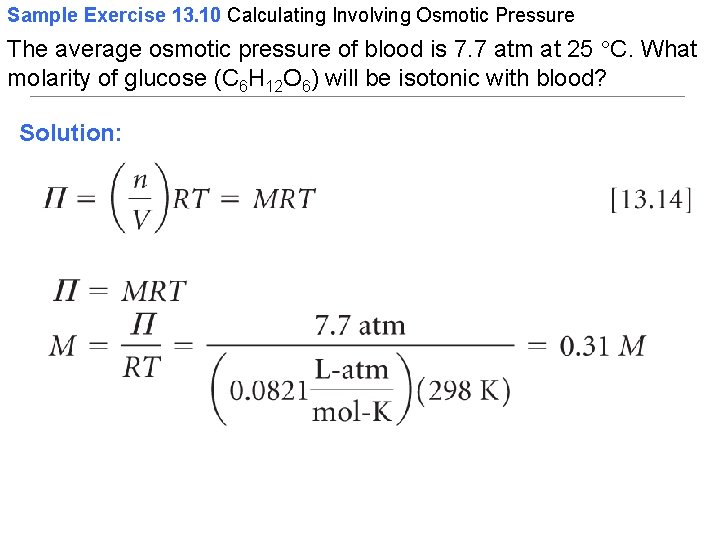
Sample Exercise 13. 10 Calculating Involving Osmotic Pressure The average osmotic pressure of blood is 7. 7 atm at 25 C. What molarity of glucose (C 6 H 12 O 6) will be isotonic with blood? Solution:

Desalination ﺗﺤﻠﻴﺔ ﺍﻟﻤﻴﺎﻩ • “Water, water everywhere, and not a drop to drink. ” Seawater has too high a concentration of Na. Cl for human consumption. • It can be desalinated through reverse osmosis.

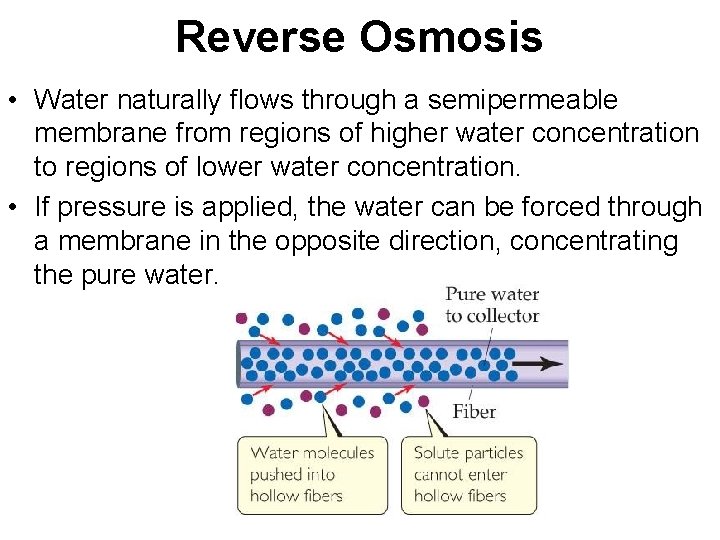
Reverse Osmosis • Water naturally flows through a semipermeable membrane from regions of higher water concentration to regions of lower water concentration. • If pressure is applied, the water can be forced through a membrane in the opposite direction, concentrating the pure water.

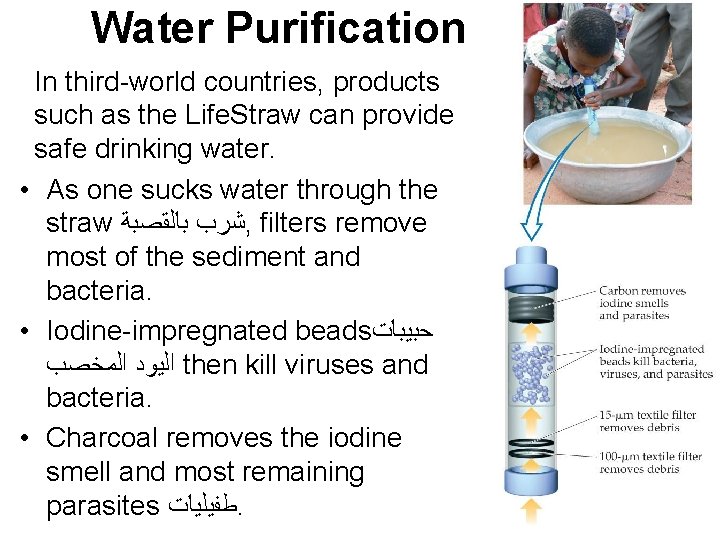
Water Purification In third-world countries, products such as the Life. Straw can provide safe drinking water. • As one sucks water through the straw ﺷﺮﺏ ﺑﺎﻟﻘﺼﺒﺔ , filters remove most of the sediment and bacteria. • Iodine-impregnated beads ﺣﺒﻴﺒﺎﺕ ﺍﻟﻴﻮﺩ ﺍﻟﻤﺨﺼﺐ then kill viruses and bacteria. • Charcoal removes the iodine smell and most remaining parasites ﻃﻔﻴﻠﻴﺎﺕ.



